Abstract
Calcium pyrophosphate deposition (CPPD) disease is a consequence of the immune response to the pathological accumulation of calcium pyrophosphate (CPP) crystals within joints. This clinically heterogeneous condition can cause significant disability, yet its management remains poorly defined. New discoveries are reshaping the therapeutic landscape beyond conventional anti-inflammatory agents—which remain the cornerstone of care—justifying this review on current standard of care and treatment advances in CPPD disease. We first address the two theoretical management goals, namely inflammation control and crystal dissolution—with attempts to address the latter having failed thus far. We then summarize the evidence supporting conventional anti-inflammatory treatments and review insights into the pathophysiology of CPPD disease, which are driving the development of novel therapeutic strategies. These include the current use of biologics (IL-1 and IL-6 inhibitors) to control inflammation and highlight the need to explore new pathways to inhibit crystal formation (e.g., selective NPP1 blockers). We present the treatments in the development pipeline for CPPD disease (including JAK inhibitors), and the therapies currently undergoing clinical trials in gout for which findings could be extended to CPPD disease given their shared pathophysiology (e.g., NLRP3 inhibitors). To support and improve research on CPPD disease treatments, clinical trial design needs to be standardized, incorporating the recent ACR/EULAR classification criteria for accurate diagnosis, careful phenotypic stratification to ensure homogeneous patient groups (although this point requires consensus), and validated core outcome domains currently being developed by the OMERACT.
1. Introduction
Calcium pyrophosphate deposition (CPPD) disease encompasses the symptoms caused by the pathological presence of CPP crystals in joints [1]. The disease can present with a variety of clinical phenotypes, ranging from the typical acute episode of arthritis which can become recurrent to chronic arthritis with and without inflammatory symptoms [2]. This very common cause of arthritis in older people is still underdiagnosed, particularly in its chronic clinical presentations, and has long been an under-researched field, with very little guidance in its management provided by international societies [3,4,5]. Consequently, management has long relied on less than a handful of anti-inflammatory drugs, based on the experience accumulated in gout, another crystal-induced inflammatory arthritis.
In recent years, global interest has reached CPPD disease, and international initiatives started structuring research focusing on CPPD disease [6,7]. Progress in the management of the disease is emerging, thanks to the recent completion of the first clinical trial performed in acute CPP crystal arthritis [8], and as new academic and industrial trials are being launched.
The aim of this review is to recapitulate what should be the current standard of care in CPPD disease, and overview current developments which will change the treatment of CPPD disease in the near future.
2. Treating the Two Sides of the Same Coin in Crystal-Induced Rheumatic Diseases: Controlling the Inflammation While Getting Rid of the Deposits
The treatment paradigm is the same in all crystal-induced arthropathies; the inflammation (and symptoms in general) needs to be treated until the crystal deposits causing these symptoms are gone. The archetype of crystal-induced arthropathies in which means are available to achieve both is gout [9]. In brief, urate lowering therapy (e.g., allopurinol) reduce serum urate levels below their supersaturation concentrations, which leads to the eventual complete dissolution of monosodium urate crystals. Drugs targeting inflammation (e.g., colchicine) aim to control inflammation caused by the presence of crystals until they are dissolved. In CPPD, the mechanisms of crystal formation are completely different than in gout, as they are mainly caused by an excessive presence of inorganic pyrophosphate (PPi) in the extra-cellular space due to increased release of ATP from certain cell types (through the ANKH anion channel) and/or an imbalance in the cleavage of ATP (performed by ENPP1) [1,10]. Attempts have been made to interfere with these mechanisms using an anion channel inhibitor (probenecid) or to stimulate PPi further cleavage to inorganic phosphate, but both failed to dissolve CPP deposits [1,11]. Therapeutic efforts in CPPD disease therefore all focus on the same side of the coin for now: controlling the immune inflammatory response to CPP crystals and potential effects on cartilage degradation.
3. The Data Behind the Use of Conventional Anti-Inflammatory Treatments
Conventional anti-inflammatory treatments (i.e., non-biologics or synthetic targeted therapies) used in the treatment of CPPD disease include colchicine, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids (systemic or intra-articular), and two disease modifying anti-rheumatic drugs (DMARDs): methotrexate and hydroxychloroquine. While the first three of the list can in theory be used for acute CPP crystal arthritis and for the treatment of the chronic phenotypes, the DMARDs are only used in the latter.
