Abstract
Bioaerosols constitute a crucial component of atmospheric particulate matter, encompassing physical and chemical aerosol properties along with biological characteristics. They can influence global ecosystems, climate change dynamics, and air quality. Notably, bioaerosols serve as a significant pathway for transmitting respiratory infectious diseases, garnering widespread attention worldwide following major pandemics such as COVID-19. Thanks to the development of high-throughput sequencing technologies, studies on bioaerosols have flourished in recent years. Understanding the interconnectedness of sources, spatial and temporal distributions, influencing factors, and health risks associated with bioaerosols is imperative for devising pollution mitigation strategies and preventing the spread of related epidemics. This review provides an overview of bioaerosol sources while elucidating distribution patterns within their community structure across various source types. Lastly, this overview offers insights into future advancements in the field of bioaerosols along with corresponding recommendations.
1. Introduction
Bioaerosols refer to airborne particles of biological origin, encompassing both living and non-living components such as pollen, fungi, protozoa, bacteria, algae, viruses, toxins, and cellular debris [1,2]. The size of bioaerosols is small, ranging from 1 to 10 μm for fungal spores, vegetable cells, pollens, and bacteria. Viruses exist in sizes smaller than 0.1 μm. Some plant pollens can be up to 100 μm [3]. On a global scale, the annual budgets of bacteria, fungi, and pollen are up to 28.1, 186, and 84 Tg/year, respectively [4,5]. Bioaerosols can influence atmospheric changes, notably acting as cloud condensation nuclei [6]. In addition, it has been recognized that pathogens are prevalent in bioaerosols and have adverse effects on human health [7]. Pathogenic bioaerosols can impact human health through respiratory, direct contact, and ingestion pathways. Moreover, they may also induce secondary infections due to their biological activity [2]. Notably, many bioaerosols possess properties that exacerbate severe oxidative stress and inflammatory responses, inducing further secondary infections [8].
Various factors can influence the structure of airborne microbial communities. For instance, environmental variables such as temperature, relative humidity, and wind speed exert profound effects on microbial concentrations and community composition [9]. Additionally, different emission sources play a crucial role in shaping the abundance and diversity of airborne microorganisms [10]. Bioaerosols originate from various sources, including soil, water, vegetation, and anthropogenic activities [2]. Soil is particularly significant, as it releases a wide range of microorganisms [11], estimated to account for 19.5% of bioaerosols [12]. Vegetation also serves as a major source, with leaves contributing to 26.8% of fungal aerosols and 45% of bacterial aerosols [2,13]. Furthermore, vegetation sources contribute significantly to allergens like pollen [14]. Water plays a vital role in determining bioaerosol characteristics due to its extensive coverage of two-thirds of the Earth’s surface. It has been estimated that oceans alone contribute between 33% and 68% of bioaerosols, with predominant genera including Bacteroides, Sphingomonas, Betaproteobacteria, Acidovorax, etc. [15,16,17]. These sources are categorized as natural origins.
Anthropogenic sources also represent significant contributors to bioaerosols. Through respiratory activities such as sneezing, coughing, and talking, an adult can release approximately 7.3 × 106 fungal genome copies and 3.7 × 107 bacterial genome copies per hour [18]. Consequently, anthropogenic activities have a greater impact on the indoor environment. Furthermore, specific human-made facilities, designed for various purposes including production, create distinct bioaerosol sources.
Researchers endeavour to quantify the contribution of each potential source to bioaerosols, yet this task remains challenging due to limited understanding of these sources. Accurately identifying the origins of airborne microorganisms and reliably estimating their impact on air quality and human health requires precise assessment of contribution rates from each microbial source [4]. To achieve this objective, several methodologies for source identification have been developed, including the Model Analysis Method (MAM), Database Analysis Method (DAM), and Correlation Analysis Method (CAM) [4]. Considering the significance of sources in relation to bioaerosols, this study presents a comprehensive overview of the knowledge pertaining to bioaerosols originating from diverse sources.
2. Natural Environment
The presence of microorganisms in the atmosphere is influenced by natural events, thereby contributing to the characteristics of bioaerosols. Bioaerosols originating from natural sources play a crucial role in the airborne chemical cycle and ecological balance, while generally exhibiting fewer pathogenic effects [2]. As shown in Figure 1, various elements of the natural environment, such as wind, rain, forests, and water bodies, contribute to the formation of bioaerosols.
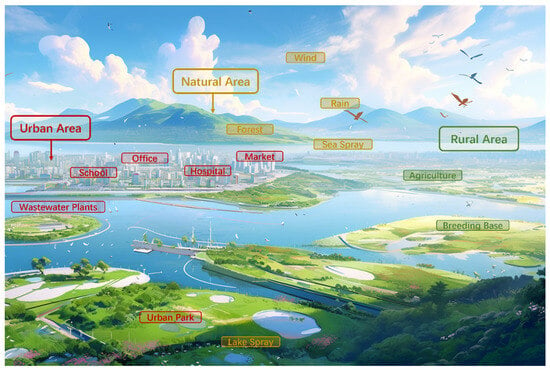
Figure 1.
The potential sources of bioaerosols. The figure was performed by Midjourny (https://www.midjourny.cn) accessed on 25 July 2024.
2.1. Wind
The wind acts as the driving force for suspending microorganisms in the air. Generally, airborne microorganisms can persist for extended periods and be transported over long distances by varying wind speeds and directions [2]. Wind speed is typically positively correlated with microbial alpha diversity abundance [19]. For example, during Saharan dust events, the dispersion of air masses stimulates a highly diverse and abundant bacterial community [20]. Dust events are one of the major sources of bioaerosols release, which can lead to health problems and impact agriculture and livestock due to the transport of organic compounds, opportunistic microbes, and trace metals [3]. The concentration of bacteria increases during dust events [21,22]. Specifically, desert dust plumes contain an abundance of bacteria, including Firmicutes (Bacillales), Bacteroidetes (Sphingobacteriales, Bacteroidales and Flavobacteriales), and Actinobacteria (Micrococcales and Corynebacteriales). Proteobacteria orders associated with dust plumes include Burkholderiales and pathogenic Neisseriales [23]. Furthermore, dust plumes may introduce bacteria from other environments, such as aquatic bacterial groups like Synechococcales and Vibrionales [21,24]. Researchers have utilized the Hybrid Single Particle Lagrangian Integrated Trajectory Model (HYSPLIT) to identify the origins of bioaerosols based on wind patterns [25]. Specific air-mass trajectories influence both bacterial concentration levels and community structure [25].
A study reviewed the contemporary research on dust storms in the Middle East region [26]. The findings indicate a global increase in annual concentrations of dust aerosols. Studies conducted in the Middle East have revealed a significant rise in respiratory and cardiovascular mortality rates, as well as hospital visits, following exposure to dust storms; however, few studies have established regional causation [26]. Another study provided evidence that episodes of desert dust intrusion lead to the combined transportation of inorganic mineral matter, inorganic pollutants, and bioaerosols [27]. Specifically, the Iberian Peninsula is affected by Saharan desert dust intrusions due to its proximity to the African continent and is seeing an increasing trend in the number of intrusion events. There is a close relationship among the conditions favouring the occurrence of intrusion episodes, the transport of particulate matter, and the transport of bioaerosols such as pollen grains, spores, and bacteria [27]. In China, a study conducted on March 15th, 2021 analyzed airborne bacteria during one of the most severe sandstorms in East Asia. The characteristics of the sandstorm were compared with those of subsequent clean and hazy days. During the sandstorm event, there was an exceptionally high concentration of particulate matter (PM) and bacterial richness. Furthermore, 10 pathogenic bacterial genera were introduced into the atmosphere by the sandstorm, posing significant risks to human health. Even after the storm subsided, small bioaerosols (0.65–1.1 μm) with a similar bacterial community remained suspended in the atmosphere, potentially causing long-lasting health hazards [28].
