Techniques for Evaluating Airborne Biocrust Diaspores: From Fundamentals to Advanced Approaches
Abstract
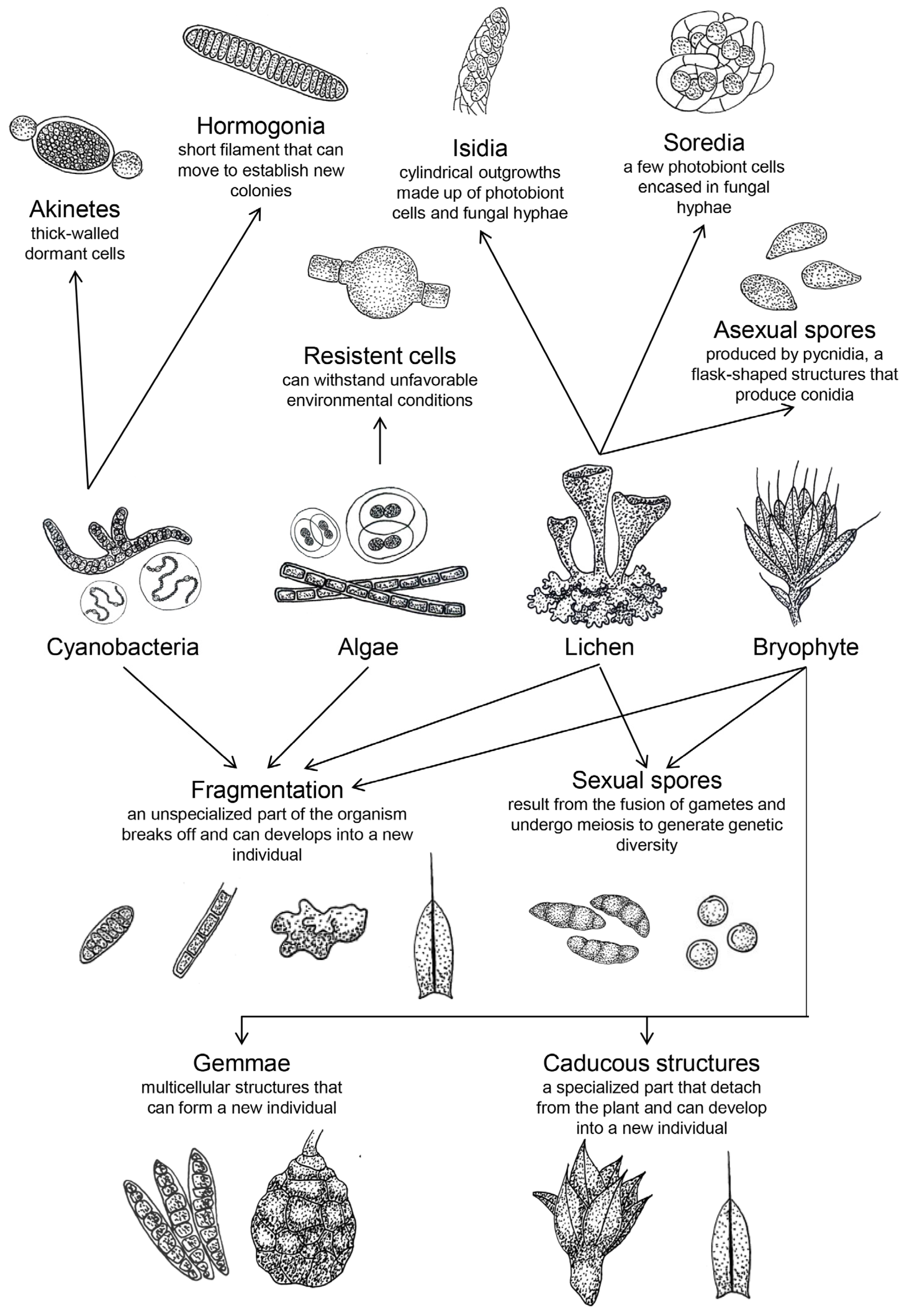
:1. Introduction
2. From Soil to Air: The Emergence and Viability of Airborne Biocrusts
3. Techniques for Sampling Airborne Biocrusts
3.1. Passive Traps
3.2. Active Traps
| Aerobiological Techniques | Organisms | References |
|---|---|---|
| Microscope glass slides | Bryophytes | [106,107,108] |
| Durham sampler | Cyanobacteria | [113] |
| Tauber trap | Bryophytes | [117,118] |
| Culture-plate sampling | Cyanobacteria, algae, bryophytes, lichens | [106,116,119,120,121] |
| Specialized trap with cloths | Bryophytes | [117] |
| Diaspore trap | Bryophytes | [55] |
| Rotorod | Cyanobacteria, algae, bryophytes, lichens | [51,54,127,128,129,130,131] |
| Hirst impact sampler | Cyanobacteria, algae, bryophytes, lichens | [56,133,134,135] |
4. From Air to Laboratory: Sifting Through Biological Airborne Samples
5. Making Your Own Research Design
- I.
- Define the objective of the experiment, clearly establishing what you wish to investigate, such as the dispersal of airborne organisms within biocrust environments.
- II.
- Review the literature, consulting previous works to understand the methodologies employed in studies related to airborne organisms also found within biocrusts, and identify those that can be applied to your research. We have compiled many of these references in Table 1, where we categorize them by organism group and the techniques employed, providing a valuable starting point for your bibliographic research.
- III.
- Select the methodologies by deciding which approaches will be used. Options may include low-cost passive techniques (e.g., Durham sampler, Tauber trap, culture-plate sampling) or active techniques that require more elaborate devices (e.g., Rotorod, Hirst impact sampler, Burkard trap), followed by traditional cultivation and classical identification of organisms. Remember that to accurately identify organisms, it is often necessary to collaborate with specialist taxonomists and utilize equipment such as stereomicroscopes and microscopes, in addition to having access to identification keys. Alternatively, you can choose advanced approaches for species identification, such as DNA metabarcoding, which provides a more comprehensive analysis of community composition. In this case, Table 2 provides a useful resource for learning more about these techniques as applied to biocrust organisms, especially by presenting data on the most commonly used primers in barcoding methods for biocrust organisms.
- IV.
- If you plan to cultivate organisms, make sure to select growth media that are suitable for their specific requirements. Always consult the literature to identify the most appropriate conditions. Additionally, ensure that the cultivation rooms have standardized temperature and photoperiod settings that are tailored to the needs of your target organisms. Maintaining these controlled conditions is essential for achieving reliable and consistent results.
- V.