3.1. Treatment of Acute CPP Crystal Arthritis
When two or fewer joints are affected, joint aspiration combined with intra-articular corticosteroid injection is an option, provided that septic arthritis has been excluded. When more than two joints are affected, however, systemic anti-inflammatory therapies are preferred. Colchicine is often used first-line, usually to smaller doses than in gout because of the older age of patients with CPPD disease, but oral NSAIDs and oral corticosteroids could also be used according to the 2011 EULAR recommendations [5]. Yet, until recently, the only available evidence for the use of colchicine in acute CPP crystal arthritis were historical studies using intravenous colchicine [12,13]. Use of parenteral ACTH was even reported as being successful [14]. The COLCHICORT trial, a randomized, open-label, controlled trial comparing oral colchicine to prednisone in acute CPP crystal arthritis has provided so far the strongest evidence to guide the treatment of acute flares [8]. This RCT randomized 112 patients to receive 1.5 mg of colchicine on day one and 1 mg on day two or 30 mg of prednisone on days one and two. Treatment response was equivalent at 24 h after treatment initiation, with a change in pain visual analogue scale (VAS) of −36 mm (±32) (/100 mm) in the colchicine group and −38 mm (±23) in the prednisone group. Safety considerations discriminated the two drugs, as 22% of patients treated with colchicine experienced diarrhea, all graded as mild, but which is more concerning in this older population (mean age of 87 years in the trial), while prednisone was generally well tolerated despite the fact that several patients had pre-existing diabetes [8]. A two-day regimen of any of the two drugs was sufficient to obtain a good treatment response on day three, but for safety considerations, the COLCHICORT trial has started a shift towards using oral prednisone as first-line therapy for acute CPP crystal arthritis. Recently, a group from Thailand conducted a randomized open-label trial comparing a 7-day course of two different doses of prednisone (10 mg vs. 30 mg daily) for the treatment of acute CPP crystal arthritis (N = 79, all cases aspirate-proven) [15]. The trial found that both doses were equally effective, with no significant differences in flare resolution time, recurrence rates over a 28-day follow-up period, or safety outcomes. This suggests that lower doses of prednisone could be considered to manage acute CPP crystal arthritis, particularly in patients who are at risk of experiencing adverse effects from higher corticosteroid doses.
3.2. Treatment of Chronic Inflammatory Phenotypes
Chronic inflammatory phenotypes include recurrent flares and/or persistent arthritis. Retrospective data from a European cohort of 128 patients showed that the prolonged use of low-dose colchicine (1 mg or 0.5 mg/day) was considered the first-line therapeutic option in referral centers for CPPD disease, and provided significant clinical improvement in 30–50% of cases [16]. This is supported by an old crossover trial of 10 patients, which suggested that daily colchicine could reduce the recurrence of flares three-fold [17]. Methotrexate (15 mg/week for three months) has been tested in recurrent acute and persistent polyarthritis in a double-blind crossover trial [18]. This trial did not show efficacy for methotrexate, but used the DAS44 as the primary endpoint, which may not be appropriate to evaluate a difference in CPPD disease. The question of the efficacy of methotrexate remains unproven as case series suggest its potential benefits [16,19]. In the European cohort, methotrexate was most often used as a second-line agent after colchicine failure and often co-prescribed with colchicine or glucocorticoids (14). A pilot double-blind crossover trial tested hydroxychloroquine in the recurrent flare phenotype and suggested a reduction in the frequency of flares, but no adequately powered confirmatory trial was ever performed [20]. Data from the European cohort, however, showed that hydroxychloroquine was rarely used in expert centers, accounting for <5% of cases [16].
4. Better Understanding the Pathophysiology of the Disease Leads to New Treatment Options
The pathophysiology of CPPD disease remains incompletely understood and is an area that is still under active research. The two key pathophysiological processes that contribute to the development of clinical disease and constitute the targets of current treatments, include the pathological formation and deposition of CPP crystals, followed by an excessive immune response to the CPP crystal deposits [1]. Historically, treatments used in CPPD disease were inspired from the clinical and basic science knowledge accumulated in gout, but bench research focused on CPPD is eventually increasing options at the bedside.
