Terrain-Integrated Soil Mapping Units (SMUs) for Precision Nutrient Management: A Case Study from Semi-Arid Tropics of India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
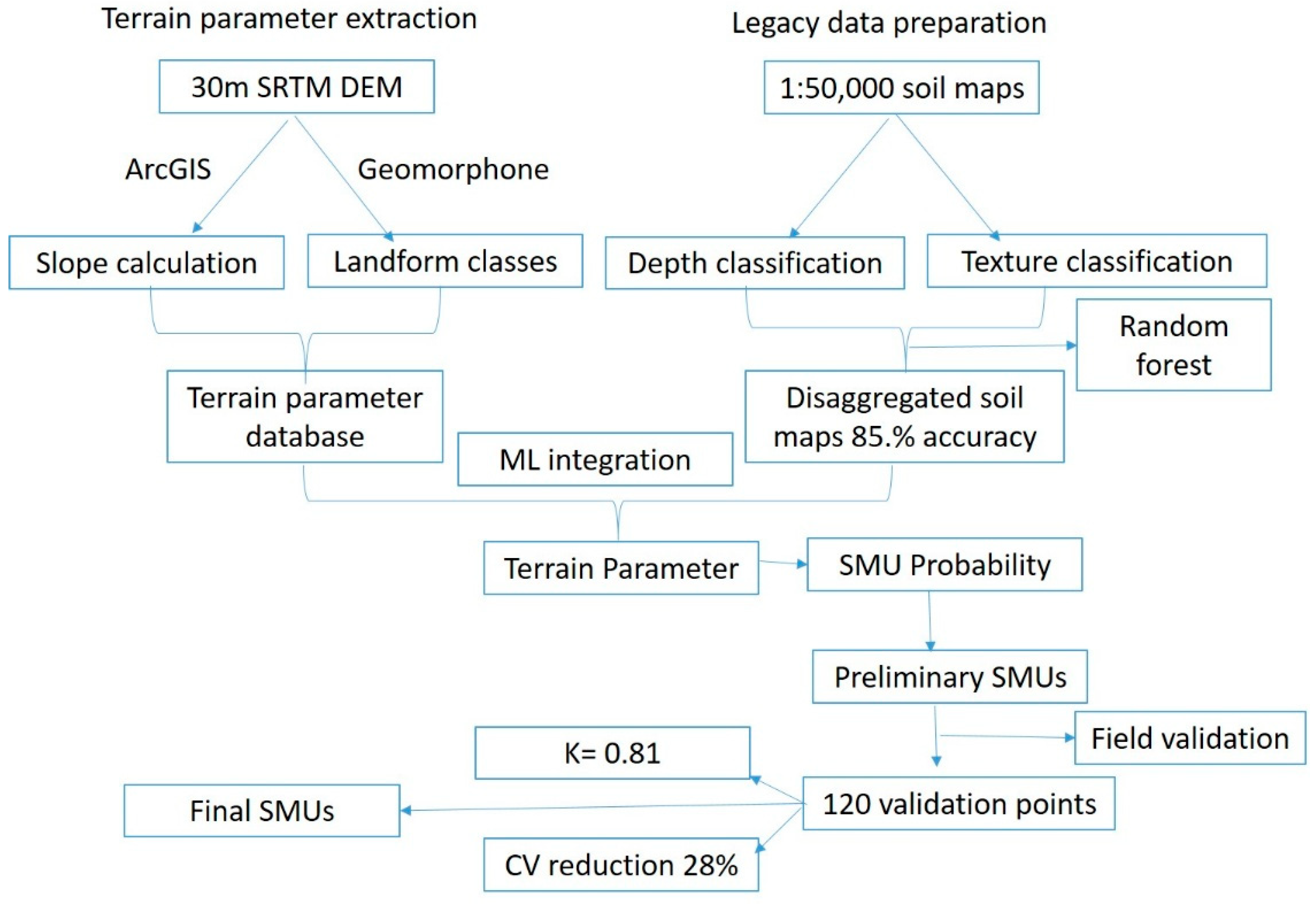
2.2. Soil Mapping Units
2.3. Surface Soil Sampling
2.4. Soil Analysis
2.5. Statistical Analysis
3. Result
3.1. Soil Mapping Units
3.2. Variability in Soil Fertility Parameters
3.3. SMUs-Fertility Parameters Relations
4. Discussion
4.1. SMU-Soil Relationships and Landscape Processes
4.2. Soil Carbon Dynamics
4.3. Management of Nutrient Management
4.4. Precision Agriculture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| Tukey HSD | Honestly Significant Difference |
| NUE | Nitrogen Use efficiency |
| CV | Coefficient of Variance |
References
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Status of the World’s Soil Resources: Main Report; FAO: Rome, Italy, 2015. [Google Scholar]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pierce, F.J.; Nowak, P. Aspects of precision agriculture. Adv. Agron. 1999, 67, 1–85. [Google Scholar]
- McBratney, A.; Whelan, B.; Ancev, T.; Bouma, J. Future directions of precision agriculture. Precis. Agric. 2005, 6, 7–23. [Google Scholar] [CrossRef]
- Jenny, H. Factors of Soil Formation: A System of Quantitative Pedology; McGraw-Hill: New York, NY, USA, 1941. [Google Scholar]
- Moore, I.D.; Gessler, P.E.; Nielsen, G.A.; Peterson, G.A. Soil attribute prediction using terrain analysis. Soil Sci. Soc. Am. J. 1993, 57, 443–452. [Google Scholar] [CrossRef]
- Pennock, D.J.; Zebarth, B.J.; De Jong, E. Landform classification and soil distribution in hummocky terrain, Saskatchewan, Canada. Geoderma 1987, 40, 297–315. [Google Scholar] [CrossRef]
- Elsenbeer, H. Hydrologic flowpaths in tropical rainforest soilscapes: A review. Hydrol. Process 2001, 15, 1751–1759. [Google Scholar] [CrossRef]
- Whelan, B.M.; McBratney, A.B. Definition and interpretation of potential management zones in Australia. In Proceedings of the 11th Australian Agronomy Conference, Geelong, Australia, 2–6 February 2003; Australian Society of Agronomy: Geelong, Australia, 2003. [Google Scholar]