- Consider adapting existing methodologies to better suit your specific needs, such as modifying sampling protocols or analytical techniques that have proven effective in related studies. If required, develop new devices to address challenges encountered during data collection, as demonstrated in previous research tackling similar issues. Notably, airborne biocrust particles exhibit relatively unknown aerodynamic behaviors compared to typical bioaerosols like pollen, fungal spores, and bacteria, which can lead to challenges with existing sampling methods. Thus, we emphasize the importance of studies focusing on the aerodynamics of these diaspores. Research utilizing wind tunnels [105], for instance, may help in investigating these dynamics and facilitating the development of more efficient sampling devices.
- VI.
- Finally, studying biocrusts is inherently a multidisciplinary task. We strongly recommend seeking partnerships with professionals from various fields, including taxonomists, ecologists, geneticists, geologists, and even physicists. Collaborating with experts from different disciplines is crucial for overcoming challenges within the study of biocrust aerobiology, such as accurately identifying the vast diversity of organisms, sampling and analysis techniques, and technical issues related to aerodynamic sizes and behavior in the atmosphere. A multidisciplinary team makes it easier to tackle the complexity of biocrusts and the various variables involved in their analysis. This collaborative approach can not only accelerate the research process but also provide more effective and innovative solutions to the diverse obstacles encountered.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, B.; Belnap, J.; Büdel, B.; Antoninka, A.J.; Barger, N.N.; Chaudhary, V.B.; Darrouzet-Nardi, A.; Eldridge, D.J.; Faist, A.M.; Ferrenberg, S.; et al. What is a biocrust? A refined, contemporary definition for a broadening research community. Biol. Rev. 2022, 97, 1768–1785. [Google Scholar] [CrossRef] [PubMed]
- Büdel, B.; Dulic, T.; Darienko, T.; Rybalka, N.; Friedl, T. Cyanobacteria and algae of biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 55–80. [Google Scholar]
- Maier, S.; Muggia, L.; Kuske, C.R.; Grube, M. Bacteria and non-lichenized fungi within biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 81–100. [Google Scholar]
- Seppelt, R.D.; Downing, A.J.; Deane-Coe, K.K.; Zhang, Y.; Zhang, J. Bryophytes within biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 101–120. [Google Scholar]
- Rosentreter, R.; Eldridge, D.J.; Westberg, M.; Williams, L.; Grube, M. Structure, composition, and function of biocrust lichen communities. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 121–138. [Google Scholar]
- Mager, D.M.; Thomas, A.D. Extracellular polysaccharides from cyanobacterial soil crusts: A review of their role in dryland soil processes. J. Arid Environ. 2011, 75, 91–97. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Greene, R.S.B. Microbiotic soil crusts: A review of their roles in soil and ecological processes in the range lands of Australia. Aust. J. Soil Res. 1994, 32, 389–415. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Felde, V.J.M.N.L.; Drahorad, S.L.; Weber, B. Microstructure and weathering processes within biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 237–255. [Google Scholar]
- Benalp, J.; Büdel, B.; Lange, O.L. Biological soil crusts: Characteristics and distribution. In Biological Soil Crusts: Structure, Function, and Management; Benalp, J., Lange, O.L., Eds.; Springer: Berlin, Germany, 2001; pp. 3–30. [Google Scholar]
- Thompson, W.A.; Eldridge, D.J.; Bonser, S.P. Structure of biological soil crust communities in Callitris glaucophylla woodlands of South Wales, Australia. J. Veg. Sci. 2006, 17, 271–280. [Google Scholar] [CrossRef]
- Issa, O.M.; Trichet, J.; Défarge, C.; Couté, A.; Valentin, C. Morphology and microstructure of microbiotic soil crusts on a tiger bush sequence (Niger, Sahel). CATENA 1999, 37, 175–196. [Google Scholar] [CrossRef]
- Benalp, J.; Weber, B.; Büdel, B. Biological soil crusts as an organizing principle in drylands. In Biological Soil Crusts as an Organizing Principle in Drylands; Weber, B., Büdel, B., Benalp, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 3–13. [Google Scholar]
- Lan, S.; Thomas, A.D.; Rakes, J.B.; Garcia-Pichel, F.; Wu, L.; Hu, C. Cyanobacterial community composition and their functional shifts associated with biocrust succession in the Gurbantunggut Desert. Environ. Microbiol. Rep. 2021, 13, 884–898. [Google Scholar] [CrossRef]
- Steven, B.; Lionard, M.; Kuske, C.R.; Vincent, W.F. High bacterial diversity of biological soil crusts in water tracks over permafrost in the high arctic polar desert. PLoS ONE 2013, 8, e71489. [Google Scholar] [CrossRef] [PubMed]
- Neher, D.A.; Walters, T.L.; Tramer, E.; Weicht, T.R.; Veluci, R.M.; Saiya-Cork, K.; Will-Wolf, S.; Toppin, J.; Traub, J.; Johansen, J.R. Biological soil crust and vascular plant communities in a sand savanna of northwestern Ohio. J. Torrey Bot. Soc. 2003, 130, 244–252. [Google Scholar] [CrossRef]
- Machado-de-Lima, N.M.; Fernandes, V.M.C.; Roush, D.; Ayuso, S.V.; Rigonato, J.; Garcia-Pichel, F.; Branco, L.H.Z. The compositionally distinct cyanobacterial biocrusts from Brazilian savanna and their environmental drivers of community diversity. Front. Microbiol. 2019, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Maya, Y.; López-Cortés, A. Cyanobacterial microbiotic crusts in eroded soils of a tropical dry forest in the Baja California Peninsula, Mexico. Geomicrobiol. J. 2002, 19, 505–518. [Google Scholar] [CrossRef]
