The Fast-Evolving Landscape of Treatments for Calcium Pyrophosphate Deposition Disease
Abstract
1. Introduction
2. Treating the Two Sides of the Same Coin in Crystal-Induced Rheumatic Diseases: Controlling the Inflammation While Getting Rid of the Deposits
3. The Data Behind the Use of Conventional Anti-Inflammatory Treatments
3.1. Treatment of Acute CPP Crystal Arthritis
3.2. Treatment of Chronic Inflammatory Phenotypes
4. Better Understanding the Pathophysiology of the Disease Leads to New Treatment Options
4.1. Pathological Formation of CPP Crystals
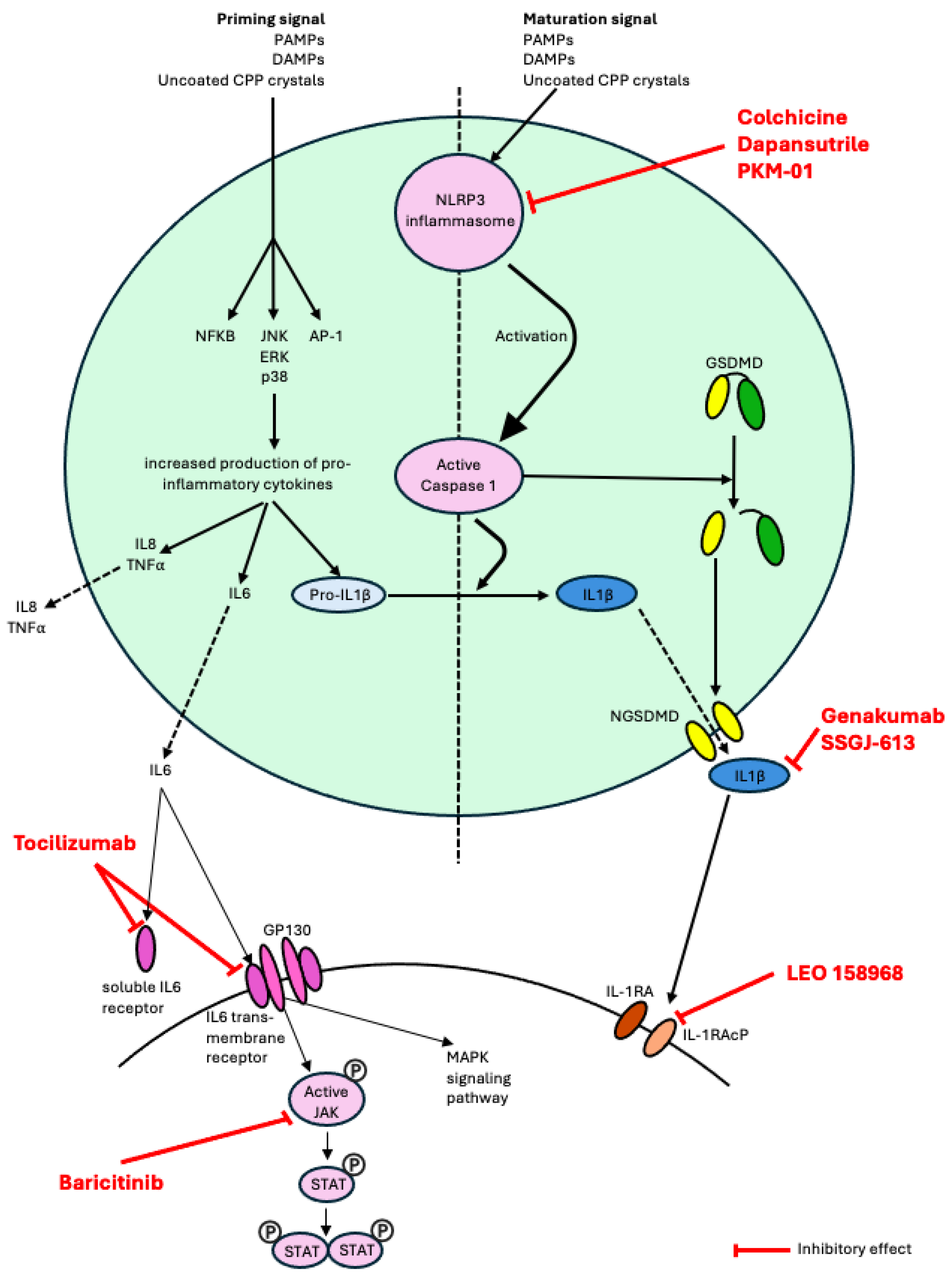
4.2. Excessive Immune Response to CPP Crystal Deposits
5. Biologics for CPPD Disease
5.1. IL-1 Inhibitors
5.2. IL-6 Inhibitors
6. Treatments in the Development Pipeline for CPPD Disease
7. What Benefits for CPPD Disease Can Be Expected from Current Drug Developments in Gout?
8. Standardizing Clinical Trial Design for New Medications for CPPD Disease
Author Contributions
Funding
Conflicts of Interest
References
- Pascart, T.; Filippou, G.; Liote, F.; Sirotti, S.; Jauffret, C.; Abhishek, A. Calcium pyrophosphate deposition disease. Lancet Rheumatol. 2024, 6, e791–e804. [Google Scholar] [CrossRef]
- Pascart, T.; Latourte, A.; Tedeschi, S.K.; Dalbeth, N.; Neogi, T.; Adinolfi, A.; Arad, U.; Andres, M.; Becce, F.; Bardin, T.; et al. Features Associated With Different Inflammatory Phenotypes of Calcium Pyrophosphate Deposition Disease: Study Using Data From the International American College of Rheumatology/EULAR Calcium Pyrophosphate Deposition Classification Criteria Cohort. Arthritis Rheumatol. 2024, 76, 1780–1788. [Google Scholar] [CrossRef]
- Abhishek, A.; Neogi, T.; Choi, H.; Doherty, M.; Rosenthal, A.K.; Terkeltaub, R. Review: Unmet Needs and the Path Forward in Joint Disease Associated With Calcium Pyrophosphate Crystal Deposition. Arthritis Rheumatol. 2018, 70, 1182–1191. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Bardin, T.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: Terminology and diagnosis. Ann. Rheum. Dis. 2011, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Doherty, M.; Pascual, E.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: Management. Ann. Rheum. Dis. 2011, 70, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Tedeschi, S.K.; Pascart, T.; Latourte, A.; Dalbeth, N.; Neogi, T.; Fuller, A.; Rosenthal, A.; Becce, F.; Bardin, T.; et al. The 2023 ACR/EULAR Classification Criteria for Calcium Pyrophosphate Deposition Disease. Arthritis Rheumatol. 2023, 75, 1703–1713. [Google Scholar] [CrossRef]
- Tedeschi, S.K. A New Era for Calcium Pyrophosphate Deposition Disease Research: The First-Ever Calcium Pyrophosphate Deposition Disease Classification Criteria and Considerations for Measuring Outcomes in Calcium Pyrophosphate Deposition Disease. Gout Urate Cryst. Depos. Dis. 2024, 2, 52–59. [Google Scholar] [CrossRef]