- Ferguson, R.B.; Hergert, G.W.; Schepers, J.S.; Gotway, C.A.; Cahoon, J.E.; Peterson, T.A. Site-specific nitrogen management of irrigated maize: Yield and soil residual nitrate effects. Soil Sci. Soc. Am. J. 2003, 67, 334–343. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. Digital soil mapping: A brief history and some lessons. Geoderma 2016, 264, 301–311. [Google Scholar] [CrossRef]
- Sanchez, P.A.; Ahamed, S.; Carré, F.; Hartemink, A.E.; Hempel, J.; Huising, J.; Lagacherie, P.; McBratney, A.B.; McKenzie, N.J.; Mendonça-Santos, M.L.; et al. Digital soil map of the world. Science 2009, 325, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Pal, D.K.; Mandal, C.; Chandran, P.; Ray, S.K.; Sarkar, D.; Velmourougane, K.; Srivastava, A.; Sidhu, G.S.; Singh, R.S.; et al. Soils of India: Historical perspective, classification and recent advances. Curr. Sci. 2013, 104, 1308–1323. [Google Scholar]
- Bhaskar, B.P. Delineation of soil management units for sustainable agriculture in Yavatmal district, Maharashtra. Agropedology 2015, 25, 1–12. [Google Scholar]
- Bhaskar, B.P. Landscape planning for agri-development at regional scale: An example from cotton growing Yavatmal district, Maharashtra, India. J. Agric. Environ. Int. Dev. 2015, 109, 235–269. [Google Scholar]
- Behrens, T.; Schmidt, K.; Zhu, A.X.; Scholten, T. The ConMap approach for terrain-based digital soil mapping. Eur. J. Soil Sci. 2010, 61, 133–143. [Google Scholar] [CrossRef]
- Adamchuk, V.I.; Hummel, J.W.; Morgan, M.T.; Upadhyaya, S.K. On-the-go soil sensors for precision agriculture. Comput. Electron. Agric. 2004, 44, 71–91. [Google Scholar] [CrossRef]
- Gebbers, R.; Adamchuk, V.I. Precision agriculture and food security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Government of Maharashtra. Maharashtra State Agriculture Policy 2018; Department of Agriculture: Mumbai, India, 2018. [Google Scholar]
- Mandal, D.K.; Mandal, C.; Singh, S.K. Agro-Ecological Regions of India (Revised Edition); NBSS&LUP Publ., no 170; ICAR-NBSS&LUP: Nagpur, India, 2015; p. 27. [Google Scholar]
- IMD. Climate of Maharashtra; Indian Meteorology Department: New Delhi, India, 2005.