- Szyja, M.; Menezes, A.G.S.; Oliveira, F.D.A.; Leal, I.; Tabarelli, M.; Büdel, B.; Wirth, R. Neglected but potent dry forest players: Ecological role and ecosystem service provision of biological soil crusts in the human-modified Caatinga. Front. Ecol. Evol. 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Figueredo, C.C.; Konell, A.H.; Maciel-Silva, A.S. A first evaluation of biological soil crusts diversity in three distinctive rocky outcrops in Brazil. Flora 2024, 320, 152613. [Google Scholar] [CrossRef]
- Seitz, S.; Nebel, M.; Goebes, P.; Käppeler, K.; Schmidt, K.; Shi, X.; Song, Z.; Webber, C.L.; Weber, B.; Scholten, T. Bryophyte-dominated biological soil crusts mitigate soil erosion in a nearly successional Chinese subtropical forest. Biogeosciences 2017, 14, 5775–5788. [Google Scholar] [CrossRef]
- Grover, H.S.; Bowker, M.A.; Fulé, P.Z. Improved, scalable techniques to cultivate fire mosses for rehabilitation. Restor. Ecol. 2020, 28, S17–S24. [Google Scholar] [CrossRef]
- Mikhailyuk, T.; Glaser, K.; Tsarenko, P.; Demchenko, E.; Karsten, U. Composition of biological soil crusts from sand dunes of the Baltic Sea Coast in the context of an integrative approach to the taxonomy of microalgae and cyanobacteria. Eur. J. Phycol. 2019, 54, 263–290. [Google Scholar] [CrossRef]
- Corbin, J.D.; Thiet, R.K. Temperate biocrusts: Mesicc ounterparts to their better-known dryland cousins. Front. Ecol. Environ. 2020, 18, 456–464. [Google Scholar] [CrossRef]
- Rodriguez-Caballero, E.; Belnap, J.; Büdel, B.; Crutzen, P.J.; Andreae, M.O.; Pöschl, U.; Weber, B. Dryland photoautotrophic soil surface communities endangered by global change. Nat. Geosci. 2018, 11, 185–189. [Google Scholar] [CrossRef]
- Mager, D.M. Carbohydrates in cyanobacterial soil crusts as a source of carbon in the southwest Kalahari, Botswana. Soil Biol. Biochem. 2010, 42, 313–318. [Google Scholar] [CrossRef]
- Miralles, I.; Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Labile carbon in biological soil crusts in the Tabernas Desert, SE Spain. Soil Biol. Biochem. 2013, 58, 1–8. [Google Scholar] [CrossRef]
- Zhou, X.; Tao, Y.; Yin, B.; Tucker, C.; Zhang, Y. Nitrogen pools in soil covered by biological soil crusts of different successional stages in a temperate desert in Central Asia. Geoderma 2020, 366, 114166. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, W.; Chen, H.; Wang, K. Moss-dominated biological soil crusts modulate soil nitrogen following vegetation restoration in a subtropical karst region. Geoderma 2019, 352, 70–79. [Google Scholar] [CrossRef]
- Baumann, K.; Glaser, K.; Mutz, J.E. Biological soil crusts of temperate forests: Their role in P cycling. Soil Biol. Biochem. 2017, 109, 156–166. [Google Scholar] [CrossRef]
- Baumann, K.; Jung, P.; Samolov, E.; Lehnert, L.W.; Büdel, B.; Karsten, U.; Bendix, J.; Achilles, S.; Schermer, M.; Matus, F.; et al. Biological soil crusts along a climatic gradient in Chile: Richness and imprints of phototrophic microorganisms in phosphorus biogeochemical cycling. Soil Biol. Biochem. 2018, 127, 286–300. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, H.; Zuo, X.; Drake, S.; Zhao, X. Biological soil crust development and its topsoil properties in the process of dune stabilization, Inner Mongolia, China. Environ. Geol. 2008, 54, 653–662. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Leys, J.F. Exploring some relationships between biological soil crusts, soil aggregation and wind erosion. J. Arid Environ. 2003, 53, 457–466. [Google Scholar] [CrossRef]
- Belnap, J. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol. Process. 2006, 20, 3159–3178. [Google Scholar] [CrossRef]
- Shi, W.; Wang, X.P.; Zhang, Y.F.; Pan, Y.X.; Hu, R.; Jin, Y.X. The effect of biological soil crusts on soil moisture dynamics under different rainfall conditions in the Tengger Desert, China. Hydrol. Process. 2018, 32, 1363–1374. [Google Scholar] [CrossRef]
- Langhans, T.M.; Storm, C.; Schwabe, A. Biological soil crusts and their microenvironment: Impact on emergence, survival and establishment of seedlings. Flora 2009, 204, 157–168. [Google Scholar] [CrossRef]
- Li, X.R.; Jia, X.H.; Long, L.Q.; Zerbe, S. Effects of biological soil crusts on seed bank, germination and establishment of two annual plant species in the Tengger Desert (N China). Plant Soil 2005, 277, 375–385. [Google Scholar] [CrossRef]
- Darby, B.J.; Neher, D.A.; Belnap, J. Soil nematode communities are ecologically more mature beneath late- than early-successional stage biological soil crusts. Appl. Soil Ecol. 2007, 35, 203–212. [Google Scholar] [CrossRef]
- Neher, D.A.; Lewins, S.A.; Weicht, T.R.; Darby, B.J. Microarthropod communities associated with biological soil crusts in the Colorado Plateau and Chihuahuan deserts. J. Arid Environ. 2009, 73, 672–677. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Maciel-Silva, A.S. Biological soil crusts and how they might colonize other worlds: Insights from these Brazilian ecosystem engineers. J. Exp. Bot. 2022, 73, 4362–4379. [Google Scholar] [CrossRef]
- Xiao, B.; Bowker, M.A.; Zhao, Y.; Chamizo, S.; Issa, O.M. Biocrusts: Engineers and architects of surface soil properties, functions, and processes in dryland ecosystems. Geoderma 2022, 424, 116015. [Google Scholar] [CrossRef]
- Green, T.A.; Proctor, M.C. Physiology of photosynthetic organisms within biological soil crusts: Their adaptation, flexibility, and plasticity. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 347–381. [Google Scholar]
- Mackelprang, R.; Vaishampayan, P.; Fisher, K. Adaptation to environmental extremes structures functional traits in biological soil crust and hypolithic microbial communities. mSystems 2022, 7, e01419-21. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Krochko, J.E. Desiccation-tolerance. In Physiological Plant Ecology II: Water Relations and Carbon Assimilation; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: NewYork, NY, USA, 1982; pp. 325–378. [Google Scholar]