- Pascart, T.; Robinet, P.; Ottaviani, S.; Leroy, R.; Segaud, N.; Pacaud, A.; Grandjean, A.; Luraschi, H.; Rabin, T.; Deplanque, X.; et al. Evaluating the safety and short-term equivalence of colchicine versus prednisone in older patients with acute calcium pyrophosphate crystal arthritis (COLCHICORT): An open-label, multicentre, randomised trial. Lancet Rheumatol. 2023, 5, e523–e531. [Google Scholar] [CrossRef]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Takei, R.; Rosenthal, A.; Pascart, T.; Reynolds, R.J.; Neogi, T.; Terkeltaub, R.; Tedeschi, S.K.; Merriman, T.R. Genome-wide association study in chondrocalcinosis reveals ENPP1 as a candidate therapeutic target in calcium pyrophosphate deposition disease. Ann. Rheum. Dis. 2025, 84, 1023–1032. [Google Scholar] [CrossRef]
- Doherty, M.; Dieppe, P. Double blind, placebo controlled trial of magnesium carbonate in chronic pyrophosphate arthropathy. Ann. Rheum. Dis. 1983, 42, 106–107. [Google Scholar] [CrossRef]
- Spilberg, I.; McLain, D.; Simchowitz, L.; Berney, S. Colchicine and pseudogout. Arthritis Rheum. 1980, 23, 1062–1063. [Google Scholar] [CrossRef]
- Tabatabai, M.R.; Cummings, N.A. Intravenous colchicine in the treatment of acute pseudogout. Arthritis Rheum. 1980, 23, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Daoussis, D.; Antonopoulos, I.; Yiannopoulos, G.; Andonopoulos, A.P. ACTH as first line treatment for acute calcium pyrophosphate crystal arthritis in 14 hospitalized patients. Jt. Bone Spine 2014, 81, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Leelasattakul, W.; Pongsittisak, W.; Manavathongchai, S.; Satpanich, P. Efficacy and safety of 10 mg versus 30 mg of oral prednisolone for acute CPP crystal arthritis: Findings of a randomized controlled trial. Clin. Rheumatol. 2024, 43, 3879–3888. [Google Scholar] [CrossRef]
- Damart, J.; Filippou, G.; Andres, M.; Cipolletta, E.; Sirotti, S.; Carboni, D.; Filippucci, E.; Diez, P.; Abhishek, A.; Latourte, A.; et al. Retention, safety and efficacy of off-label conventional treatments and biologics for chronic calcium pyrophosphate crystal inflammatory arthritis. Rheumatology 2024, 63, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Alvarellos, A.; Spilberg, I. Colchicine prophylaxis in pseudogout. J. Rheumatol. 1986, 13, 804–805. [Google Scholar]
- Finckh, A.; Mc Carthy, G.M.; Madigan, A.; Van Linthoudt, D.; Weber, M.; Neto, D.; Rappoport, G.; Blumhardt, S.; Kyburz, D.; Guerne, P.A. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: No significant effect in a randomized crossover trial. Arthritis Res. Ther. 2014, 16, 458. [Google Scholar] [CrossRef]
- Andres, M.; Sivera, F.; Pascual, E. Therapy for CPPD: Options and Evidence. Curr. Rheumatol. Rep. 2018, 20, 31. [Google Scholar] [CrossRef]