2.2. Rainfall
Rainfall can serve as a driving force for the generation of bioaerosols. When raindrops impact the soil, tiny bubbles form within them and rupture at the interface between the raindrop and air, releasing minute water jets that subsequently fragment into aerosols containing soil-associated bacteria within the atmospheric column [29]. Approximately 0.01% of bacteria are released when a raindrop hits the soil surface during rainfall events. The estimated range of bacterial dispersion from soil under rainfall influence is between 1.2 × 1022 and 8.5 × 1023 cells per year [30]. Raindrops release a substantial number of bioaerosol particles from plant and soil surfaces into the atmosphere, thereby increasing microbial concentrations. Moreover, research has demonstrated that raindrops transport bioaerosols from high altitudes to surface environments [31]. Jang et al. (2018) demonstrated that heavy rainfall appeared to induce alterations in the composition of airborne bacteria at a suburban site, characterized by an increase in the relative abundance of non-spore forming Actinobacteria and a decrease in the relative abundance of Firmicutes within the atmospheric column. The occurrence of frequent rainfall events emerged as a contributing factor influencing the composition of the airborne bacterial community. The observed rise in pathogenic and phytopathogenic Actinobacteria sequences in post-rain air samples suggests that rainfall may potentially exert adverse effects on both plant health and human wellbeing [32].
2.3. Forests
The forest presents a promising environment for microbial inhibition. Different types of forests exert distinct effects on bioaerosols. Forested areas are less impacted by human activities and host unique ecosystems. Variations in plant species contribute to the diverse microbial communities found on leaf surfaces, thereby endowing forests with an exceptionally unique assemblage of microorganisms [33]. For example, the Amazon rainforest exhibits a higher abundance of bioaerosols originating from leaves rather than soil, owing to its extensive vegetation coverage [34]. Forests also have higher concentrations of airborne fungi and pollen compared to urban areas. Airborne fungal communities can account for up to 45% in tropical rainforests, compared to only 6% in urban environments [35,36,37]. Li et al. (2020) analyzed primary biological aerosol particles (PBAPs) using scanning electron microscopy (SEM, Zeiss Ultra 55, Jena, Germany) and transmission electron microscopy (TEM, JEM-2100, JEOL Ltd., Tokyo, Japan) in the boreal forest of Lesser Khingan Mountain, China. Their results indicated that PBAPs were dominated by C, N, O, P, K, and Si elements, with phosphorus serving as a key marker distinguishing PBAPs from non-PBAPs. They estimated that PBAPs accounted for 47% of the airborne particle mass concentration, while mineral dust and other particles accounted for 43% and 10%, respectively, suggesting that large boreal forests may represent a significant source of PBAPs in the atmosphere. Furthermore, there was a higher frequency and concentration of PBAPs observed during nighttime compared to daytime [38].
2.4. Mountains
The mountainous region, characterized by an abundance of vegetation and fertile soil, harbours a substantial concentration of bioaerosols [39,40]. For instance, the concentration of Cladosporium was detected at 1.2 × 103 CFU/m3 in mountainous regions [39]. Mu et al. (2020) investigated the airborne microbiota in mountainous areas, revealing that bacteria from surface soil and leaf surfaces contributed significantly to the airborne microbial community [40]. Xu et al. (2019) identified gram-negative bacteria, including Burkholderia, Delftia, Bradyrhizobium, and Methylobacterium, in PM2.5 at Mountain Tai. Local air masses originating from the Shandong peninsula and its adjacent areas exhibited the highest bacterial concentration loading (602 cells m−3) and a greater abundance of potential pathogens at the sampling site [25]. A study conducted on bacterial bioaerosols collected passively from rain and dry deposition at high-elevation sites in the Sierra Nevada over a period of 3 years revealed that wet and dry bioaerosols shared up to 65% of operational taxonomic units (OTUs) and 82% of bacterial genera. Notably, Oxalobacteraceae were more abundant in wet deposition, dominated by Noviherbaspirillum and Massilia. These findings suggest that the bacterial composition of bioaerosols collected through passive natural deposition at high elevations is similar to the bacterial microbiome found in the free troposphere [41]. Fungal abundance values were recorded as 9.4 × 104 copies m−3 for PM2.5 and 1.3 × 105 copies m−3 for PM1 at the summit of Mountain Tai. Most fungal sequences belonged to Ascomycota and Basidiomycota, known for actively releasing spores into the atmosphere. Glomerella and Zasmidium showed increased abundance in larger particles during autumn, while Penicillium, Bullera, and Phaeosphaeria exhibited higher levels in smaller particles during winter [42]. In another study conducted in typical mountainous terrain in Jinan, China, cell counts ranged from 6.83 × 105 ± 1.27 × 104 (non-polluted air, NP) to 2.32 × 106 ± 3.56 × 104 (heavily polluted air, HP) cells per cubic meter of air. The proportion of viable apoptotic and necrotic cells showed a positive correlation with PM2.5 levels. Burkholderia cenocepacia (36.6%) was identified as the predominant human pathogen in heavily polluted air; this gram-negative bacterium is associated with potentially fatal respiratory infections in cystic fibrosis patients [43].
2.5. Water Bodies
Water bodies, including rivers, lakes, and marine environments, harbour a diverse array of microorganisms. When foams or bubbles rupture in the water due to external forces such as wind and water currents, a substantial quantity of microorganisms is released into the atmosphere [4]. Notably, water bodies are susceptible to nutrient-induced eutrophication. Wave breaking generates aerosols that carry harmful algal blooms and toxins from the water body into the atmosphere, posing a threat to public health [44]. These negative effects may have broader implications. A study was conducted to investigate daily and seasonal variations in the qualitative and quantitative distribution of airborne cyanobacteria and microalgae during both vegetative and non-vegetative seasons in the coastal zone of the Baltic Sea. Samples were collected using a Tisch six-stage microbiological impactor (Tisch Environmental, Inc., Cleves, OH, USA) from January to December 2020. The results revealed year-round presence of cyanobacteria and microalgae, with maximum abundance observed in July (1685 cells m−3). Furthermore, it was confirmed that these microorganisms have the ability to produce MC-LR toxin, which can adversely affect human health. MC-LR was detected in various species maintained at our Culture Collection of Airborne Algae (CCAA) as well as in air samples, with the highest concentrations recorded in Synechococcus sp. CCAA 46 (420 fg cell−1) and an air sample taken in May (mean concentration of 0.95 μg L−1). This research enhances our understanding of cyanobacteria and microalgae present in the atmospheric environment along the southern Baltic Sea coastal zone [45].