- McBratney, A.B.; Santos, M.L.M.; Minasny, B. Digital soil mapping: A new paradigm for soil survey and land evaluation. Geoderma 2003, 117, 3–52. [Google Scholar] [CrossRef]
- USGS EarthExplorer. Available online: https://earthexplorer.usgs.gov (accessed on 25 May 2020).
- ICAR-NBSS&LUP. Soil Map of Maharashtra; ICAR-NBSS&LUP: Nagpur, India, 2015. [Google Scholar]
- Zhu, A.X.; Liu, J.; Du, F.; Qi, F.; Qin, C. Predictive soil mapping with limited data using fuzzy logic and spatial data mining. Geoderma 2010, 158, 199–206. [Google Scholar] [CrossRef]
- Singh, K.; Tiwary, P.; Mandal, D.; Das, D.K. Delineation of soil mapping units using terrain attributes and satellite data. Agropedology 2017, 27, 45–53. [Google Scholar]
- NRSC. Land Use/Land Cover Atlas of India; National Remote Sensing Centre: Hyderabad, India, 2019.
- Tiwari, G.; Singh, A.; Chatterjee, R.S.; Srivastava, Y.K. Use of hyperspectral data for digital soil mapping. NDT 2020, 12, 123–145. [Google Scholar]
- Wani, S.P.; Garg, K.K.; Pathak, P.; Sahrawat, K.L. Soil health management for sustainable agriculture. Agric. Syst. 2016, 145, 1–10. [Google Scholar]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; p. 109. [Google Scholar]
- Zhou, W.; Han, G.; Liu, M.; Li, X. Effects of soil pH and texture on soil carbon and nitrogen in soil profiles under different land uses in Mun River Basin. Northeast. Thail. Peer J. 2019, 7, e7880. [Google Scholar] [CrossRef] [PubMed]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, Part-2. Chemical and Microbiological Properties; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid method for the estimation of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ. 1954, 939, 1–19. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Williams, C.H.; Steinbergs, A. Soil sulphur fraction as chemical indices of available sulphur in soils. Aust. J. Agric. Res. 1959, 10, 340–352. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Novell, W.A. Development of DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Anselin, L. Local indicators of spatial association-LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Anselin, L. The Moran Scatterplot as an ESDA tool to assess local instability in spatial association. In Spatial Analytical Perspectives on GIS; Taylor & Francis: Oxford, UK, 1996; Volume 4, p. 111. [Google Scholar]
- Wilding, L.P. Spatial variability: Its documentation, accommodation and implication to soil survey. In Soil Spatial Variability; Nielsen, D.R., Bouma, J., Eds.; Pudoc: Wagenigen, The Netherlands, 1985; pp. 166–189. [Google Scholar]
- Esri. ArcGIS Desktop: Release 10.3; Environmental Systems Research Institute: Redlands, CA, USA, 2014. [Google Scholar]
- Webster, R.; Oliver, M.A. Geostatistics for Environmental Scientists, 2nd ed.; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Lagacherie, P.; McBratney, A.B. Spatial soil information systems and spatial soil inference systems: Perspectives for digital soil mapping. Geoderma 2007, 140, 369–387. [Google Scholar]
- Rossiter, D.G. Digital soil resource inventories: Status and prospects. Soil Use Manag. 2004, 20, 296–301. [Google Scholar] [CrossRef]