- Rothschild, B.; Yakubov, L.E. Evidence for an effect of hydroxychloroquine in chronic pyrophosphate deposition disease. J. Clin. Rheumatol. 1996, 2, 170. [Google Scholar] [CrossRef]
- Szeri, F.; Niaziorimi, F.; Donnelly, S.; Fariha, N.; Tertyshnaia, M.; Patel, D.; Lundkvist, S.; van de Wetering, K. The Mineralization Regulator ANKH Mediates Cellular Efflux of ATP, Not Pyrophosphate. J. Bone Miner. Res. 2022, 37, 1024–1031. [Google Scholar] [CrossRef]
- Cheng, P.T.; Pritzker, K.P. The effect of calcium and magnesium ions on calcium pyrophosphate crystal formation in aqueous solutions. J. Rheumatol. 1981, 8, 772–782. [Google Scholar]
- Zhang, Y.; Brown, M.A. Genetic studies of chondrocalcinosis. Curr. Opin. Rheumatol. 2005, 17, 330–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Johnson, K.; Russell, R.G.; Wordsworth, B.P.; Carr, A.J.; Terkeltaub, R.A.; Brown, M.A. Association of sporadic chondrocalcinosis with a -4-basepair G-to-A transition in the 5′-untranslated region of ANKH that promotes enhanced expression of ANKH protein and excess generation of extracellular inorganic pyrophosphate. Arthritis Rheum. 2005, 52, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Szeri, F.; Lundkvist, S.; Donnelly, S.; Engelke, U.F.H.; Rhee, K.; Williams, C.J.; Sundberg, J.P.; Wevers, R.A.; Tomlinson, R.E.; Jansen, R.S.; et al. The membrane protein ANKH is crucial for bone mechanical performance by mediating cellular export of citrate and ATP. PLoS Genet. 2020, 16, e1008884. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Qazi, U.; Bernstein, M.; Charniak, A.; Gohr, C.; Mitton-Fitzgerald, E.; Ortiz, A.; Cardinal, L.; Kaell, A.T.; Rosenthal, A.K. Mutations in osteoprotegerin account for the CCAL1 locus in calcium pyrophosphate deposition disease. Osteoarthr. Cartil. 2018, 26, 797–806. [Google Scholar] [CrossRef]
- Abhishek, A.; Doherty, S.; Maciewicz, R.; Muir, K.; Zhang, W.; Doherty, M.; Valdes, A.M. The association between ANKH promoter polymorphism and chondrocalcinosis is independent of age and osteoarthritis: Results of a case-control study. Arthritis Res. Ther. 2014, 16, R25. [Google Scholar] [CrossRef]
- Bartels, C.M.; Singh, J.A.; Parperis, K.; Huber, K.; Rosenthal, A.K. Validation of Administrative Codes for Calcium Pyrophosphate Deposition: A Veterans Administration Study. J. Clin. Rheumatol. 2015, 21, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Pritzker, K.; Firestein, G.S.; Terkeltaub, R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J. Immunol. 2005, 174, 5016–5023. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Renaudin, F.; Orliaguet, L.; Castelli, F.; Fenaille, F.; Prignon, A.; Alzaid, F.; Combes, C.; Delvaux, A.; Adimy, Y.; Cohen-Solal, M.; et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann. Rheum. Dis. 2020, 79, 1506–1514. [Google Scholar] [CrossRef]