3. Anthropogenic Sources
Anthropogenic activities play a significant role in shaping the composition of airborne microbiota The human body harbours approximately 3.9 × 1013 bacteria, which substantially contribute to indoor bioaerosols, given that humans spend more than 80% of their time indoors [2]. As previously mentioned, individuals tend to spend extended periods in specific occupational environments in pursuit of productivity. Consequently, these associated facilities, influenced by anthropogenic activities, also contribute notably to the airborne microbiota.
3.1. Urban, Suburban, and Rural Areas
The community structure of bioaerosols shows significant variation along the urbanization gradient, influenced by anthropogenic activities [46,47]. Urban areas are characterized by diverse land-use patterns designed to meet human production and lifestyle needs. Specific land-use practices create unique “microenvironments” that potentially influence the generation, propagation, transport, re-suspension, diffusion, aerosolization, and intermolecular interactions of airborne microbiota [4]. Urban sources can shape the community structures of airborne microorganisms. Compared to natural sources, human-made facilities in urban atmospheres harbour various pathogenic bioaerosols. Pathogenic bioaerosol abundance is generally high in urban environments and positively correlates with the level of urbanization [48], resulting in severe bioaerosol pollution that poses potential risks to air quality, ecosystems, and human health [2]. Airborne microbial community diversity negatively correlates with levels of urbanization due to potential influences on environmental contamination [49,50]. Bioaerosol characteristics in urban areas are affected by seasonality and geographic location [51]. The core bacterial phyla include Proteobacteria, Actinobacteria, Firmicutes, Bacteroidetes, and Cyanobacteria [51]. However, some ambient environmental factors induce variations in microbial community structures; for instance, the proportion of Proteobacteria can range from 17% to 75% within the total aerobiome [51].
Regarding the sources of bioaerosols in urban areas, research has revealed significant variations in total bacterial levels among bioaerosols, with average pollution levels of 1.86 × 105 and 4.35 × 104 cfu m−3 in spring and summer, respectively, surpassing those of airborne fungi. Bioaerosol samples predominantly contained opportunistic pathogenic bacteria such as Burkholderia-Paraburkholderia, Staphylococcus, and Acinetobacter, as well as fungi like Alternaria, Penicillium, and Cladosporium. Furthermore, a total of 21 subtypes of antibiotic resistance genes (ARGs) were detected in the Pearl River Estuary area, with tetA gene showing the highest abundance, followed by aminoglycoside resistance genes and mobile genetic elements [52]. Another noteworthy aspect is that modern life provides convenient facilities within indoor environments in urban areas where subway systems harbour bacterial species including Bacillus, Micrococcus, and Pseudomonas, along with human skin bacterial species like Staphylococcus and human opportunistic pathogens such as S. haemolyticus and S. epidermidis [53]. Urban parks typically consist of green spaces that play an essential role in city functioning; however, closely proximity to these green spaces was associated with higher concentrations of airborne fungi [4]. Many airborne microbes originate from various sources [54]. For example, Kasprzyk et al. (2021) identified dominant genera including an allergenic type called Cladosporium sp., which was more prevalent than other genera across all studied parks [54].
In terms of suburban areas, Chen et al. (2021) investigated the bacterial community structure in PM2.5 in the suburban atmosphere of Beijing [55]. Airborne bacteria can exhibit relative stability within the local environment during the same season but across different years. Although variations in pollution levels result in distinct changes in the relative abundances of different bacterial species, no significant statistical differences were observed [55]. Xu et al. (2021) demonstrated that the suburban areas of Shanghai harboured high and stable bacterial concentrations during summer (1.53 ± 1.21 × 103 cells/m3) and winter (4.19 ± 1.18 × 103 cells/m3) [46]. Lin et al. (2024) quantified airborne microorganisms at a suburban site in Guangzhou, southern China, during the winter and spring periods of 2021. The average concentration was 1.82 ± 1.33 × 106 cells/m3, corresponding to a mass concentration level of 0.42 ± 0.30 μg/m3, which was comparable to, but slightly lower than, the concentration of proteins (0.81 ± 0.48 μg/m3). Both concentrations were significantly higher than the average saccharide concentration (19.93 ± 11.53 ng/m3). Strong correlations among these three components were observed during the winter period, with a biological outbreak in late March leading to a substantial increase in airborne microorganisms and subsequent rises in protein and saccharide levels during springtime [56].
In rural areas, variations in agricultural land were attributed to the cultivation of different main crops and farming methods [4]. A correlation was discovered between the impact of fungi released during harvesting and specific grain types, as well as the cultivated land [57]. Furthermore, fertilization practices were found to influence airborne microorganisms [58]. Researchers have reported higher concentrations of fungal spores in rural locations compared to urban areas [39]. Shen et al. (2019) reported that bioaerosols in rural regions, specifically at the Wangdu site, had high levels of viable bioPM (0.79 × 105 ± 1.4 × 105 m−3) and endotoxins (15.1 ± 23.96 EU/m3). The dominant airborne microbiota included Enterococcus (65%) and Paenibacillus (10%) [59]. Bao et al. (2023) identified Methylobacterium, belonging to the order Rhizobiales, which is indicative of vegetation-related genera for rural sites [60].
3.2. Occupational Areas
Due to the significant volume of waste produced, various measures have been implemented to manage waste degradation, including wastewater treatment, landfill facilities, and composting. In recent years, an increasing amount of research has been conducted on the bioaerosols emitted from these facilities. Wastewater treatment plants (WWTPs), which play a crucial role in urban areas, are known to release pathogenic bioaerosols through mechanical aeration, bubble aeration, and sludge dehydration treatment units [61,62]. Studies have reported that culturable airborne bacteria concentrations within WWTPs ranged from 5.1 × 101 to 6.9 × 103 CFU/m3, while airborne fungi ranged from 6.3 × 102 to 3.9 × 103 CFU/m3 [63,64]. Moreover, these wastewater treatment units often harbour harmful microorganisms, toxins, and metabolites that can be released into the air [65]. The adverse effects of wastewater are associated with the spread of bioaerosols to neighbouring regions. Individuals residing near WWTPs may experience negative impacts at receptor sites [66,67]. Wang et al. (2023) reported that bacterial concentrations ranged from 50 ± 5 to 1296 ± 261 CFU/m3, with the highest concentrations observed in the biochemical reaction tank. The dominant bacteria identified in bioaerosols included Bacteroides, Cetobacterium, Romboutsia, Lactobacillus, and Turicibacter. Regarding fungi species composition, Aspergillus, Alternaria, Cladosporium, and Fusarium were found to be dominant. Pathogenic microorganisms such as Escherichia and Aspergillus were detected throughout all stages of the treatment process. Principal component analysis revealed similarities in bacterial composition across different technological processes, while fungal species composition varied significantly. The microbial composition of sludge and bioaerosols exhibited relatively close resemblance. Source tracking results indicated that sludge served as the primary source of airborne bacteria in the sludge dewatering house and also contributed to airborne fungi in both plate-frame pressure filtration tanks and sloping-plate sedimentation tanks. Non-carcinogenic risk levels at each stage were found to be low, ranging from 1.22 × 10−9 to 3.99 × 10−2 [68].