4.1. Pathological Formation of CPP Crystals
The complexation of calcium ions and inorganic pyrophosphate (PPi), after the cleavage of ATP by the ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) to adenosine monophosphate (AMP) and PPi, results in CPP crystal formation, preferentially on the cartilage matrix [1,21]. This process is enhanced when PPi concentrations are increased in the joint space. In vitro, magnesium supplementation has a growth inhibitory effect on CPP crystals [22]. The only randomized study on magnesium supplementation in CPPD disease was conducted in 1983 in 38 patients with chronic CPPD (with or without hypomagnesemia), and was a pilot study comparing oral magnesium (30 mmol/day) to placebo over a 6 month follow-up period [11]. In this study, there was a uniform trend towards improvement in those taking magnesium (on pain and objective assessment at 6 months), but also a pronounced placebo effect. Radiological appearance of chondrocalcinosis did not change over the six months follow-up. It is probable that radiographic change in CPPD could not be observed on such a short time period, and that the mechanism of action of magnesium, favoring the inorganic phosphate (Pi) in the PPi/Pi balance, would slow down CPPD but not promote CPP crystal dissolution. Thus, the in vivo efficacy of magnesium supplementation on preventing further CPP crystal deposition needs to be further explored, and its potential improvement on inflammatory symptoms needs to be confirmed.
The first discovered genetic mutations were identified in familial cases of CPPD disease [23,24]. Gain-of-function mutations in the ANKH gene, which encodes a transmembrane transporter of ATP, result in increased extracellular accumulation of PPi [21,25]. Conversely, TNFRSF11B, the gene encoding osteoprotegerin—a receptor from the tumor necrosis factor family—has been linked to CPPD through loss-of-function mutations, without any associated abnormalities in pyrophosphate metabolism, suggesting an indirect relationship between this mutation and the development of CPPD disease [26]. An association between a functional polymorphism in the 5′ UTR of ANKH and CPPD was also found a few years ago [27]. To date, these findings have not translated to therapeutic solutions.
A recent genome-wide association study (GWAS) of an administrative database was conducted to identify new candidate causal genes in 536 Africans and 2468 Europeans [10]. In this study, radiographic chondrocalcinosis (identified by Phecode 274.21) was used as a surrogate marker for CPPD disease, found to be reliable in a previous study in the Veterans Affairs database [28]. Two genome-wide significant loci, both on chromosome 6, and within the ENPP1 (associated with increased ENPP1 expression) and RNF144B genes, were found in both Africans and Europeans. ENPP1 encodes ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (NPP1), and NPP1 is a transmembrane glycoprotein that hydrolyses nucleoside triphosphates, primarily adenosine triphosphate (ATP), to produce AMP and PPi. Therefore, the authors suggest that the selective NPP1 inhibitors developed for infectious disease and cancer could be tested as treatment for CPPD disease.
4.2. Excessive Immune Response to CPP Crystal Deposits
Several studies (predominantly in vitro) have demonstrated that CPP crystals induce an acute inflammatory response similar to that observed in gout flares. Like MSU crystals, CPP crystals can directly trigger inflammation via crystal-cell membrane contact or via toll-like receptors (TLRs), mostly TLR2 and TLR4 [29]. This induces a strong innate immune response by macrophages, through the activation of the NLRP3 inflammasome via mitogen-activated protein kinases and NFκB, which results in the production and secretion of IL-1β, a potent pro-inflammatory cytokine [30,31,32]. IL-1β in turn activates various cells (fibroblasts, endothelial cells, synoviocytes) to produce other pro-inflammatory cytokines (including IL-8, IL-6, TNF-α) that recruit neutrophils from the blood into the joint cavity, further amplifying the inflammatory response. CPP crystals also inhibit neutrophil apoptosis and stimulate neutrophils to release myeloperoxidases, IL-1β, IL-8, IL-6, and neutrophil extracellular traps (NETs) resulting in damage to bystander cells and tissues [33]. Moreover, CPP crystals exert direct catabolic effects on chondrocytes and synoviocytes, leading to the production of destructive matrix metalloproteinases and prostaglandins [33]. The net effect is acute inflammation of the joint and periarticular tissues resulting in the clinical manifestation of synovitis. Treatments targeting various components of the inflammatory cascade initiated by CPP crystals have emerged in recent years and will be discussed in the following sections.
5. Biologics for CPPD Disease
Acute CPP crystal arthritis is rarely difficult to treat, as mentioned above with the use of colchicine, NSAIDs, and corticosteroids (systemic or intra-articular), however biologics can be considered when these first-line treatments have failed due to side effects, lack of efficacy or contra-indications [34]. Biologics may also be considered in chronic forms of CPPD, in case of failure of one or more of the above-mentioned drugs, namely long-term colchicine, methotrexate and hydroxychloroquine [1,35]. In some cases, these biologics are combined with long-term colchicine and methotrexate [16]. Despite increasing evidence, for now all the biologics prescribed for both acute and chronic forms are off-label [16], and in the most up-to-date management recommendations of CPPD—the 2011 European League Against Rheumatism (EULAR) recommendations—the use of biologics was not considered [5]. Despite promising efficacy data, careful assessment of safety will be essential in future studies of biologics for CPPD [16].