- Campillo-Gimenez, L.; Renaudin, F.; Jalabert, M.; Gras, P.; Gosset, M.; Rey, C.; Sarda, S.; Collet, C.; Cohen-Solal, M.; Combes, C.; et al. Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-kappaB Activation and MAPK Pathways. Front. Immunol. 2018, 9, 2248. [Google Scholar] [CrossRef]
- Voulgari, P.V.; Venetsanopoulou, A.I.; Drosos, A.A. Recent advances in the therapeutic management of calcium pyrophosphate deposition disease. Front. Med. 2024, 11, 1327715. [Google Scholar] [CrossRef]
- Dumusc, A.; Pazar Maldonado, B.; Benaim, C.; Zufferey, P.; Aubry-Rozier, B.; So, A. Anakinra compared to prednisone in the treatment of acute CPPD crystal arthritis: A randomized controlled double-blinded pilot study. Jt. Bone Spine 2021, 88, 105088. [Google Scholar] [CrossRef]
- Cipolletta, E.; Di Matteo, A.; Grassi, W.; Filippucci, E. Treatment of acute CPP crystal arthritis: What are we missing? Comment on: “Anakinra compared to prednisone in the treatment of acute CPPD crystal arthritis: A randomized controlled double-blinded pilot study” by Dumusc A. et al. Joint Bone Spine. 2020;88:105088. Jt. Bone Spine 2021, 88, 105217. [Google Scholar] [CrossRef]
- Antoniadou, C.; Fytanidis, N.; Devetzis, V.; Kantartzi, K.; Papagoras, C. Anakinra for Refractory Pseudogout in Patients with End-stage Renal Disease on Haemodialysis. Mediterr. J. Rheumatol. 2024, 35, 58–62. [Google Scholar] [CrossRef]
- Cipolletta, E.; Di Matteo, A.; Scanu, A.; Isidori, M.; Di Battista, J.; Punzi, L.; Grassi, W.; Filippucci, E. Biologics in the treatment of calcium pyrophosphate deposition disease: A systematic literature review. Clin. Exp. Rheumatol. 2020, 38, 1001–1007. [Google Scholar] [CrossRef]
- Verhoeven, F.; Prati, C.; Godfrin-Valnet, M.; Guillot, X.; Wendling, D. IL1 blockade in crystal-induced arthritis: Impact of disease duration and the inflammatory syndrome. Comments on the article by Couderc M. et al. “Efficacy of anakinra in articular chondrocalcinosis”. Jt. Bone Spine 2013, 80, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Pascart, T.; Richette, P. IL-6: A new target in crystal-induced arthritides—A narrative review. Jt. Bone Spine 2025, 92, 105935. [Google Scholar] [CrossRef] [PubMed]
- Quilis, N.; Andres, M.; Vela, P.; Pascual, E. Interleukin-6 pathway blockade as an option for managing refractory cases of crystal arthritis: Two cases report. Jt. Bone Spine 2018, 85, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Ea, H.K.; Frazier, A.; Blanchard, A.; Liote, F.; Marotte, H.; Bardin, T.; Richette, P. Tocilizumab in symptomatic calcium pyrophosphate deposition disease: A pilot study. Ann. Rheum. Dis. 2020, 79, 1126–1128. [Google Scholar] [CrossRef]