- Dillon, J.G.; Castenholz, R.W. Scytonemin, a cyanobacterial sheath pigment, protects against UVC radiation: Implications for early photosynthetic life. J. Phycol. 1999, 35, 673–681. [Google Scholar] [CrossRef]
- Aigner, S.; Remias, D.; Karsten, U.; Holzinger, A. Unusual phenolic compounds contribute to ecophysiological performance in the purple-colored green alga Zygogonium ericetorum (Zygnematophyceae, Streptophyta) from a high-alpine habitat. J. Phycol. 2013, 49, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Kappen, L. Response to extreme environments. In The Lichens; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: New York, NY, USA, 1973; pp. 311–380. [Google Scholar]
- Frahm, J.P. Diversity, life strategies, origins and distribution of tropical inselbergs bryophytes. An. Del Inst. Biol. Ser. Botánica 1996, 67, 73–86. [Google Scholar]
- Vanderpoorten, A.; Goffinet, B. Introduction to Bryophytes; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Warren, S.D.; St. Clair, L.L.; Stark, L.R.; Lewis, L.A.; Pombubpa, N.; Kurbessoian, T.; Stajich, J.E.; Aanderud, Z.T. Reproduction and dispersal of biological soil crust organisms. Front. Ecol. Evol. 2019, 7, 344. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rai, A.K.; Singh, S.; Brown, R.M., Jr. Airborne algae: Their present status and relevance. J. Phycol. 2007, 43, 615–627. [Google Scholar] [CrossRef]
- Sharma, N.K.; Singh, S. Differential aerosolization of algal and cyanobacterial particles in the atmosphere. Indian J. Microbiol. 2010, 50, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.W. Ecological strategies of lichens. Lichenologist 1990, 22, 149–162. [Google Scholar] [CrossRef]
- Laaka-Lindberg, S.; Korpelainen, H.; Pohjamo, M. Dispersal of asexual propagules in bryophytes. J. Hattori Bot. Lab. 2003, 93, 319–330. [Google Scholar] [CrossRef]
- Marshall, W.A. Aerial dispersal of lichen soredia in the maritime Antarctic. New Phytol. 1996, 134, 523–530. [Google Scholar] [CrossRef]
- Robinson, S.C.; Miller, N.G. Bryophyte diversity on Adirondack alpine summits is maintained by dissemination and establishment of vegetative fragments and spores. Bryologist 2013, 116, 382–391. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Sandrone, S.; Matteucci, E.; Appolonia, L.; Piervittori, R. Spores of lichen-forming fungi in the mycoaerosol and their relationships with climate factors. Sci. Total Environ. 2014, 466, 26–33. [Google Scholar] [CrossRef]
- Hutsemekers, V.; Dopagne, C.; Vanderpoorten, A. How far and how fast do bryophytes travel at the landscape scale? Divers. Distrib. 2008, 14, 483–492. [Google Scholar] [CrossRef]
- Muñoz, J.; Felicísimo, Á.M.; Cabezas, F.; Burgaz, A.R.; Martínez, I. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science 2004, 304, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Frahm, J.P. Diversity, dispersal and biogeography of bryophytes (mosses). In Protist Diversity and Geographical Distribution; Foissner, W., Hawksworth, D.L., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 8, pp. 43–50. [Google Scholar]
- Bowker, M.A.; Belnap, J.; Büdel, B.; Sannier, C.; Pietrasiak, N.; Eldridge, D.J.; Rivera-Aguilar, V. Controls on distribution patterns of biological soil crusts at micro- to global scales. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 173–197. [Google Scholar]
- Chu, W.L.; Tneh, S.Y.; Ambu, S. A survey of airborne algae and cyanobacteria within the indoor environment of an office building in Kuala Lumpur, Malaysia. Grana 2013, 52, 207–220. [Google Scholar] [CrossRef]
- Warren, S.D.; St. Clair, L.L.; Leavitt, S.D. Aerobiology and passive restoration of biological soil crusts. Aerobiologia 2019, 35, 45–56. [Google Scholar] [CrossRef]
- Warren, S.D.; St. Clair, L.L. Atmospheric transport and mixing of biological soil crust microorganisms. AIMS Environ. Sci. 2021, 8, 498–516. [Google Scholar] [CrossRef]
- Sahu, N.; Tangutur, A.D. Airborne algae: Overview of the current status and its implications on the environment. Aerobiologia 2015, 31, 89–97. [Google Scholar] [CrossRef]
- Després, V.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Lee, R.E. Cyanobacteria. In Phycology, 4th ed.; Lee, R.E., Ed.; Cambridge University Press: New York, NY, USA, 2018; pp. 33–84. [Google Scholar]
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Lee, R.E. Basic characteristics of the algae. In Phycology, 4th ed.; Lee, R.E., Ed.; Cambridge University Press: New York, NY, USA, 2018; pp. 3–29. [Google Scholar]
- Guiry, M.D. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef]
- Lee, R.E. Chlorophyta. In Phycology, 4th ed.; Lee, R.E., Ed.; Cambridge University Press: New York, NY, USA, 2018; pp. 139–226. [Google Scholar]
- Lee, R.E. Heterokontophyta—Bacillariophyceae. In Phycology, 4th ed.; Lee, R.E., Ed.; Cambridge University Press: New York, NY, USA, 2018; pp. 369–408. [Google Scholar]
- Edlund, M.B.; Stoermer, E.F. Ecological, evolutionary, and systematic significance of diatom life histories. J. Phycol. 1997, 33, 897–918. [Google Scholar] [CrossRef]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. Corrections and amendments to the 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota. Bryologist 2017, 120, 58–69. [Google Scholar] [CrossRef]
- Lutzoni, F.; Miadlikowska, J. Lichens. Curr. Biol. 2009, 19, R502–R503. [Google Scholar] [CrossRef]
- Jahns, H.M.; Galun, M. The Lichen Thallus. In Handbook of Lichenology; Galun, M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 1, pp. 95–143. [Google Scholar]
- Blackwell, M.; Alexopoulos, C.J.; Mims, C.W. Introductory Mycology; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Heinken, T. Dispersal patterns of terricolous lichens by thallus fragments. Lichenologist 1999, 34, 603–612. [Google Scholar] [CrossRef]