5.1. IL-1 Inhibitors
The use of IL-1 inhibitors stems from our understanding of the pathophysiological mechanisms of crystal-induced inflammation, from retrospective case series, and from the promising results of a randomized yet interrupted Swiss trial [30,34,36]. The Swiss trial was a double-blind, randomized placebo-controlled trial, which evaluated the efficacy and safety of a three-day course of anakinra 100 mg/d versus prednisone 30 mg/d to treat acute CPP crystal arthritis, and the primary outcome was the change from baseline of patients’ pain evaluation of the affected joint at 72 h post-treatment [34]. It was unfortunately halted after treating 15 patients due to recruitment issues, but showed similar decrease in VAS pain at 72 h in both groups [34]. Anakinra 100 mg/day for 3 days, or every other day for 5 days, is usually the first-line biologic for the treatment of acute CPP crystal arthritis in the case of contraindication or non-response to conventional treatments [1,16]. In chronic phenotypes of CPPD disease, the efficacy of IL-1 inhibitors is limited except in the management of acute recurrent forms associated with elevated acute phase—reactant levels [16,37,38]. Three patients were treated with canakinumab in the European cohort of chronic CPPD disease, with similar results to anakinra [16].
5.2. IL-6 Inhibitors
IL-6 inhibitors have only been described in the chronic form of the disease, predominantly with tocilizumab and more rarely with sarilumab [16,39]. Promising results were initially reported in small case series of 2 and 11 patients, respectively, with severe refractory forms (including failure of anakinra) [40,41]. A recent retrospective French cohort of 55 patients (70% persistent forms and 25% recurrent forms) who received tocilizumab (mainly 8 mg/kg/month intravenously, otherwise subcutaneous 162 mg/week) has been described [42]. A significant improvement in pain was observed at 3 and 6 months, prompting the submission of a protocol for a prospective study on