Landfill plants are a significant area of interest for studying bioaerosols. Previous research has indicated that the primary sources of pathogenic bioaerosol release in landfill plants are waste pretreatment units and leachate treatment units [2]. Composting is a microbial-driven process involving waste degradation. Throughout the composting process, different stages such as fresh waste delivery, shredding, compost pile turning, and compost screening result in the release of various bacteria and fungi. Mbareche et al. (2017) reported a higher prevalence of Eurotiomycetes in carcass compost, while Sordariomycetes were found to be dominant in domestic compost. Both types of compost exhibited diverse profiles of bioaerosols, indicating the presence of several newly identified pathogenic fungi emitted from composting plants. Analysis of both air and compost samples revealed the presence of members belonging to families Herpotrichiellaceae and Gymnoascaceae, which are known to cause human diseases. Moreover, certain fungi were detected at higher concentrations in the air compared to those found in the compost [69].
Hospitals play a crucial role in urban areas, where patients are highly susceptible to nosocomial infections, which affect approximately 15% of hospitalized individuals. Additionally, specific sections within hospitals dedicated to infectious diseases may act as potential reservoirs for microbial dissemination. Moreover, a significant proportion of microbes isolated from hospitals exhibit elevated levels of resistance to treatment, due to the presence of antibiotic resistance genes (ARGs), which seem to be favoured by the selective pressures exerted by this particular environment on microorganisms [70].
Breeding bases refer to facilities for animal cultivation, where animal manure serves as a significant source of pathogenic bioaerosol release. Farming activities involving animals such as poultry and pigs can result in high concentrations of culturable microorganisms in the form of bioaerosols, ranging from 103 to 105 CFU/m3 [71], thereby impacting the surrounding environment [4]. While various studies have demonstrated a decrease in bioaerosol concentration with increasing distance downwind, levels at the periphery of these facilities may still reach as high as 105 CFU/m3 [72], posing potential health risks to workers due to exposure to elevated microbial concentrations [5,73]. Thorne et al. (2009) found that regardless of swine operation type (hoop or conventional), endotoxin concentrations downwind of swine operations were associated with adverse health effects. Despite extensive research on biological air pollution levels related to animal production operations, knowledge gaps remain regarding the specific sources and factors influencing the abundance and composition of bioaerosols [74]. Kumari and Choi (2015) conducted an analysis on airborne microorganism abundance and composition in piggeries equipped with three different types of manure removal systems. Their findings revealed that facilities employing deep-pit manure removal systems with slats exhibited higher levels of airborne biotic contaminants compared to those utilizing scraper and deeplitter bed removal systems [57].
Farming activities, including the handling of animal excrement and feed, present potential risks to human health due to the presence of pathogenic bacteria including Bacillus, Corynebacterium, and Streptococcus [58,75,76]. In a study by Peng et al. (2023), distinct particle morphology was observed in the piggery, with elliptically deposited particles suspected to be bacterial components. Analysis of full-length 16S rRNA revealed that most airborne bacteria in both fattening and gestation houses belonged to the Bacilli class. Beta diversity analysis and inter-sample comparisons indicated a significantly higher relative abundance of certain bacteria in PM2.5 compared to PM10 within the same pig house (p < 0.01). Moreover, significant differences were observed in the bacterial composition of inhalable particles between fattening and gestation houses (p < 0.01). Fast expectation–maximization microbial source tracking (FEAST) identified feces as a major potential source contributing to airborne bacteria in pig houses (contribution range: 52.64–80.58%) [77]. Certain agricultural facilities also influence the composition of airborne microbial communities. For instance, Nie et al. (2023) reported average concentrations of 1.67 × 103 cells/m3 (PM2.5) and 2.38 × 103 cells/m3 (PM10) for airborne bacteria, while the mean concentrations of airborne fungi reached 1.49 × 102 cells/m3 (PM2.5) and 3.19 × 102 cells/m3 (PM10) in a vegetable plastic greenhouse (VPGS). The predominant bacterial genera identified in VPGS were Ralstonia, Alcanivorax, Pseudomonas, Bacillus, and Acinetobacter, whereas frequently detected fungal genera included Botrytis, Alternaria, Fusarium, Sporobolomyces, and Cladosporium [78].
Another anthropogenic activity associated with agriculture is biomass burning, which was found to result in similar bacterial and fungal community structures in both PM1 and PM2.5 fractions, as indicated by wide range particle spectrometer analysis. Among bacteria, Pseudomonas accounted for 18.06% and 21.29% of the community in PM1 and PM2.5, respectively. Alternaria comprised up to 69.01% and 72. 76% of the fungal community in PM1 and PM2.5, respectively. The abundance of rare species revealed a disparity among bacterial communities, with Bacilli constituting a higher proportion in PM1 (2.4%) compared to PM2.5 (1.8%), while Defluviicoccus exhibited higher abundance in PM2.5 (2.5%) than in PM1.0 (0.5%). These differences may be attributed to variations in cell size and growth patterns. Quantitative PCR analysis demonstrated that microbial cell numbers were higher in PM2.5 compared to PM1, with bacterial cells outnumbering fungal cells by an order of magnitude. However, despite lower cell numbers, fungi displayed greater mass concentration and contribution to particulate matter than bacteria did, highlighting the underestimated role of fungi as atmospheric aerosols. Airborne microorganisms from alpine areas remain insufficiently characterized; hence, this study provides valuable insights into the impact of biomass burning on air quality [79].
3.3. Indoor Environments
Numerous indoor environments, including households, schools, offices, and commercial complexes, have received significant attention from bioaerosol studies. One recent study revealed notable disparities in bioaerosol concentrations across different buildings and climate zones. Bacterial concentrations were significantly higher in residences (536 ± 647 CFU/m3) compared to schools, offices, and hospitals, due to variations in built environments and human activities. Schools exhibited the highest mean value of fungal concentration (826 ± 955 CFU/m3), attributed to their larger landscaping areas. Bacterial concentrations were also significantly lower in the cold zone (307 ± 506 CFU/m3) and hot summer/cold winter zone (214 ± 180 CFU/m3) compared to the other three climate zones. Fungal concentrations were notably higher in the severe cold zone (709 ± 900 CFU/m3) and hot summer/warm winter zone (1094 ± 832 CFU/m3) than in the remaining three climate zones. Additionally, indoor airborne bacterial concentration decreased with lower indoor temperature (T) and increased air exchange rate, whereas indoor airborne fungi decreased with lower relative humidity (RH). Furthermore, a higher air exchange rate mitigated the impact of occupant density on indoor bacterial concentration [80].
The assessment of airborne microbial communities in public buildings with intricate architecture and diverse human activities, such as educational institutions like schools and universities, is of significant importance in delineating potential health risks associated with fungal contamination [81]. Furthermore, seasonal variation can also influence the airborne microbial community. For instance, Wu et al. (2020) conducted a study on the concentration of airborne fungi in various rooms at the University of Xi’an, China, and observed that seasonal variation had a significant impact on fungal levels in all analyzed areas except for the reading room [82]. Lu et al. (2022) identified a total of 667 fungal strains categorized into 160 species and 73 genera based on morphological and molecular analysis. The most abundant fungal genera were Alternaria, Cladosporium, and Aspergillus, accounting for 38.57%, 21.49%, and 5.34%, respectively; while the most frequently occurring species was A. alternata (21%), followed by A. tenuissima (12.4%) and C. cladosporioides (9.3%). Fungal concentrations ranged from 0 to 150 CFU/m3 in specific environments, with significantly higher levels outdoors compared to indoors [81].