- Vobis, G.; Hawksworth, D.L.; Cole, G.T.; Kendrick, B. Conidial lichen-forming fungi. In Biology of Conidial Fungi; Cole, G.T., Kendrick, B., Eds.; Academic Press: San Diego, CA, USA, 1981; Volume 1, pp. 245–273. [Google Scholar]
- Marshall, W.A. Seasonality in Antarctic airborne fungal spores. Appl. Environ. Microbiol. 1997, 63, 2240–2245. [Google Scholar] [CrossRef] [PubMed]
- Maciel-Silva, A.S.; Pôrto, K.C. Reproduction in Bryophytes. In Reproductive Biology of Plants; Ramawat, K.G., Merillon, J.M., Shivanna, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 57–81. [Google Scholar]
- Glime, J.M. Adaptive strategies: Vegetative vs sexual diaspores. In Bryophyte Ecology; Glime, J.M., Ed.; Michigan Technological University: Houghton, MI, USA, 2017; Volume 1, pp. 1–61. [Google Scholar]
- Frey, W.; Kürschner, H. Asexual reproduction, habitat colonization and habitat maintenance in bryophytes. Flora 2011, 206, 173–184. [Google Scholar] [CrossRef]
- Vanderpoorten, A.; Patiño, J.; Désamoré, A.; Laenen, B.; Górski, P.; Papp, B.; Holá, E.; Korpelainen, H.; Hardy, O. To what extent are bryophytes efficient dispersers? J. Ecol. 2019, 107, 2149–2154. [Google Scholar] [CrossRef]
- Patiño, J.; Vanderpoorten, A. Bryophyte biogeography. Crit. Rev. Plant Sci. 2018, 37, 175–209. [Google Scholar] [CrossRef]
- Smith, R.J.; Stark, L.R. Habitat vs. dispersal constraints on bryophyte diversity in the Mojave Desert, USA. J. Arid Environ. 2014, 102, 76–81. [Google Scholar] [CrossRef]
- van Zanten, B.O.; Pócs, T. Distribution and dispersal of bryophytes. Adv. Bryol. 1981, 1, 479–562. [Google Scholar]
- van Zanten, B.O.; Gradstein, S.R. Experimental dispersal geography of neotropical liverworts. Beih. Zur Nova Hedwig. 1988, 90, 41–94. [Google Scholar]
- Büdel, B.; Weber, B.; Kühl, M.; Pfanz, H.; Sültemeyer, D.; Wessels, D. Reshaping of sandstone surfaces by cryptoendolithic cyanobacteria: Bioalkalization causes chemical weathering in arid landscapes. Geobiology 2004, 2, 261–268. [Google Scholar] [CrossRef]
- Prospero, J.M.; Blades, E.; Mathison, G.; Naidu, R. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 2005, 21, 1–19. [Google Scholar] [CrossRef]
- Studlar, S.M.; Eddy, C.; Spencer, J. Survival of four mosses from West Virginia after two hours in the stratosphere. Evansia 2007, 24, 17–21. [Google Scholar] [CrossRef]
- Estébanez, B.; Medina, N.G.; Caparrós, R.; Monforte, L.; Del-Castillo-Alonso, M.Á.; Martínez-Abaigar, J.; Núñez-Olivera, E. Spores potentially dispersed to longer distances are more tolerant to ultraviolet radiation: A case study in the moss genus Orthotrichum. Am. J. Bot. 2018, 105, 996–1008. [Google Scholar] [CrossRef]
- Saxena, V.K. Evidence of the biogenicnuclei involvement in Antarctic coastal clouds. J. Phys. Chem. 1983, 87, 4130–4134. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hashimoto, H.; Nakagawa, T.; Shibata, S. Survivability of moss and fungal spores in tests simulating conditions of the ISS outer wall. Biol. Sci. Space 2011, 25, 83–92. [Google Scholar] [CrossRef]
- Nicholson, K.W. Physical aspects of bioaerosol sampling and deposition. In Bioaerosols Handbook; Cox, C.S., Wathes, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 27–53. [Google Scholar]
- Zimmerman, N.J.; Reist, P.C.; Turner, A.G. Comparison of two biological aerosol sampling methods. Appl. Environ. Microbiol. 1987, 53, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, H.E. The importance of airborne algae and protozoa. J. Air Pollut. Control Assoc. 1969, 19, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, A. Some observations on the size and shape of lichen ascospores in relation to ecology and taxonomy. New Phytol. 1981, 89, 667–678. [Google Scholar] [CrossRef]
- Mogensen, G.S. The biological significance of morphological characters in bryophytes: The spore. Bryologist 1981, 84, 187–207. [Google Scholar] [CrossRef]
- Lacey, M.E.; West, J.S. Introduction to aerobiology. In The Air Spora; Lacey, M.E., West, J.S., Eds.; Springer: Boston, MA, USA, 2006; pp. 1–14. [Google Scholar]
- Mogensen, G. The Spore. In New Manual of Bryology; Schuster, R.M., Ed.; The Hattori Botanical Laboratory: Nichinan, Japan, 1983; Volume 1, pp. 325–342. [Google Scholar]
- Sherwood, A.R.; Wade, R.M.; Conklin, K.Y. Seasonality of tropical airborne algae: A 16-month study based on high-through put sequencing in the Hawaiian Islands. Grana 2020, 59, 354–365. [Google Scholar] [CrossRef]
- Žilka, M.; Tropeková, M.; Zahradníková, E.; Kováčik, Ľ.; Ščevková, J. Temporal variation in the spectrum and concentration of airborne microalgae and cyanobacteria in the urban environments of inland temperate climate. Environ. Sci. Pollut. Res. 2023, 30, 97616–97628. [Google Scholar] [CrossRef] [PubMed]
- Gallenmüller, F.; Langer, M.; Poppinga, S.; Kassemeyer, H.H.; Speck, T. Spore liberation in mosses revisited. AoB Plants 2018, 10, plx075. [Google Scholar] [CrossRef] [PubMed]
- Johansson, V.; Lönnell, N.; Sundberg, S.; Hylander, K. Release thresholds for moss spores: The importance of turbulence and sporophyte length. J. Ecol. 2014, 102, 721–729. [Google Scholar] [CrossRef]
- Lacey, M.E.; West, J.S. Airsampling techniques. In The Air Spora; Lacey, M.E., West, J.S., Eds.; Springer: Boston, MA, USA, 2006; pp. 35–47. [Google Scholar]
- Rudolph, E.D. Local dissemination of plant propagules in Antarctica. In Antarctic Ecology; Holdgate, M.W., Ed.; Academic Press: London, UK, 1970; pp. 812–817. [Google Scholar]
- Zhang, Z.Q.; Min, H.X.; Wang, Z.H.; Zhang, Z.H. Study on spore release of Polytrichum commune Hedw. var. commune by synergetic effects of sub-hygroscopic movement and wind. Plant Biol. 2021, 23, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Stoneburner, A.; Lane, D.M.; Anderson, L.E. Spore dispersal distances in Atrichum angustatum (Polytrichaceae). Bryologist 1992, 95, 324–328. [Google Scholar] [CrossRef]