- Carrabin, S.; Houze, M.; Jauffret, C.; Bardin, T.; Ea, H.; Lioté, F.; Richette, P.; Pascart, T.; Latourte, A. Efficacy and Safety of Tocilizumab in the Treatment of Chronic Inflammatory Forms of CPPD: Retrospective Study of 55 Cases. Arthritis Rheumatol. 2024, 76, 5202–5203. [Google Scholar]
- Tian, Y.; He, X.; Li, R.; Wu, Y.; Ren, Q.; Hou, Y. Recent advances in the treatment of gout with NLRP3 inflammasome inhibitors. Bioorg. Med. Chem. 2024, 112, 117874. [Google Scholar] [CrossRef]
- Marchetti, C.; Swartzwelter, B.; Gamboni, F.; Neff, C.P.; Richter, K.; Azam, T.; Carta, S.; Tengesdal, I.; Nemkov, T.; D’Alessandro, A.; et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, E1530–E1539. [Google Scholar] [CrossRef]
- Kluck, V.; Jansen, T.; Janssen, M.; Comarniceanu, A.; Efde, M.; Tengesdal, I.W.; Schraa, K.; Cleophas, M.C.P.; Scribner, C.L.; Skouras, D.B.; et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: An open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020, 2, e270–e280. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; Braverman, G.; Yue, L.; Fields, T. Pipeline Therapies for Gout. Curr. Rheumatol. Rep. 2024, 26, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.E.; Wu, A.; Zhou, H.; Pham, M.A.; Lin, S.; McNulty, R. Targeting the NLRP3 inflammasome for inflammatory disease therapy. Trends Pharmacol. Sci. 2025, 46, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Stack, J.; McCarthy, G. Calcium pyrophosphate deposition (CPPD) disease-Treatment options. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101720. [Google Scholar] [CrossRef]
- Hojen, J.F.; Kristensen, M.L.V.; McKee, A.S.; Wade, M.T.; Azam, T.; Lunding, L.P.; de Graaf, D.M.; Swartzwelter, B.J.; Wegmann, M.; Tolstrup, M.; et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat. Immunol. 2019, 20, 1138–1149. [Google Scholar] [CrossRef]
- Gronberg, C.; Rattik, S.; Tran-Manh, C.; Zhou, X.; Rius Rigau, A.; Li, Y.N.; Gyorfi, A.H.; Dickel, N.; Kunz, M.; Kreuter, A.; et al. Combined inhibition of IL-1, IL-33 and IL-36 signalling by targeting IL1RAP ameliorates skin and lung fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis. 2024, 83, 1156–1168. [Google Scholar] [CrossRef]
- Lema, D.A.; Jakobsson, G.; Daoud, A.; Elias, D.; Talor, M.V.; Rattik, S.; Gronberg, C.; Kalinoski, H.; Jaensson Gyllenback, E.; Wang, N.; et al. IL1RAP Blockade with a Monoclonal Antibody Reduces Cardiac Inflammation and Preserves Heart Function in Viral and Autoimmune Myocarditis. Circ. Heart Fail. 2024, 17, e011729. [Google Scholar] [CrossRef] [PubMed]
- Grassot, J.; Marzouki, F.; Hervé, R.; Beckler, M.; Semerano, L.; Bessis, N.; Rivière, E.; Sanson, C.; Pouliquen, G.; Pouletty, P.; et al. Intra-articular Treatment Combining Sustained Release Colchicine Encapsulated in Microspheres, and Ropivacaine, Is Effective in Inflammatory Arthritis in Rats. Arthritis Rheumatol. 2024, 76, 547–548. [Google Scholar]