tocilizumab in chronic CPPD disease. The intravenous form of tocilizumab seems to provide better results than the subcutaneous form [41]. Two patients were treated with sarilumab in the European cohort after tocilizumab failure, both experiencing initial treatment response, and were secondarily lost to follow-up [16].
6. Treatments in the Development Pipeline for CPPD Disease
Therapies currently undergoing clinical trials in CPPD disease are shown in Table 1 and Figure 1. Colchicine for the treatment of chronic phenotypes of CPPD disease needs further validation and a trial of daily colchicine versus placebo to reduce interleukin-18 levels is about to begin in the United States (CRYSTALLIZE trial, NCT06855433). An open-label trial of baricitinib 4 mg qd vs. standard-of-care (colchicine, methylprednisolone, methotrexate or hydroxychloroquine) with a primary outcome measure using markers of inflammation on the synovial biopsy is currently under way in Italy (BAPTIST trial, NCT06768294). The TociCCAre study is a French academic randomized, double-blind placebo-controlled 16 weeks trial of 8 mg/kg monthly infusions of tocilizumab, expected to be launched at the end of 2025.

Table 1.
Therapies currently undergoing clinical trials in CPPD disease.
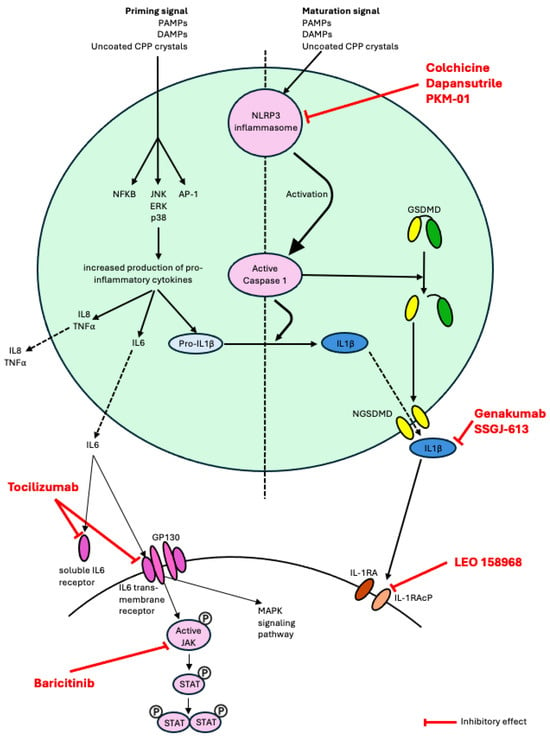
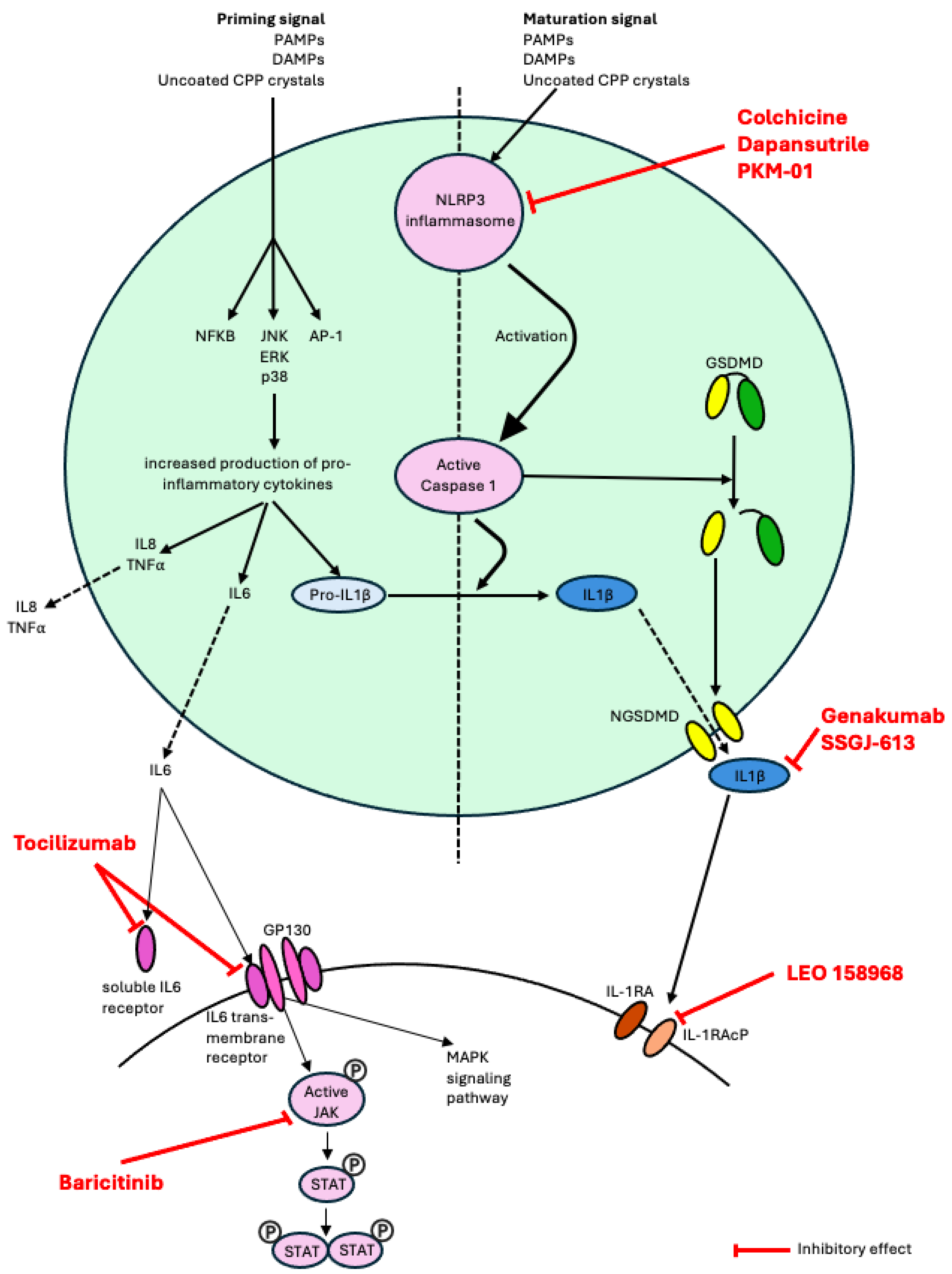
Figure 1.
Schematic representation of the role in pathophysiology of the therapies currently undergoing clinical trials in CPPD disease and in gout (pipeline drugs for CPPD disease). Abbreviations: NLRP3, nucleotide-binding domain, leucine-rich-containing family, pryrin domain-containing 3; MAPK, mitogen-activated protein kinase; PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; TNF, tumor necrosis factor; GSDMD, Gasdermine D; NGSDMD, N-terminal Gasdermine D; IL-1β, interleukin-1β; GP130, Glycoprotein 130; JAK, janus kinase; STAT, signal transducer and activator of transcription; IL-1RA, interleukin-1 receptor; IL-1RAcP, interleukin-1 receptor accessory protein.