- Nuntiis, P.; Maggi, O.; Mandrioli, P.; Ranalli, G.; Sorlini, C. Monitoring the biological aerosol. In Cultural Heritage and Aerobiology; Mandrioli, P., Caneva, G., Sabbioni, C., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 1–10. [Google Scholar]
- Durham, O.C. The volumetric incidence of atmospheric allergens: IV. A proposed standard method of gravity sampling, counting, and volumetric interpolation of results. J. Allergy 1946, 17, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Kawashima, S.; Kobayashi, S.; Nakajima, H.; Noguchi, I.; Matsuda, M.; Nakamura, Y.; Hayashi, T. Evaluating inaccurate pollen concentrations caused by turbulence using passive sampler. Aerobiologia 2022, 38, 1–12. [Google Scholar] [CrossRef]
- Takahashi, Y. Current state of Japanese cedar (Cryptomeria japonica D.Don) pollen information and future directions for its airborne allergen determination and improved pollen monitoring. Aerobiologia 2024, 2, 1–17. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Calderon-Ezquerro, M.C. Metagenomic survey of bacterial diversity in the atmosphere of Mexico City using different sampling methods. Environ. Pollut. 2018, 235, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Tauber, H. A static non-overload pollen collector. New Phytol. 1974, 73, 359–369. [Google Scholar] [CrossRef]
- Hicks, S.; Hyvarinen, V.P. Sampling modern pollen deposition by means of “Tauber Traps”: Some considerations. Pollen Spores 1986, 28, 219–242. [Google Scholar]
- Selkirk, P.M. Vegetative reproduction and dispersal of bryophytes on subantarctic Macquarie Islandand in Antarctica. J. Hattori Bot. Lab. 1984, 55, 105–111. [Google Scholar] [CrossRef]
- Sundberg, S. Spore rain in relation to regional sources and beyond. Ecography 2013, 36, 364–373. [Google Scholar] [CrossRef]
- Lewandowska, A.U.; Śliwińska-Wilczewska, S.; Woźniczka, D. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea. Mar. Pollut. Bull. 2017, 125, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.F.; Eggleston, P.M. Airborne algae and cyanobacteria. Grana 1989, 28, 63–66. [Google Scholar] [CrossRef]
- Ross-Davis, A.L.; Frego, K.A. Propagule sources of forest floor bryophytes: Spatiotemporal compositional patterns. Bryologist 2004, 107, 88–97. [Google Scholar] [CrossRef]
- Santos, M.F.; Carvalho, G.M.; Sérgio, C.; Ormonde, J.; Leitão, M.T. Plantas criptogâmicas na atmosfera de Coimbra, Portugal. An. Jard. Bot. Madr. 1996, 54, 30–36. [Google Scholar]
- Wiśniewska, K.A.; Śliwińska-Wilczewska, S.; Lewandowska, A.U. The first characterization of airborne cyanobacteria and microalgae in the Adriatic Sea Region. PLoS ONE 2020, 15, e0238808. [Google Scholar] [CrossRef] [PubMed]
- Cundill, P.R. A new design of pollen trap for modern pollen studies. J. Biogeogr. 1986, 13, 83–98. [Google Scholar] [CrossRef]
- Perkins, W.A. The Rotorod Sampler; 2nd Semiannual Report; Aerosol Laboratory, Stanford University: Stanford, CA, USA, 1957. [Google Scholar]
- Webster, F.X. Collection Efficiency of the Rotorod FP Sampler; Tech. Rept. 98; Metronics Associates Inc.: Palo Alto, CA, USA, 1963. [Google Scholar]
- Errington, F.P.; Powell, E.O. A cyclone separator for aerosol sampling in the field. Epidemiol. Infect. 1969, 67, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Chalmers, M.O. Airborne dispersal of Antarctic terrestrial algae and cyanobacteria. Ecography 1997, 20, 585–594. [Google Scholar] [CrossRef]
- Wynn-Williams, D.D. Photofading retardant for epifluorescence microscopy in soil micro-ecological studies. Soil Biol. Biochem. 1985, 17, 739–746. [Google Scholar] [CrossRef]
- Marshall, W.; Convey, P. Dispersal of moss propagules on Signy Island, Maritime Antarctic. Polar Biol. 1997, 18, 376–383. [Google Scholar] [CrossRef]
- Sharma, K.N.; Rai, A.K.; Singh, S. Meteorological factors affecting the diversity of airborne algae in an urban atmosphere. Ecography 2006, 29, 766–772. [Google Scholar] [CrossRef]
- Sharma, N.K.; Singh, S.; Rai, A.K. Diversity and seasonal variation of viable algal particles in the atmosphere of a subtropical city in India. Environ. Res. 2006, 102, 252–259. [Google Scholar] [CrossRef]
- Hirst, J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952, 39, 259–265. [Google Scholar] [CrossRef]
- de Oliveira, S.M.; Duijm, E.; Stech, M.; Ruijgrok, J.; Polling, M.; Barbosa, C.G.G.; Cerqueira, G.R.; Nascimento, A.H.M.; Godoi, R.H.M.; Taylor, P.E.; et al. Life is in the air: An expedition into the Amazonian atmosphere. Front. Ecol. Evol. 2022, 10, 789791. [Google Scholar] [CrossRef]
- Ščevková, J.; Tropeková, M.; Dušička, J.; Štefániková, N.; Žilka, M.; Zahradníková, E.; Kováč, J.; Mišíková, K. Moss spores: Overlooked airborne bioparticles in an urban environment. Environ. Sci. Pollut. Res. 2024, 31, 58010–58020. [Google Scholar] [CrossRef]
- Magyar, D.; Eszéki, E.R.; Oros, G.; Szécsi, Á.; Kredics, L.; Hatvani, L.; Körmöczi, P. The air spora of an orchid greenhouse. Aerobiologia 2011, 27, 121–134. [Google Scholar] [CrossRef]
- Lacey, M.E.; West, J.S. Using a Burkard Trap. In The Air Spora; Lacey, M.E., West, J.S., Eds.; Springer: Boston, MA, USA, 2006; pp. 49–58. [Google Scholar]
- May, K.R. The cascade impactor: An instrument for sampling coarse aerosols. J. Sci. Instrum. 1945, 22, 187. [Google Scholar] [CrossRef]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.; Śliwińska-Wilczewska, S.; Savoie, M.; Lewandowska, A.U. Quantitative and qualitative variability of airborne cyanobacteria and microalgae and their toxins in the coastal zone of the Baltic Sea. Sci. Total Environ. 2022, 826, 154152. [Google Scholar] [CrossRef]