Offices are also considered as significant sources of concern due to their impact on indoor air quality in the workplace. In 1998, the Hong Kong Environmental Protection Department (HKEPD) published a consultancy report on Indoor Air Quality at workplaces, which included measurement results of various indoor contaminants in 40 office premises across Hong Kong. The report’s assessment of indoor air quality indicators encompassed bioaerosols such as bacteria, fungi, protozoa, pollen, and animal dander. However, despite establishing a threshold level of 1000 CFU/m3 for indoor bioaerosols, the report did not provide specific details regarding the measurement protocol or daily exposure profile [83].
Commercial indoor spaces, such as shopping complexes and market areas, attract a significant influx of individuals, increasing airborne bacterial concentrations [4]. Markets, particularly Chinese wet markets renowned for their live poultry trade, have been identified as significant sources for the dissemination and horizontal transfer of bacterial and viral pathogens. The findings revealed that Corynebacterium minutissimum and other pathogenic bacteria accounted for 0.81–8.02% of the total microbial community across various air samples. Moreover, the relative concentration (copies/ng DNA) of the four antibiotic resistance genes (ARGs) quantified in this study was comparable to those observed in municipal wastewater systems. Poultry manure was recognized as a key contributor to microbial contamination within wet markets. These findings are supported by both source tracking based on microbial composition and quantification of airborne microbial density. Additionally, a range of Firmicutes and Bacteroidetes indicators associated with poultry area contamination were identified, which can facilitate more convenient monitoring methods for assessing airborne pollution originating from poultry areas [84]. The prolonged presence of airborne microbes in indoor environments has led to an increased awareness of the detrimental impact of indoor microorganisms on human health. Apart from directly affecting human health, indoor airborne bacteria can also synergistically interact with non-biological factors (such as volatile organic chemicals, inorganic particulate matter, ventilation issues, etc.) to trigger ‘sick building syndrome’, characterized by symptoms such as headache, dizziness, and cough [85]. The Air Conditioning (AC) system emerged as one of the sources, potentially harbouring a diverse range of microbial contaminants. A study revealed the inherent hazards associated with bacteria, fungi, and pollen [86]. The prevalence of AC systems in various indoor environments, such as households, hospitals, classrooms, and offices, necessitates prioritizing their study in relation to indoor airborne environments.
4. Conclusions
This review presents a comprehensive overview of the specific sources that exert influence on the community structures of bioaerosols, elucidating their diverse origins from both natural and anthropogenic sources that collectively shape the structural composition of the bioaerosol community. Bioaerosols are influenced by various factors, such as their origins, land-use patterns, meteorological conditions, air quality, and climate change during their atmospheric transport and dispersion, resulting in spatial and temporal variations in their community structure. Moreover, owing to the influence of human activity on urban air quality, monitoring efforts have detected numerous pathogens that exacerbate air pollution. The abundance of available data can and should be utilized as proxies to inform public health guidance regarding potential occupational and environmental exposures among workers, and to promote environmental justice when situating biogenic industries in proximity to residential areas.
Author Contributions
Conceptualization, C.N. and Y.Q. (Yuqi Qiu); methodology, T.P.; validation, C.N., Y.Q. (Yuqi Qiu) and T.P.; formal analysis, C.N. and Y.Q. (Yunhan Qin); investigation, C.N. and Y.Q. (Yunhan Qin); resources, C.N. and Y.Q. (Yunhan Qin); data curation, C.N. and Y.Q. (Yuqi Qiu); writing—original draft preparation, C.N. and Y.Q. (Yuqi Qiu); writing—review and editing, C.N., Y.Q. (Yuqi Qiu) and Y.Q. (Yunhan Qin); visualization, C.N. and Y.Q. (Yuqi Qiu); supervision, C.N.; project administration, C.N.; funding acquisition, C.N. and Y.Q. (Yunhan Qin). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project (24-4-4-zrjj-46-jch) supported by Qingdao Natural Science Foundation, the Doctoral Research Fund of Qingdao University (DC2400001227).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The figure was performed on Midjourny platform (https://www.midjourny.cn, accessed on 25 July 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sajjad, B.; Hussain, S.; Rasool, K.; Hassan, M.; Almomani, F. Comprehensive insights into advances in ambient bioaerosols sampling, analysis and factors influencing bioaerosols composition. Environ. Pollut. 2023, 336, 122473. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, X.; Yao, X.; Zhao, Y.; Tang, Y.; Zhao, Z.; Wei, Y.; Mehmood, T.; Luo, X. Sources, compositions, spatio-temporal distributions, and human health risks of bioaerosols: A review. Atmos. Res. 2024, 305, 107453. [Google Scholar] [CrossRef]
- Ruiz-Gil, T.; Acuña, J.J.; Fujiyoshi, S.; Tanaka, D.; Noda, J.; Maruyama, F.; Jorquera, M.A. Airborne bacterial communities of outdoor environments and their associated influencing factors. Environ. Int. 2020, 145, 106156. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, Y.; Bai, W.; Hou, J.; Ma, T.; Zeng, X.; Zhang, L.; An, T. The source and transport of bioaerosols in the air: A review. Front. Environ. Sci. Eng. 2021, 15, 44. [Google Scholar] [CrossRef]
- Després, V.; Huffman, J.A.; Burrows, S.M.; Hoose, C.; Safatov, A.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus. Ser. B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Qiu, G.; Zhang, X.; DeMello, A.J.; Yao, M.; Cao, J.; Wang, J. On-site airborne pathogen detection for infection risk mitigation. Chem. Soc. Rev. 2023, 52, 8531–8579. [Google Scholar] [CrossRef]
- Reinmuth-Selzle, K.; Kampf, C.J.; Lucas, K.; Lang-Yona, N.; Fröhlich-Nowoisky, J.; Shiraiwa, M.; Lakey, P.S.J.; Lai, S.; Liu, F.; Kunert, A.T.; et al. Air Pollution and Climate Change Effects on Allergies in the Anthropocene: Abundance, Interaction, and Modification of Allergens and Adjuvants. Environ. Sci. Technol. 2017, 51, 4119–4141. [Google Scholar] [CrossRef]