7. What Benefits for CPPD Disease Can Be Expected from Current Drug Developments in Gout?
Anti-inflammatory therapies currently in development for gout that might be extended to CPPD disease, given the shared pathophysiology of crystal-induced inflammatory arthritis, are shown in Table 2 and Figure 1.

Table 2.
Pipeline drugs for CPPD disease—therapies currently undergoing clinical trials in gout. Findings could be extended to CPPD disease given the shared pathophysiology of crystal-induced inflammatory arthritis.
In recent years, there has been significant interest in developing small molecule inhibitors that directly target the NLRP3 inflammasome as a novel therapeutic approach for the management of gout. One such example is dapansutrile (OLT1177), a β-sulfonyl nitrile compound that selectively inhibits the NLRP3 inflammasome by directly binding the protein and inhibiting its ATPase activity [43]. A phase 1 study showed that dapansutrile is safe in humans at effective plasma levels greater than 100 times that needed to inhibit the activation of the NLRP3 inflammasome in vitro [44]. Subsequently, an open-label phase 2a clinical trial of dapansutrile showed promising results with oral administration providing substantial analgesic effects and a satisfactory safety profile among patients experiencing a monoarticular gout flare [45]. Currently, a multicentre phase 2/3 randomized, double-blind, placebo-controlled safety and efficacy study of dapansutrile is underway (NCT05658575). In this trial, patients with gout flare onset within 96 h are being randomized to receive dapansutrile tablets vs. placebo for 7 days without concurrent use of anti-inflammatory medications (NSAIDs, colchicine, steroids) with the primary outcome defined as change in pain score (measured on a 100 mm VAS) at the target joint 72 h post treatment initiation. Several other agents with NLRP3 inhibitor properties are also under study in ex vivo and mouse models of gout [43,46,47]. Given that CPP crystals also cause inflammation by activating the NLRP3 inflammasome [30], small molecule NLPR3 inhibitors could be effective in the management of CPPD disease and are worthy of further investigation [48].
There has also been growing interest in the development of antibodies that target the IL-1 receptor accessory protein (IL-1RAcP), the shared subunit for the IL-1/IL-33/IL-36 isoform receptors, in the management of various autoimmune/inflammatory conditions. Monoclonal antibodies blocking IL-1RAcP have shown promise in murine models of inflammatory diseases including monosodium urate peritonitis, allergic airway inflammation, psoriasis, systemic sclerosis and myocarditis [49,50,51]. Currently, a humanized anti-IL1RAcP antibody (CAN10) is undergoing testing in a phase 1 clinical trial in healthy volunteers and subjects with plaque psoriasis (NCT06143371). Given the critical role of IL-1 signaling in the pathophysiology of gout and CPPD disease, IL-1RAcP inhibitors may eventually emerge as another therapeutic agent for crystal arthritis, and a phase 1b trial of LEO 158968 in gout flares is undergoing (NCT06444867). Novel anti-IL-1β monoclonal antibodies, genakumab and SSGJ-613, are also undergoing Phase 2/3 trials in gout and their findings could potentially be extended to CPPD disease.
Finally, new intra-articular therapies for crystal arthritis are also under investigation. Recently, a French group has developed an injectable agent (PKM-01), in which ropivacaine (a local anesthetic) is combined with colchicine within a microsphere composed of biodegradable polylactic-co-glycolic acid (PLGA) polymer. This specific formulation allows for the sustained release of colchicine over 7–10 days, i.e., the duration of a typical flare. This treatment has been tested in a murine model of acute inflammatory arthritis and demonstrated a rapid positive effect on pain, inflammation and joint destruction, superior to that seen with intra-articular colchicine or intra-articular dexamethasone. Moreover, colchicine levels in the blood following PKM-01 injection showed low levels below the threshold of toxicity, reinforcing the safety of this therapy and potential use in patients with comorbidities and contraindications to oral therapies [52]. A phase 2 clinical trial in patients experiencing gout flares has been cleared by the FDA. If positive findings are seen in the gout population, then the therapy could also be extended to CPPD disease.
8. Standardizing Clinical Trial Design for New Medications for CPPD Disease
Well conducted clinical trials will be essential to determine the safety and efficacy of medications for CPPD disease. Previous studies in CPPD disease have been limited by small sample size, a lack of clear definition for CPPD disease, and a lack of standardized outcome measures. Fortunately, there has been major progress in the research tools needed for high quality clinical trial design in CPPD disease in the last five years.