- Tedeschi, S.K.; Becce, F.; Pascart, T.; Guermazi, A.; Budzik, J.F.; Dalbeth, N.; Filippou, G.; Iagnocco, A.; Kohler, M.J.; Laredo, J.D.; et al. Imaging Features of Calcium Pyrophosphate Deposition Disease: Consensus Definitions from an International Multidisciplinary Working Group. Arthritis Care Res. 2023, 75, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Sirotti, S.; Becce, F.; Sconfienza, L.M.; Terslev, L.; Naredo, E.; Zufferey, P.; Pineda, C.; Gutierrez, M.; Adinolfi, A.; Serban, T.; et al. Reliability and Diagnostic Accuracy of Radiography for the Diagnosis of Calcium Pyrophosphate Deposition: Performance of the Novel Definitions Developed by an International Multidisciplinary Working Group. Arthritis Rheumatol. 2023, 75, 630–638. [Google Scholar] [CrossRef]
- Filippou, G.; Sirotti, S.; Cipolletta, E.; Filippucci, E. Optimizing the Use of Ultrasound in Calcium Pyrophosphate Deposition (CPPD): A Review from the Ground Up. Gout Urate Cryst. Depos. Dis. 2024, 2, 17–33. [Google Scholar] [CrossRef]
- Laurent, V.; Filippou, G.; Sirotti, S.; Pascart, T. Advanced imaging techniques in crystal arthritis. Ther. Adv. Musculoskelet. Dis. 2025, 17, 1759720X251316097. [Google Scholar] [CrossRef]
- Cipolletta, E.; Rozza, D.; Andres, M.; Ottaviani, S.; Pascart, T.; Calvo-Aranda, E.; Chiarvetto Peralta, M.V.; Muto, P.; Calabuig, I.; Gómez-Sabater, S.; et al. Development and internal-external cross-validation of a patient-reported definition for acute calcium pyrophosphate crystal arthritis. Rheumatology 2025, 64, 2609–2617. [Google Scholar] [CrossRef]
- Cai, K.; Fuller, A.; Hensey, O.; Grossberg, D.; Christensen, R.; Shea, B.; Singh, J.A.; Abhishek, A.; Tedeschi, S.; Dalbeth, N. Outcome domains reported in calcium pyrophosphate deposition studies: A scoping review by the OMERACT CPPD working group. Semin. Arthritis Rheum. 2020, 50, 719–727. [Google Scholar] [CrossRef]
- Fuller, A.; Cai, K.; Diaz-Torne, C.; Filippou, G.; Pascart, T.; Hensey, O.; Grossberg, D.; Christensen, R.; Shea, B.; Singh, J.A.; et al. Outcome domains reported by patients, caregivers, healthcare professionals and stakeholders for calcium pyrophosphate deposition (CPPD): A content analysis based on semi-structured qualitative interviews from the OMERACT CPPD working group. Semin. Arthritis Rheum. 2021, 51, 650–654. [Google Scholar] [CrossRef]
- Cai, K.; Fuller, A.; Zhang, Y.; Hensey, O.; Grossberg, D.; Christensen, R.; Shea, B.; Singh, J.A.; McCarthy, G.M.; Rosenthal, A.K.; et al. Towards development of core domain sets for short term and long term studies of calcium pyrophosphate crystal deposition (CPPD) disease: A framework paper by the OMERACT CPPD working group. Semin. Arthritis Rheum. 2021, 51, 946–950. [Google Scholar] [CrossRef]