- Eaton, S.; Zúñiga, C.; Czyzewski, J.; Ellis, C.; Genney, D.R.; Haydon, D.; NosratMirzai, R.; Yahr, R. A method for the direct detection of airborne dispersal in lichens. Mol. Ecol. Resour. 2018, 18, 240–250. [Google Scholar] [CrossRef]
- Hughes, E.O.; Gorham, P.R.; Zehnder, A. Toxicity of a unialgal culture of Microcystis aeruginosa. Can. J. Microbiol. 1958, 4, 225–236. [Google Scholar] [CrossRef]
- Allen, M.M. Simple conditions for the growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories, and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research: Oslo, Norway, 1972. [Google Scholar]
- Gromov, B.V.; Titova, N.N. Algal culture collection of the laboratory of microbiology. In Kul’tivirovanie Kollektsionnykh Shtammov Vodoroslei (Cultivation of Algal Collection Strains); Leningrad University Press: Leningrad, Russia, 1983; pp. 3–57. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies on marine planktonic diatoms I. Cyclotella nana Hustedt and Detonula confervacea (Cleve). Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Walne, P.R. Studies on the Food Value of Nineteen Genera of Algae to Juvenile Bivalves of the Genera Ostrea, Crassostrea, Mercenaria and Mytilus; Fishery Investigations; H.M. Stationery Office: London, UK, 1970. [Google Scholar]
- Temraleeva, A.D.; Dronova, S.A.; Moskalenko, S.V.; Didovich, S.V. Modern methods for isolation, purification, and cultivation of soil cyanobacteria. Microbiology 2016, 85, 389–399. [Google Scholar] [CrossRef]
- Watanabe, M. Freshwater culture media. In Algal Culturing Techniques; Anderson, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 13–20. [Google Scholar]
- Harrison, P.J.; Berges, J.A. Marine Culture Media. In Algal Culturing Techniques; Anderson, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 21–34. [Google Scholar]
- Castenholz, R.W. Culturing methods for cyanobacteria. Methods Enzymol. 1988, 167, 68–93. [Google Scholar]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae culture quality indicators: A review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J. Cyanoprokaryota 3. Teil: Heterocytous Genera. In Süßwasserflora von Mitteleuropa; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer: Berlin, Germany, 2013; Volume 19/3, pp. 1–1130. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1: Chroococcales. In Süsswasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Heynig, H., Mollenheuer, D., Eds.; Springer: Berlin, Germany, 1999; Volume 1, pp. 1–548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Oscillatoriales. In Süßwasserflora von Mitteleuropa 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier: Berlin, Germany, 2005. [Google Scholar]
- Huber-Pestalozzi, G. Das Phytoplankton des Süsswassers, 3. Teil: Cryptophyceen, Chloromonadien, Peridineen. In Die Binnengewässer; Thienemann, A., Ed.; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1950. [Google Scholar]
- Cox, E.J. Identification of Freshwater Diatoms From Live Material; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Stocker-Wörgötter, E. Experimental Cultivation of Lichens and Lichen Symbionts. Can. J. Bot. 1995, 73, 579–589. [Google Scholar] [CrossRef]
- Ahmadjian, V. Laboratory culture of lichens and lichen symbionts. In Proceedings of the Symposium on Tissue Culture of Lichen and Bryophyte; Nippon Paint: Osaka, Japan, 1987; pp. 1–13. [Google Scholar]
- Lilly, V.G.; Barnett, H.L. Physiology of the Fungi; McGraw-Hill: New York, NY, USA, 1951. [Google Scholar]
- Ahmadjian, V. The Lichen Symbiosis; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Hamada, N. The effect of various culture conditions on depside production by an isolated lichen mycobiont. Bryologist 1989, 92, 310–313. [Google Scholar] [CrossRef]
- Accorinti, J. Cultivo Unialgaly Masivo de Scenedesmus obliquus: Técnicas de Obtención. Comun. Mus. Argent. Cien. Nat. 1960, 1, 21–29. [Google Scholar]
- Nehira, K. Germination and protonemata. In Methods in Bryology, 1st ed.; Glime, J.M., Ed.; The Hattori Botanical Laboratory: Nichinan, Japan, 1988; pp. 113–117. [Google Scholar]
- Duckett, J.G.; Burch, J.; Fletcher, P.W.; Matcham, H.W.; Read, D.J.; Russell, A.J.; Pressel, S. In Vitro Cultivation of Bryophytes: A Review of Practicalities, Problems, Progress and Promise. J. Bryol. 2004, 26, 3–20. [Google Scholar] [CrossRef]
- Silva-e-Costa, J.D.C.; Luizi-Ponzo, A.P.; Resende, C.F.D.; Peixoto, P.H.P. Spore germination, early development and some notes on the effects of in vitro culture medium on Frullania ericoides (Nees)Mont. (Frullaniaceae, Marchantiophyta). Acta Bot. Brasil. 2017, 31, 19–28. [Google Scholar] [CrossRef]
- Egunyomi, A. Comparative culture studies on the spores and gemmae of Octoblepharum albidum Hedw. J. Hattori Bot. Lab. 1978, 44, 25–30. [Google Scholar] [CrossRef]
- Maciel-Silva, A.S. Asexual regeneration and its implications for local bryophyte establishment in a Brazilian tropical rainforest. Botany 2017, 95, 45–52. [Google Scholar] [CrossRef]
- Maciel-Silva, A.S.; Porto, K.C.; Simabukuro, E.A. Effect of light and water availability on spore germination and protonemal growth of the neotropical moss Thamniopsis incurva (Pilotrichaceae). Cryptogam. Bryol. 2009, 30, 243–257. [Google Scholar]
- Ogbimi, A.Z.; Owoeye, Y.B.; Ibiyemi, V.O.; Bofede, A.V. Effects of pH, photoperiod, and nutrients on germination and growth of Calymperes erosum C.Muell. gemmaling. J. Bot. 2014, 2014, 159457. [Google Scholar] [CrossRef]
- Benson-Evans, K. Some aspects of spore formation and germination in Cryptothallus mirabilis V. Malmb. Trans. Br. Bryol. Soc. 1960, 3, 729–735. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Erdtman, G. The acetolysis method: A revised description. Sven. Bot. Tidskr. 1960, 39, 561–564. [Google Scholar]