The ACR/EULAR CPPD disease classification criteria was published in 2023 and will allow investigators to enroll participants into CPPD trials with a high degree of certainty regarding the disease classification [6,7]. As part of the classification criteria project, imaging definitions for CPPD have also been developed and subsequently validated [53,54]. Developing the use of advanced techniques (US and CT/DECT) with higher sensitivity, and to some extent higher specificity, for CPPD will help identify patients classified as having CPPD disease [55,56].
For trials of CPPD disease, a reliable definition of acute CPP crystal arthritis has been needed. In a multi-national study, patient-reported definitions of acute CPP crystal arthritis have been developed [57]. These definitions include patient self-reported acute CPP crystal arthritis, patient self-reported joint swelling and patient self-reported joint warmth. The definitions perform well compared with independent rheumatologist opinion as the reference standard.
To date, there has been wide variation in the outcomes reported in clinical studies of CPPD [58]. While people with CPPD consider joint pain and ability to complete daily tasks as the most important outcomes [59], most CPPD clinical studies have focused on imaging evidence of joint damage and joint calcification [58]. The Outcomes Measures in Rheumatology (OMERACT) CPPD working group has developed core outcome domains for clinical trials using established OMERACT methodology [60,61,62]. Initial work highlighted the importance of developing separate core domain sets for clinical trials of an individual flare of acute CPP crystal arthritis and for clinical trials of the chronic and/or recurrent manifestations of CPPD disease [60]. In consensus exercises with patient research partners and health professionals/researchers, joint pain, swelling and tenderness, duration of acute CPP crystal arthritis flare, physical function, and patient global assessment of disease activity were strongly supported as core outcome domains for an individual flare of acute CPP crystal arthritis [62]. For chronic and/or recurrent manifestations of CPPD disease, joint pain, joint tenderness, joint swelling, acute CPP crystal arthritis flare, physical function, and global assessment of disease activity were strongly supported [61]. These findings have informed the development of core domain sets which will be presented to the wider OMERACT community for voting and endorsement. Subsequent work will be needed to identify valid instruments for each of the core domains. Ultimately, this work will ensure that clinical trials of new medications and treatment strategies report outcomes of importance to people with CPPD disease and improve comparability of findings between studies.
Author Contributions
Conceptualization, T.P.; Writing—Original Draft Preparation, C.J., V.T., N.D. and T.P.; Writing—Review & Editing, C.J., V.T., N.D. and T.P.; Supervision, N.D. and T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This review article received no external funding. V.T. is funded by the Health Research Council of New Zealand for her research in CPPD disease.
Conflicts of Interest
N.D. has received consulting fees, speaker fees or grants from Novartis, Horizon, Selecta, Arthrosi, LG Chem, JPI, PTC Therapeutics, Protalix, Unlocked Labs, Hikma, Dexcel Pharma, Shanton Pharma, Sobi, Avalo, Biomarin, Crystalys, SK Chemicals, Medcryst, Convergence Bio outside the submitted work. T.P. has received advisory fees from Avalo Therapeutics and Novartis. C.J. and V.T. have no conflicts of interest to declare.
References
- Pascart, T.; Filippou, G.; Liote, F.; Sirotti, S.; Jauffret, C.; Abhishek, A. Calcium pyrophosphate deposition disease. Lancet Rheumatol. 2024, 6, e791–e804. [Google Scholar] [CrossRef]
- Pascart, T.; Latourte, A.; Tedeschi, S.K.; Dalbeth, N.; Neogi, T.; Adinolfi, A.; Arad, U.; Andres, M.; Becce, F.; Bardin, T.; et al. Features Associated With Different Inflammatory Phenotypes of Calcium Pyrophosphate Deposition Disease: Study Using Data From the International American College of Rheumatology/EULAR Calcium Pyrophosphate Deposition Classification Criteria Cohort. Arthritis Rheumatol. 2024, 76, 1780–1788. [Google Scholar] [CrossRef]