- Sarkar, D.; Das, T.H.; Singh, R.S.; Subba Rao, A. Soils of alluvial plains of Indo-Gangetic region. Agropedology 2001, 11, 1–8. [Google Scholar]
- Sehgal, J. Pedology: Concepts and Applications; Kalyani Publishers: New Delhi, India, 1996. [Google Scholar]
- Bhattacharyya, T.; Pal, D.K.; Mandal, C.; Velayutham, M. Soil Atlas of India; NBSS&LUP: Nagpur, India, 2008. [Google Scholar]
- Kharche, V.K.; Gaikwad, S.T.; Patil, N.G.; Gaikwad, M.S. Digital terrain analysis for delineation of physiographic units in parts of Maharashtra. J. Indian Soc. Remote Sens. 2012, 40, 567–575. [Google Scholar]
- Das, D.K.; Mandal, D.; Mandal, C.; Mandal, B. Characterization and classification of soils on different landforms of dryland ecosystem. Agropedology 1992, 2, 29–36. [Google Scholar]
- Pal, D.K.; Bhattacharyya, T.; Chandran, P.; Ray, S.K. Significance of soil colour in the assessment of land quality and productivity. Curr. Sci. 2009, 96, 543–547. [Google Scholar]
- NBSSLUP. Manual on Soil Resource Mapping using Remote Sensing and GIS Techniques; NBSS Publ. No. 85; National Bureau of Soil Survey and Land Use Planning: Nagpur, India, 2000. [Google Scholar]
- Dadhwal, V.K.; Singh, R.P.; Dutta, S.; Parihar, J.S. Remote sensing-based crop inventory: A review of Indian experience. Trop. Ecol. 2002, 43, 107–122. [Google Scholar]
- Vasu, D.; Singh, S.K.; Tiwary, P.; Chandran, P.; Ray, S.K.; Duraisami, V.P. Pedogenic processes and soil–landform relationships for identification of yield-limiting soil properties. Soil Res. 2016, 55, 273. [Google Scholar] [CrossRef]
- Chinchmalatpure, A.R.; Brij-Lal; Challa, O.; Sehgal, J. Available micronutrient status of soils on different parent materials and landforms in a micro watershed of Wunna catchment near Nagpur (Maharashtra). Agropedology 2000, 10, 53–58. [Google Scholar]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Börjesson, G.; Menichetti, L.; Kirchmann, H.; Kätterer, T. Soil microbial community structure affected by 53 years of nitrogen fertilisation and different organic amendments. Biol. Fertil. Soils. 2012, 48, 245–257. [Google Scholar] [CrossRef]
- Mau, R.L.; Liu, C.M.; Aziz, M.; Schwartz, E.; Dijkstra, P.; Marks, J.C.; Price, L.B.; Keim, P.; Hungate, B.A. Linking soil bacterial biodiversity and soil carbon stability. ISME J. 2015, 9, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Chattaraj, S.; Jangir, A.; Tiwari, G.; Dash, B.; Daripa, A.; Naitam, R.K. Geospatial variability mapping of soil nutrients for site specific input optimization in a part of central India. Agron. J. 2022, 114, 1489–1499. [Google Scholar] [CrossRef]
- Iticha, B.; Takele, C. Soil–landscape variability: Mapping and building detail information for soil management. Soil Use Manag. 2018, 34, 111–123. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mile. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Manns, H.R.; Martin, R.C. Cropping system yield stability in response to plant diversity and soil organic carbon in temperate ecosystems. Agroecol. Sustain. Food Syst. 2018, 42, 724–750. [Google Scholar] [CrossRef]
- De Datta, S.K.; Buresh, R.J. Integrated nitrogen management in irrigated rice. Adv. Soil Sci. 1989, 10, 143–169. [Google Scholar]