- Shammi, M.; Rahman, M.M.; Tareq, S.M. Distribution of Bioaerosols in Association with Particulate Matter: A Review on Emerging Public Health Threat in Asian Megacities. Front. Environ. Sci. 2021, 9, 698215. [Google Scholar] [CrossRef]
- Mirskaya, E.; Agranovski, I.E. Sources and mechanisms of bioaerosol generation in occupational environments. Crit. Rev. Microbiol. 2018, 44, 739–758. [Google Scholar] [CrossRef]
- Archer, S.D.J.; Lee, K.C.; Caruso, T.; Alcami, A.; Araya, J.G.; Cary, S.C.; Cowan, D.A.; Etchebehere, C.; Gantsetseg, B.; Gomez-Silva, B.; et al. Contribution of soil bacteria to the atmosphere across biomes. Sci. Total Environ. 2023, 871, 162137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Li, H.; Giles, M.; Neilson, R.; Yang, X.R.; Su, J.Q. Microbial Flow Within an Air-Phyllosphere-Soil Continuum. Front. Microbiol. 2020, 11, 615481. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, Y.; Xie, W.; Lu, R.; Mu, F.; Bai, W.; Du, S. Temporal-spatial variations of fungal composition in PM2.5 and source tracking of airborne fungi in mountainous and urban regions. Sci. Total Environ. 2020, 708, 135027. [Google Scholar] [CrossRef] [PubMed]
- Manirajan, B.A.; Maisinger, C.; Ratering, S.; Rusch, V.; Schwiertz, A.; Cardinale, M.; Schnell, S. Diversity, specificity, co-occurrence and hub taxa of the bacterial-fungal pollen microbiome. FEMS Microbiol. Ecol. 2018, 94, fiy112. [Google Scholar] [CrossRef] [PubMed]
- Mayol, E.; Arrieta, J.M.; Jimenez, M.A.; Martinez-Asensio, A.; Garcias-Bonet, N.; Dachs, J.; Gonzalez-Gaya, B.; Royer, S.J.; Benitez-Barrios, V.M.; Fraile-Nuez, E.; et al. Long-range transport of airborne microbes over the global tropical and subtropical ocean. Nat. Commun. 2017, 8, 201. [Google Scholar] [CrossRef]
- Xia, X.; Wang, J.; Ji, J.; Zhang, J.; Chen, L.; Zhang, R. Bacterial Communities in Marine Aerosols Revealed by 454 Pyrosequencing of the 16S rRNA Gene. J. Atmos. Sci. 2015, 72, 2997–3008. [Google Scholar] [CrossRef]
- Ma, M.; Zhen, Y.; Mi, T. Characterization of Bacterial Communities in Bioaerosols over Northern Chinese Marginal Seas and the Northwestern Pacific Ocean in Spring. J. Appl. Meteorol. Clim. 2019, 58, 903–917. [Google Scholar] [CrossRef]
- Qian, J.; Hospodsky, D.; Yamamoto, N.; Nazaroff, W.W.; Peccia, J. Size-resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air 2012, 22, 339–351. [Google Scholar] [CrossRef]
- Uetake, J.; Tobo, Y.; Uji, Y.; Hill, T.C.J.; DeMott, P.J.; Kreidenweis, S.M.; Misumi, R. Seasonal Changes of Airborne Bacterial Communities Over Tokyo and Influence of Local Meteorology. Front. Microbiol. 2019, 10, 1572. [Google Scholar] [CrossRef]
- Federici, E.; Petroselli, C.; Montalbani, E.; Casagrande, C.; Ceci, E.; Moroni, B.; La Porta, G.; Castellini, S.; Selvaggi, R.; Sebastiani, B.; et al. Airborne bacteria and persistent organic pollutants associated with an intense Saharan dust event in the Central Mediterranean. Sci. Total Environ. 2018, 645, 401–410. [Google Scholar] [CrossRef]
- Maki, T.; Hara, K.; Iwata, A.; Lee, K.C.; Kawai, K.; Kai, K.; Kobayashi, F.; Pointing, S.B.; Archer, S.; Hasegawa, H.; et al. Variations in airborne bacterial communities at high altitudes over the Noto Peninsula (Japan) in response to Asian dust events. Atmos. Chem. Phys. 2017, 17, 11877–11897. [Google Scholar] [CrossRef]
- Jeon, E.M.; Kim, H.J.; Jung, K.; Kim, J.H.; Kim, M.Y.; Kim, Y.P.; Ka, J. Impact of Asian dust events on airborne bacterial community assessed by molecular analyses. Atmos. Environ. 2011, 45, 4313–4321. [Google Scholar] [CrossRef]
- Griffin, D.W. Atmospheric Movement of Microorganisms in Clouds of Desert Dust and Implications for Human Health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Abd Aziz, A.; Lee, K.; Park, B.; Park, H.; Park, K.; Choi, I.; Chang, I.S. Comparative study of the airborne microbial communities and their functional composition in fine particulate matter (PM2.5) under non-extreme and extreme PM2.5 conditions. Atmos. Environ. 2018, 194, 82–92. [Google Scholar] [CrossRef]
- Xu, C.; Wei, M.; Chen, J.; Zhu, C.; Li, J.; Xu, X.; Wang, W.; Zhang, Q.; Ding, A.; Kan, H.; et al. Profile of inhalable bacteria in PM2.5 at Mt. Tai, China: Abundance, community, and influence of air mass trajectories. Ecotoxicol. Environ. Saf. 2019, 168, 110–119. [Google Scholar] [CrossRef]
- Soleimani, Z.; Teymouri, P.; Darvishi Boloorani, A.; Mesdaghinia, A.; Middleton, N.; Griffin, D.W. An overview of bioaerosol load and health impacts associated with dust storms: A focus on the Middle East. Atmos. Environ. 2020, 223, 117187. [Google Scholar] [CrossRef]
- Rodríguez-Arias, R.M.; Rojo, J.; Fernández-González, F.; Pérez-Badia, R. Desert dust intrusions and their incidence on airborne biological content. Review and case study in the Iberian Peninsula. Environ. Pollut. 2023, 316, 120464. [Google Scholar] [CrossRef]
- Xia, F.; Chen, Z.; Tian, E.; Mo, J. A super sandstorm altered the abundance and composition of airborne bacteria in Beijing. J. Environ. Sci. 2024, 144, 35–44. [Google Scholar] [CrossRef]
- Joung, Y.S.; Ge, Z.; Buie, C.R. Bioaerosol generation by raindrops on soil. Nat. Commun. 2017, 8, 14668. [Google Scholar] [CrossRef]
- Núñez, A.; Amo De Paz, G.; Rastrojo, A.; Ferencova, Z.; Gutiérrez-Bustillo, A.M.; Alcamí, A.; Moreno, D.A.; Guantes, R. Temporal patterns of variability for prokaryotic and eukaryotic diversity in the urban air of Madrid (Spain). Atmos. Environ. 2019, 217, 116972. [Google Scholar] [CrossRef]
- Kang, S.M.; Heo, K.J.; Lee, B.U. Why Does Rain Increase the Concentrations of Environmental Bioaerosols during Monsoon? Aerosol Air Qual. Res. 2015, 15, 2320–2324. [Google Scholar] [CrossRef]
- Jang, G.I.; Hwang, C.Y.; Cho, B.C. Effects of heavy rainfall on the composition of airborne bacterial communities. Front. Environ. Sci. Eng. 2018, 12, 12. [Google Scholar] [CrossRef]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.F.C.; Mathai, P.P.; Pauliquevis, T.; Balsanelli, E.; Pedrosa, F.O.; Souza, E.M.; Baura, V.A.; Monteiro, R.A.; Cruz, L.M.; Souza, R.A.F.; et al. Influence of seasonality on the aerosol microbiome of the Amazon rainforest. Sci. Total. Environ. 2021, 760, 144092. [Google Scholar] [CrossRef]