- Silva-e-Costa, J.D.C.; Luizi-Ponzo, A.P. Spores of Plagiochila (Dumort.)Dumort.: The taxonomic relevance of morphology and ultrastructure. Acta Bot. Bras. 2019, 33, 391–404. [Google Scholar] [CrossRef]
- Passarella, M.D.A.; Luizi-Ponzo, A.P. Palynology of Amphidium Schimp. (Amphidiaceae M.Stech): Can spore morphology circumscribe the genus? Acta Bot. Bras. 2019, 33, 135–140. [Google Scholar] [CrossRef]
- Frahm, J.P. Dicranaceae: Campylopodioideae, Paraleucobryoideae. Flora Neotrop. 1991, 54, 1–237. [Google Scholar]
- Zander, R.H. Genera of the Pottiaceae: Mosses of Harsh Environments; Buffalo Society of Natural Sciences: Buffalo, NY, USA, 1993; Volume 32, pp. 1–378. [Google Scholar]
- Spence, J.R. Bryaceae. In Flora of North America; Flora North America Editorial Committee, Ed.; Volume 28: Bryophyta: Mosses Part 2; Oxford University Press: New York, NY, USA, 2015; pp. 117–191. [Google Scholar]
- Hebert, P.D.N.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Goyat, N.; Malik, A.; Singh, S.; Duhan, J.S. DNA Metabarcoding: Simplifying Biodiversity. Adv. Zool. Bot. 2023, 11, 466–474. [Google Scholar] [CrossRef]
- Valentini, A.; Pompanon, F.; Taberlet, P. DNA Barcoding for Ecologists. Trends Ecol. Evol. 2009, 24, 110–117. [Google Scholar] [CrossRef]
- Leontidou, K.; Vernesi, C.; De Groeve, J.; Cristofolini, F.; Vokou, D.; Cristofori, A. DNA Metabarcoding of airborne p ollen: New protocols for improved taxonomic identification of environmental samples. Aerobiologia 2018, 34, 63–74. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. Biodiversity of pollen in indoor air samples as revealed by DNA metabarcoding. Nord. J. Bot. 2017, 35, 602–608. [Google Scholar] [CrossRef]
- Bell, K.L.; De Vere, N.; Keller, A.; Richardson, R.T.; Gous, A.; Burgess, K.S.; Brosi, B.J. Pollen DNA barcoding: Current applications and future prospects. Genome 2016, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Banchi, E.; Ametrano, C.G.; Tordoni, E.; Stanković, D.; Ongaro, S.; Tretiach, M.; Pallavicini, A.; Muggia, L. Environmental DNA assessment of airborne plant and fungal seasonal diversity. Sci. Total Environ. 2020, 738, 140249. [Google Scholar] [CrossRef]
- Banchi, E.; Ametrano, C.G.; Stanković, D.; Verardo, P.; Moretti, O.; Gabrielli, F.; Lazzarin, S.; Borney, M.; Tassan, F.; Tretiach, M.; et al. DNA metabarcoding uncovers fungal diversity of mixed airborne samples in Italy. PLoS ONE 2018, 13, e0194489. [Google Scholar] [CrossRef]
- Abrego, N.; Furneaux, B.; Hardwick, B.; Somervuo, P.; Palorinne, I.; Aguilar-Trigueros, C.A.; Andrew, N.R.; Babiy, U.V.; Bao, T.; Bazzano, G.; et al. Airborne DNA reveals predictable spatial and seasonal dynamics of fungi. Nature 2024, 631, 835–842. [Google Scholar] [CrossRef]
- Singh, H.W.; Wade, R.M.; Sherwood, A.R. Diurnal patterns of airborne algae in the Hawaiian islands: A preliminary study. Aerobiologia 2018, 34, 363–373. [Google Scholar] [CrossRef]
- Câmara, P.E.A.S.; Stech, M.; Convey, P.; Šantl-Temkiv, T.; Pinto, O.H.B.; Bones, F.L.V.; Lopes, F.A.C.; Rodrigues, L.A.D.C.; Carvalho-Silva, M.; Rosa, L.H. Assessing aerial biodiversity over Keller Peninsula, King George Island, Maritime Antarctica, using DNA metabarcoding. Antarct. Sci. 2024, 36, 37–46. [Google Scholar] [CrossRef]
- Hadziavdic, K.; Lekang, K.; Lanzen, A.; Jonassen, I.; Thompson, E.M.; Troedsson, C. Characterization of the 18Sr RNA Gene for Designing Universal Eukaryote Specific Primers. PLoS ONE 2014, 9, e87624. [Google Scholar] [CrossRef] [PubMed]
- Antil, S.; Abraham, J.S.; Sripoorna, S.; Maurya, S.; Dagar, J.; Makhija, S.; Bhagat, P.; Gupta, R.; Sood, U.; Lal, R.; et al. DNA barcoding, an effective tool for species identification: A review. Mol. Biol. Rep. 2023, 50, 761–775. [Google Scholar] [CrossRef] [PubMed]
- DelCampo, E.M.; DelHoyo, A.; Royo, C.; Casano, L.M.; Álvarez, R.; Barreno, E. A single primer pair gives a specific ortholog amplicon in a wide range of Cyanobacteria and plastid-bearing organisms: Applicability in inventory of reference material from collections and phylogenetic analysis. Mol. Phylogenet. Evol. 2010, 57, 1323–1328. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Belnap, J.; Zhang, B.; Bu, C.; Zhang, Y. Practices of biological soil crust rehabilitation in China: Experiences and challenges. Restor. Ecol. 2020, 28, S45–S55. [Google Scholar] [CrossRef]
- Zhao, Y.; Bowker, M.A.; Zhang, Y.; Zaady, E. Enhanced recovery of biological soil crusts after disturbance. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 499–523. [Google Scholar]


| Organisms | Primers | References |
|---|---|---|
| Cyanobacteria | EMP; UPA marker; 23SU1/23SU2; Cya359f/Cya781r | [101,188,192,193] |
| Algae | Euk1391f/EukBr; ITS3 and ITS4; UPA marker; Euk528f/CHLO02r | [101,133,188,189,193] |
| Lichens | Prb1F, Pcn1F, Nla1F, Npa2F, Dg1R, and Psax3F | [140] |
| Bryophytes | ITS3 and ITS4 | [133,189] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.F.; Maciel-Silva, A.S. Techniques for Evaluating Airborne Biocrust Diaspores: From Fundamentals to Advanced Approaches. Aerobiology 2025, 3, 1. https://doi.org/10.3390/aerobiology3010001
Oliveira MF, Maciel-Silva AS. Techniques for Evaluating Airborne Biocrust Diaspores: From Fundamentals to Advanced Approaches. Aerobiology. 2025; 3(1):1. https://doi.org/10.3390/aerobiology3010001
Chicago/Turabian StyleOliveira, Mateus Fernandes, and Adaíses Simone Maciel-Silva. 2025. "Techniques for Evaluating Airborne Biocrust Diaspores: From Fundamentals to Advanced Approaches" Aerobiology 3, no. 1: 1. https://doi.org/10.3390/aerobiology3010001
APA StyleOliveira, M. F., & Maciel-Silva, A. S. (2025). Techniques for Evaluating Airborne Biocrust Diaspores: From Fundamentals to Advanced Approaches. Aerobiology, 3(1), 1. https://doi.org/10.3390/aerobiology3010001