- Khaitov, B.; Allanov, K.; Islam, K.R.; Park, K.W. Bio-inoculant improves nitrogen-use efficiency and cotton yield in saline soils. J. Plant. Nutr. Soil Sci. 2019, 182, 393–400. [Google Scholar] [CrossRef]
- Sharma, R.P.; Chattaraj, S.; Vasu, D.; Karthikeyan, K.; Tiwary, P.; Naitam, R.K.; Dash, B.; Tiwari, G.; Jangir, A.; Daripa, A.; et al. Spatial variability assessment of soil fertility in black soils of central India using geostatistical modeling. Arch. Agron. Soil Sci. 2020, 67, 876–888. [Google Scholar] [CrossRef]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D.; Havlin, J.L. Soil Fertility and Fertilizer, 5th ed.; Prentice-Hall of India: New Delhi, India, 1995; p. 684. [Google Scholar]
- Bhaskar, B.P.; Sarkar, D.; Mandal, C.; Bobade, S.V.; Gaikwad, M.S.; Gaikwad, S.S.; Nimkar, A.M.; Bhattacharyya, T. Reconnaissance Soil Survey of Yavatmal District, Maharashtra, India; NBSS Publ. No. 1059; NBSS & LUP: Nagpur, India, 2014; p. 208. [Google Scholar]
- Fu, W.; Zhao, K.; Jiang, P.; Ye, Z.; Tunney, H.; Zhang, C. Field-scale variability of soil test phosphorus and other nutrients in grasslands under long-term agricultural managements. Soil Res. 2013, 51, 503–512. [Google Scholar] [CrossRef]
- Simonsson, M.; Hillier, S.; Öborn, I. Changes in Clay Minerals and Potassium Fixation Capacity as a Result of Release and Fixation of Potassium in Long-Term Field Experiments. Geoderma 2009, 151, 109–120. [Google Scholar] [CrossRef]
- Li, J.; Richter, D.D.; Mendoza, A.; Heine, P. Four-decade responses of soil trace elements to an aggrading old-field forest: B, Mn, Zn, Cu, and Fe. Ecology 2008, 89, 2911–2923. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Weight | Rationale | Reference |
|---|---|---|---|
| Landform | 0.40 | Controls hydrology | [10] |
| Slope | 0.30 | Affects erosion | [29] |
| Depth | 0.20 | Rooting limitation | [30] |
| Texture | 0.10 | Modifies processes | [16] |
| Metric | This Study (2023) | Bhaskar (2015) [17] | Singh et al. (2017) [30] | Improvement |
|---|---|---|---|---|
| Spatial Resolution | 10-ha grids | 25-ha grids | 15-ha grids | +150% vs. [17] |
| Within-SMU CV (%) | 28.1 ± 3.2 | 38.4 ± 5.6 | 32.7 ± 4.1 | 26.8% reduction vs. [17] |
| Boundary Accuracy (κ) | 0.81 | 0.65 | 0.72 | +24.6% vs. [17] |
| Parameters Integrated | 4 (Terrain + Soil + ML + Field) | 2 (Soil only) | 3 (Soil + Terrain) | +100% vs. [17] |
| Validation Points | 120 | 80 | 100 | +50% vs. [17] |
| Parameters | Unit | Threshold Value | Adopted by |
|---|---|---|---|
| OC | (%) | 0.50–0.75 | [37] |
| AvN | kg ha−1 | 280–560 | [38] |
| AvP | 22–56 | [39] | |
| AvK | 140–336 | [40] | |
| AvS | 10–20 | [41] | |
| DTPA-Fe | mg kg−1 | ≤4.5 | [42] |
| DTPA-Mn | ≤1.0 | ||
| DTPA-Zn | ≤0.6 | ||
| DTPA-Cu | ≤0.2 |
| SMU | Area (%) | Depth | Texture | pH | SOC (%) | AvN (kg ha−1) | Management Implication |
|---|---|---|---|---|---|---|---|
| 1 | 35 | Very deep | Clay | 7.9 | 0.9 | 121 | Optimal for cotton |
| 4 | 8 | Deep | Clay loam | 7.6 | 1.0 | 148 | High N variability (CV = 53%) * |
| 7 | 5 | Mod. shallow | Clay | 7.6 | 1.1 | 128 | High SOC hotspot |
| 13 | 3 | Shallow | Clay loam | 7.7 | 0.9 | 116 | Zn-deficient (0.4 mg kg−1) ** |
| Variables | pH | EC | OC | AvN | AvP | AvK | AvS | DTPA-Fe | DTPA-Mn | DTPA-Zn | DTPA-Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||