- Bowers, R.M.; McLetchie, S.; Knight, R.; Fierer, N. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME J. 2011, 5, 601–612. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Frohlich-Nowoisky, J.; Pickersgill, D.A.; Despres, V.R.; Poschl, U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA 2009, 106, 12814–12819. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Xu, L.; Zhang, J.; Yuan, Q.; Ding, X.; Hu, W.; Fu, P.; Zhang, D. Overview of primary biological aerosol particles from a Chinese boreal forest: Insight into morphology, size, and mixing state at microscopic scale. Sci. Total Environ. 2020, 719, 137520. [Google Scholar] [CrossRef]
- Haas, D.; Ilieva, M.; Fritz, T.; Galler, H.; Habib, J.; Kriso, A.; Kropsch, M.; Ofner-Kopeinig, P.; Reinthaler, F.F.; Strasser, A.; et al. Background concentrations of airborne, culturable fungi and dust particles in urban, rural and mountain regions. Sci. Total Environ. 2023, 892, 164700. [Google Scholar] [CrossRef]
- Mu, F.; Li, Y.; Lu, R.; Qi, Y.; Xie, W.; Bai, W. Source identification of airborne bacteria in the mountainous area and the urban areas. Atmos. Res. 2020, 231, 104676. [Google Scholar] [CrossRef]
- Triadó-Margarit, X.; Caliz, J.; Reche, I.; Casamayor, E.O. High similarity in bacterial bioaerosol compositions between the free troposphere and atmospheric depositions collected at high-elevation mountains. Atmos. Environ. 2019, 203, 79–86. [Google Scholar] [CrossRef]
- Xu, C.; Wei, M.; Chen, J.; Zhu, C.; Li, J.; Lv, G.; Xu, X.; Zheng, L.; Sui, G.; Li, W.; et al. Fungi diversity in PM2. 5 and PM1 at the summit of Mt. Tai: Abundance, size distribution, and seasonal variation. Atmos. Chem. Phys. 2017, 17, 11247–11260. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, Q.; Fu, X.; Zheng, L.; Dong, J.; Wang, J.; Guo, S. Feedback of airborne bacterial consortia to haze pollution with different PM2.5 levels in typical mountainous terrain of Jinan, China. Sci. Total Environ. 2019, 695, 133912. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.M.; Stanley, R.K. Airborne Algae: A Rising Public Health Risk. Environ. Sci. Technol. 2023, 57, 5501–5503. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Śliwińska-Wilczewska, S.; Savoie, M.; Lewandowska, A.U. Quantitative and qualitative variability of airborne cyanobacteria and microalgae and their toxins in the coastal zone of the Baltic Sea. Sci. Total Environ. 2022, 826, 154152. [Google Scholar] [CrossRef]
- Xu, C.; Chen, J.; Wang, Z.; Chen, H.; Feng, H.; Wang, L.; Xie, Y.; Wang, Z.; Ye, X.; Kan, H.; et al. Diverse bacterial populations of PM2.5 in urban and suburb Shanghai, China. Front. Environ. Sci. Eng. 2021, 15, 37. [Google Scholar] [CrossRef]
- Lin, W.; Wang, P.; Tien, C.; Chen, W.; Yu, Y.; Hsu, L. Changes in airborne fungal flora along an urban to rural gradient. J. Aerosol Sci. 2018, 116, 116–123. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, L.; Wu, D.; Xie, J.W.; Li, J.; Fu, X.W.; Cong, Z.Y.; Fu, P.Q.; Zhang, Y.; Luo, X.S.; et al. Global airborne bacterial community-interactions with Earth’s microbiomes and anthropogenic activities. Proc. Natl. Acad. Sci. USA 2022, 119, e2204465119. [Google Scholar] [CrossRef]
- Abrego, N.; Crosier, B.; Somervuo, P.; Ivanova, N.; Abrahamyan, A.; Abdi, A.; Hamalainen, K.; Junninen, K.; Maunula, M.; Purhonen, J.; et al. Fungal communities decline with urbanization-more in air than in soil. ISME J. 2020, 14, 2806–2815. [Google Scholar] [CrossRef]
- Calderón-Ezquerro, M.D.C.; Serrano-Silva, N.; Brunner-Mendoza, C. Aerobiological study of bacterial and fungal community composition in the atmosphere of Mexico City throughout an annual cycle. Environ. Pollut. 2021, 278, 116858. [Google Scholar] [CrossRef]
- Franchitti, E.; Caredda, C.; Anedda, E.; Traversi, D. Urban Aerobiome and Effects on Human Health: A Systematic Review and Missing Evidence. Atmosphere 2022, 13, 1148. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, Y.; Ye, Z.; Li, G.; Wang, W.; An, T. Pollution profiles of antibiotic resistance genes associated with airborne opportunistic pathogens from typical area, Pearl River Estuary and their exposure risk to human. Environ. Int. 2020, 143, 105934. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, H.; Yao, M. Microbial emission levels and diversities from different land use types. Environ. Int. 2020, 143, 105988. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, I.; Grinn-Gofroń, A.; Ćwik, A.; Kluska, K.; Cariñanos, P.; Wójcik, T. Allergenic fungal spores in the air of urban parks. Aerobiologia 2021, 37, 39–51. [Google Scholar] [CrossRef]
- Chen, H.; Du, R.; Zhang, Y.; Du, P.; Zhang, S.; Ren, W.; Yang, M. Evolution of PM2.5 bacterial community structure in Beijing’s suburban atmosphere. Sci. Total Environ. 2021, 799, 149387. [Google Scholar] [CrossRef]
- Lin, X.; Pei, C.; Liu, T.; Shu, Q.; Hong, D.; Huang, Z.; Zhang, Y.; Lai, S. Characterizing atmospheric biological aerosols at a suburban site in Guangzhou, southern China by airborne microbes, proteins and saccharides. Sci. Total Environ. 2023, 883, 163543. [Google Scholar] [CrossRef]
- Kumari, P.; Choi, H.L. Manure removal system influences the abundance and composition of airborne biotic contaminants in swine confinement buildings. Environ. Monit. Assess. 2015, 187, 537. [Google Scholar] [CrossRef]
- Finn, D.R.; Maldonado, J.; Martini, F.; Yu, J.; Penton, C.R.; Fontenele, R.S.; Schmidlin, K.; Kraberger, S.; Varsani, A.; Gile, G.H.; et al. Agricultural practices drive biological loads, seasonal patterns and potential pathogens in the aerobiome of a mixed-land-use dryland. Sci. Total Environ. 2021, 798, 149239. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, Y.; Niu, M.; Zhou, F.; Wu, Y.; Wang, J.; Zhu, T.; Wu, Y.; Wu, Z.; Hu, M.; et al. Characteristics of biological particulate matters at urban and rural sites in the North China Plain. Environ. Pollut. 2019, 253, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, Y.; Wang, F.; Xu, Z.; Zhou, S.; Sun, R.; Wu, X.; Yan, K. East Asian monsoon manipulates the richness and taxonomic composition of airborne bacteria over China coastal area. Sci. Total Environ. 2023, 875, 162581. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, B.; Han, Y.; Yang, L.; Zhang, S.; Yang, K.; Yu, F. The pollution of bioaerosols in hospital sewage purification process: Composition characteristics, seasonal variation and risk assessment. J. Clean. Prod. 2023, 419, 138301. [Google Scholar] [CrossRef]