- Zhang, Y.; Tedeschi, S.K.; Abhishek, A.; Hensey, O.; Grossberg, D.; Cai, K.; Shea, B.; Singh, J.A.; Christensen, R.; Serban, T.; et al. Core domain set for chronic and/or recurrent manifestations of calcium pyrophosphate deposition disease: OMERACT delphi survey to establish consensus. Semin. Arthritis Rheum. 2025, 72, 152669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tedeschi, S.K.; Abhishek, A.; Hensey, O.; Grossberg, D.; Cai, K.; Shea, B.; Singh, J.A.; Christensen, R.; Serban, T.; et al. Core domain set for studies of acute calcium pyrophosphate crystal arthritis: OMERACT delphi survey to establish consensus. Semin. Arthritis Rheum. 2025, 72, 152670. [Google Scholar] [CrossRef] [PubMed]
| Medication | Mechanism of Action | Route & Dose | Clinical Trials |
|---|---|---|---|
| Colchicine | Prevents microtubule assembly thereby disrupting inflammasome activation, microtubule-based inflammatory cell chemotaxis, generation of leukotrienes and cytokines, and phagocytosis | Oral, 0.6 mg daily | Phase 2 (NCT06855433) |
| Baricitinib | JAK 1/JAK 2 inhibitor | Oral, 4 mg daily | Phase 2 (NCT06768294) |
| Tocilizumab | IL-6R inhibitor | Intravenous, 8 mg/kg monthly | Phase 2 (pending) |
| Medication | Mechanism of Action/Structure | Route | Clinical Trials |
|---|---|---|---|
| Dapansutrile | NLRP3 inflammasome inhibitor | Oral | Phase 2/3 (NCT05658575) |
| LEO 158968 | IL-1RAcP inhibitor | Subcutaneous | Phase 1b (NCT06444867) |
| Genakumab | Anti-IL-1β monoclonal antibody | Subcutaneous | Phase 2/3 (NCT05936268, NCT05983445) |
| SSGJ-613 | Recombinant anti-IL-1β monoclonal antibody | Subcutaneous | Phase 3 (NCT06169891) |
| PKM-01 | Microsphere containing ropivacaine and colchicine | Intra-articular | Phase 2 (pending) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, V.; Jauffret, C.; Dalbeth, N.; Pascart, T. The Fast-Evolving Landscape of Treatments for Calcium Pyrophosphate Deposition Disease. Gout Urate Cryst. Depos. Dis. 2025, 3, 22. https://doi.org/10.3390/gucdd3040022
Tai V, Jauffret C, Dalbeth N, Pascart T. The Fast-Evolving Landscape of Treatments for Calcium Pyrophosphate Deposition Disease. Gout, Urate, and Crystal Deposition Disease. 2025; 3(4):22. https://doi.org/10.3390/gucdd3040022
Chicago/Turabian StyleTai, Vicky, Charlotte Jauffret, Nicola Dalbeth, and Tristan Pascart. 2025. "The Fast-Evolving Landscape of Treatments for Calcium Pyrophosphate Deposition Disease" Gout, Urate, and Crystal Deposition Disease 3, no. 4: 22. https://doi.org/10.3390/gucdd3040022
APA StyleTai, V., Jauffret, C., Dalbeth, N., & Pascart, T. (2025). The Fast-Evolving Landscape of Treatments for Calcium Pyrophosphate Deposition Disease. Gout, Urate, and Crystal Deposition Disease, 3(4), 22. https://doi.org/10.3390/gucdd3040022