| EC | 0.16 | 1 | |||||||||
| OC | −0.02 | 0.71 ** | 1 | ||||||||
| AvN | −0.49 | 0.12 | 0.47 | 1 | |||||||
| AvP | −0.25 | 0.34 | 0.60 * | 0.41 | 1 | ||||||
| AvK | 0.04 | 0.79 ** | 0.83 ** | 0.55 * | 0.58 * | 1 | |||||
| AvS | 0.03 | 0.77 ** | 0.65 ** | 0.56 * | 0.41 | 0.81 ** | 1 | ||||
| DTPA-Fe | −0.48 | 0.48 | 0.62 * | 0.38 | 0.08 | 0.45 | 0.31 | 1 | |||
| DTPA-Mn | −0.55 | 0.36 | 0.69 ** | 0.76 ** | 0.34 | 0.57 * | 0.46 | 0.85 ** | 1 | ||
| DTPA-Zn | 0.00 | 0.51 | 0.86 ** | 0.26 | 0.68 ** | 0.67 ** | 0.37 | 0.45 | 0.52 * | 1 | |
| DTPA-Cu | 0.05 | 0.22 | 0.47 | −0.16 | −0.14 | 0.10 | −0.15 | 0.66 ** | 0.41 | 0.48 | 1 |
| Soil Properties | Kriging Type | Fitted Model | Range (m) | Nugget (Co) | Partial Sill (C) | Sill (Co + C) | N:S ratio | RMSE ** |
|---|---|---|---|---|---|---|---|---|
| pH | Ordinary | Exponential | 1173 | 0.12 | 0.2 | 0.3 | 0.38 | 0.6 |
| EC | Simple | Exponential | 1599 | 0.15 | 0.17 | 0.3 | 0.47 | 0.1 |
| SOC | Ordinary | Exponential | 656 | 0.04 | 0.09 | 0.1 | 0.31 | 0.1 |
| N | Ordinary | Exponential | 608 | 115 | 308 | 423.0 | 0.27 | 59.0 |
| P | Ordinary | Spherical | 608 | 80 | 343 | 423.0 | 0.19 | 11.2 |
| K | Ordinary | Spherical | 608 | 120,560 | 176,740 | 297,300.0 | 0.41 | 102.8 |
| S | Ordinary | Spherical | 608 | 50 | 67 | 117.0 | 0.43 | 5.6 |
| Zn | Simple | Exponential | 2815 | 0.38 | 0.47 | 0.9 | 0.45 | 0.3 |
| Cu | Simple | Exponential | 2902 | 0.39 | 0.46 | 0.9 | 0.46 | 0.7 |
| Fe | Ordinary | Exponential | 1931 | 32 | 38 | 70.0 | 0.46 | 1.7 |
| Mn | Ordinary | Exponential | 1324 | 220 | 271 | 491.0 | 0.45 | 2.7 |
| SMU Group | Key Constraint | Recommended Practice | Expected Benefit |
|---|---|---|---|
| Summit (6–7) | Erosion, low SOC | Contour hedgerows + compost | 40% soil loss reduction [33] |
| Slope (4–5) | N leaching | Polymer-coated urea | 25% NUE improvement [64] |
| Valley (14–15) | Zn deficiency | ZnSO4 @ 25 kg ha−1 | 0.5–1.2 t ha−1 yield gain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, G.; Sharma, R.P.; Chattaraj, S.; Jangir, A.; Dash, B.; Malav, L.C.; Yadav, B.; Daripa, A. Terrain-Integrated Soil Mapping Units (SMUs) for Precision Nutrient Management: A Case Study from Semi-Arid Tropics of India. NDT 2025, 3, 19. https://doi.org/10.3390/ndt3030019
Tiwari G, Sharma RP, Chattaraj S, Jangir A, Dash B, Malav LC, Yadav B, Daripa A. Terrain-Integrated Soil Mapping Units (SMUs) for Precision Nutrient Management: A Case Study from Semi-Arid Tropics of India. NDT. 2025; 3(3):19. https://doi.org/10.3390/ndt3030019
Chicago/Turabian StyleTiwari, Gopal, Ram Prasad Sharma, Sudipta Chattaraj, Abhishek Jangir, Benukantha Dash, Lal Chand Malav, Brijesh Yadav, and Amrita Daripa. 2025. "Terrain-Integrated Soil Mapping Units (SMUs) for Precision Nutrient Management: A Case Study from Semi-Arid Tropics of India" NDT 3, no. 3: 19. https://doi.org/10.3390/ndt3030019
APA StyleTiwari, G., Sharma, R. P., Chattaraj, S., Jangir, A., Dash, B., Malav, L. C., Yadav, B., & Daripa, A. (2025). Terrain-Integrated Soil Mapping Units (SMUs) for Precision Nutrient Management: A Case Study from Semi-Arid Tropics of India. NDT, 3(3), 19. https://doi.org/10.3390/ndt3030019