- Kataki, S.; Patowary, R.; Chatterjee, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Kamboj, D.V. Bioaerosolization and pathogen transmission in wastewater treatment plants: Microbial composition, emission rate, factors affecting and control measures. Chemosphere 2022, 287 Pt 3, 132180. [Google Scholar] [CrossRef]
- Kowalski, M.; Wolany, J.; Pastuszka, J.S.; Płaza, G.; Wlazło, A.; Ulfig, K.; Malina, A. Characteristics of airborne bacteria and fungi in some Polish wastewater treatment plants. Int. J. Environ. Sci. Technol. 2017, 14, 2181–2192. [Google Scholar] [CrossRef]
- Xu, G.; Han, Y.; Li, L.; Liu, J. Characterization and source analysis of indoor/outdoor culturable airborne bacteria in a municipal wastewater treatment plant. J. Environ. Sci. 2018, 74, 71–78. [Google Scholar] [CrossRef]
- Hsiao, T.; Lin, A.Y.; Lien, W.; Lin, Y. Size distribution, biological characteristics and emerging contaminants of aerosols emitted from an urban wastewater treatment plant. J. Hazard. Mater. 2020, 388, 121809. [Google Scholar] [CrossRef] [PubMed]
- Michałkiewicz, M. Wastewater Treatment Plants as a Source of Bioaerosols. Pol. J. Environ. Stud. 2019, 28, 2261–2271. [Google Scholar] [CrossRef]
- Sialve, B.; Gales, A.; Hamelin, J.; Wery, N.; Steyer, J.P. Bioaerosol emissions from open microalgal processes and their potential environmental impacts: What can be learned from natural and anthropogenic aquatic environments? Curr. Opin. Biotech. 2015, 33, 279–286. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yang, K.; Yang, L.; Zhang, S.; Ba, Y.; Zhou, G. The bioaerosols generated from the sludge treatment process: Bacterial and fungal variation characteristics, source tracking, and risk assessment. Sci. Total Environ. 2023, 903, 166193. [Google Scholar] [CrossRef]
- Mbareche, H.; Veillette, M.; Bonifait, L.; Dubuis, M.; Benard, Y.; Marchand, G.; Bilodeau, G.J.; Duchaine, C. A next generation sequencing approach with a suitable bioinformatics workflow to study fungal diversity in bioaerosols released from two different types of composting plants. Sci. Total Environ. 2017, 601–602, 1306–1314. [Google Scholar] [CrossRef]
- Núñez, A.; García, A.M. The aerobiome in a hospital environment: Characterization, seasonal tendencies and the effect of window opening ventilation. Build. Environ. 2023, 230, 110024. [Google Scholar] [CrossRef]
- Martin, E.; Kampfer, P.; Jackel, U. Quantification and identification of culturable airborne bacteria from duck houses. Ann. Occup. Hyg. 2010, 54, 217–227. [Google Scholar] [PubMed]
- Dungan, R.S.; Leytem, A.B.; Verwey, S.A.; Bjorneberg, D.L. Assessment of bioaerosols at a concentrated dairy operation. Aerobiologia 2010, 26, 171–184. [Google Scholar] [CrossRef]
- Sowiak, M.; Bródka, K.; Buczyńska, A.; Cyprowski, M.; Kozajda, A.; Sobala, W.; Szadkowska-Stańczyk, I. An assessment of potential exposure to bioaerosols among swine farm workers with particular reference to airborne microorganisms in the respirable fraction under various breeding conditions. Aerobiologia 2012, 28, 121–133. [Google Scholar] [CrossRef]
- Thorne, P.S.; Ansley, A.C.; Perry, S.S. Concentrations of bioaerosols, odors, and hydrogen sulfide inside and downwind from two types of swine livestock operations. J. Occup. Environ. Hyg. 2009, 6, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Skóra, J.; Matusiak, K.; Wojewódzki, P.; Nowak, A.; Sulyok, M.; Ligocka, A.; Okrasa, M.; Hermann, J.; Gutarowska, B. Evaluation of Microbiological and Chemical Contaminants in Poultry Farms. Int. J. Environ. Res. Public Health 2016, 13, 192. [Google Scholar] [CrossRef]
- Dai, P.; Shen, D.; Tang, Q.; Huang, K.; Li, C. PM2.5 from a broiler breeding production system: The characteristics and microbial community analysis. Environ. Pollut. 2020, 256, 113368. [Google Scholar] [CrossRef]
- Peng, S.; Luo, M.; Long, D.; Liu, Z.; Tan, Q.; Huang, P.; Shen, J.; Pu, S. Full-length 16S rRNA gene sequencing and machine learning reveal the bacterial composition of inhalable particles from two different breeding stages in a piggery. Ecotoxicol. Environ. Saf. 2023, 253, 114712. [Google Scholar] [CrossRef]
- Nie, C.; Geng, X.; Ouyang, H.; Wang, L.; Li, Z.; Wang, M.; Sun, X.; Wu, Y.; Qin, Y.; Xu, Y.; et al. Abundant bacteria and fungi attached to airborne particulates in vegetable plastic greenhouses. Sci. Total Environ. 2023, 857, 159507. [Google Scholar] [CrossRef]
- Wei, M.; Xu, C.; Xu, X.; Zhu, C.; Li, J.; Lv, G. Size distribution of bioaerosols from biomass burning emissions: Characteristics of bacterial and fungal communities in submicron (PM1.0) and fine (PM2.5) particles. Ecotoxicol. Environ. Saf. 2019, 171, 37–46. [Google Scholar] [CrossRef]
- Wang, S.; Qian, H.; Sun, Z.; Cao, G.; Ding, P.; Zheng, X. Comparison of airborne bacteria and fungi in different built environments in selected cities in five climate zones of China. Sci. Total Environ. 2023, 860, 160445. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Almeida, L.C.S.D.; Pecoraro, L. Environmental Factors Affecting Diversity, Structure, and Temporal Variation of Airborne Fungal Communities in a Research and Teaching Building of Tianjin University, China. J. Fungi 2022, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Y.; Tian, Y.; Li, A.; Li, Y.; Xiong, J.; Gao, R. On-site investigation of the concentration and size distribution characteristics of airborne fungi in a university library. Environ. Pollut. 2020, 261, 114138. [Google Scholar] [CrossRef] [PubMed]
- Law, A.K.Y.; Chau, C.K.; Chan, G.Y. Characteristics of bioaerosol profile in office buildings in Hong Kong. Build. Environ. 2001, 36, 527–541. [Google Scholar] [CrossRef]
- Gao, X.; Shao, M.; Luo, Y.; Dong, Y.; Ouyang, F.; Dong, W.; Li, J. Airborne bacterial contaminations in typical Chinese wet market with live poultry trade. Sci. Total Environ. 2016, 572, 681–687. [Google Scholar] [CrossRef]
- Fan, L.; Han, X.; Wang, X.; Li, L.; Gong, S.; Qi, J.; Li, X.; Ge, T.; Liu, H.; Ye, D.; et al. Levels, distributions and influential factors of residential airborne culturable bacteria in 12 Chinese cities: Multicenter on-site survey among dwellings. Environ. Res. 2022, 212, 113425. [Google Scholar] [CrossRef]
- Geng, X.; Nie, C.; Wang, L.; Li, L.; Li, D.; Nishino, A.; Chen, J. ITS and 16S rRNA Gene Revealed Multitudinous Microbial Contaminations of Residential Air Conditioning Filters in Megacity Shanghai, China. Environ. Health 2024, 2, 34–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).