- Abhishek, A.; Neogi, T.; Choi, H.; Doherty, M.; Rosenthal, A.K.; Terkeltaub, R. Review: Unmet Needs and the Path Forward in Joint Disease Associated With Calcium Pyrophosphate Crystal Deposition. Arthritis Rheumatol. 2018, 70, 1182–1191. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Bardin, T.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: Terminology and diagnosis. Ann. Rheum. Dis. 2011, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Doherty, M.; Pascual, E.; Barskova, V.; Guerne, P.A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: Management. Ann. Rheum. Dis. 2011, 70, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Tedeschi, S.K.; Pascart, T.; Latourte, A.; Dalbeth, N.; Neogi, T.; Fuller, A.; Rosenthal, A.; Becce, F.; Bardin, T.; et al. The 2023 ACR/EULAR Classification Criteria for Calcium Pyrophosphate Deposition Disease. Arthritis Rheumatol. 2023, 75, 1703–1713. [Google Scholar] [CrossRef]
- Tedeschi, S.K. A New Era for Calcium Pyrophosphate Deposition Disease Research: The First-Ever Calcium Pyrophosphate Deposition Disease Classification Criteria and Considerations for Measuring Outcomes in Calcium Pyrophosphate Deposition Disease. Gout Urate Cryst. Depos. Dis. 2024, 2, 52–59. [Google Scholar] [CrossRef]
- Pascart, T.; Robinet, P.; Ottaviani, S.; Leroy, R.; Segaud, N.; Pacaud, A.; Grandjean, A.; Luraschi, H.; Rabin, T.; Deplanque, X.; et al. Evaluating the safety and short-term equivalence of colchicine versus prednisone in older patients with acute calcium pyrophosphate crystal arthritis (COLCHICORT): An open-label, multicentre, randomised trial. Lancet Rheumatol. 2023, 5, e523–e531. [Google Scholar] [CrossRef]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Takei, R.; Rosenthal, A.; Pascart, T.; Reynolds, R.J.; Neogi, T.; Terkeltaub, R.; Tedeschi, S.K.; Merriman, T.R. Genome-wide association study in chondrocalcinosis reveals ENPP1 as a candidate therapeutic target in calcium pyrophosphate deposition disease. Ann. Rheum. Dis. 2025, 84, 1023–1032. [Google Scholar] [CrossRef]
- Doherty, M.; Dieppe, P. Double blind, placebo controlled trial of magnesium carbonate in chronic pyrophosphate arthropathy. Ann. Rheum. Dis. 1983, 42, 106–107. [Google Scholar] [CrossRef]
- Spilberg, I.; McLain, D.; Simchowitz, L.; Berney, S. Colchicine and pseudogout. Arthritis Rheum. 1980, 23, 1062–1063. [Google Scholar] [CrossRef]
- Tabatabai, M.R.; Cummings, N.A. Intravenous colchicine in the treatment of acute pseudogout. Arthritis Rheum. 1980, 23, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Daoussis, D.; Antonopoulos, I.; Yiannopoulos, G.; Andonopoulos, A.P. ACTH as first line treatment for acute calcium pyrophosphate crystal arthritis in 14 hospitalized patients. Jt. Bone Spine 2014, 81, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Leelasattakul, W.; Pongsittisak, W.; Manavathongchai, S.; Satpanich, P. Efficacy and safety of 10 mg versus 30 mg of oral prednisolone for acute CPP crystal arthritis: Findings of a randomized controlled trial. Clin. Rheumatol. 2024, 43, 3879–3888. [Google Scholar] [CrossRef]
- Damart, J.; Filippou, G.; Andres, M.; Cipolletta, E.; Sirotti, S.; Carboni, D.; Filippucci, E.; Diez, P.; Abhishek, A.; Latourte, A.; et al. Retention, safety and efficacy of off-label conventional treatments and biologics for chronic calcium pyrophosphate crystal inflammatory arthritis. Rheumatology 2024, 63, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Alvarellos, A.; Spilberg, I. Colchicine prophylaxis in pseudogout. J. Rheumatol. 1986, 13, 804–805. [Google Scholar]
- Finckh, A.; Mc Carthy, G.M.; Madigan, A.; Van Linthoudt, D.; Weber, M.; Neto, D.; Rappoport, G.; Blumhardt, S.; Kyburz, D.; Guerne, P.A. Methotrexate in chronic-recurrent calcium pyrophosphate deposition disease: No significant effect in a randomized crossover trial. Arthritis Res. Ther. 2014, 16, 458. [Google Scholar] [CrossRef]
- Andres, M.; Sivera, F.; Pascual, E. Therapy for CPPD: Options and Evidence. Curr. Rheumatol. Rep. 2018, 20, 31. [Google Scholar] [CrossRef]
- Rothschild, B.; Yakubov, L.E. Evidence for an effect of hydroxychloroquine in chronic pyrophosphate deposition disease. J. Clin. Rheumatol. 1996, 2, 170. [Google Scholar] [CrossRef]
- Szeri, F.; Niaziorimi, F.; Donnelly, S.; Fariha, N.; Tertyshnaia, M.; Patel, D.; Lundkvist, S.; van de Wetering, K. The Mineralization Regulator ANKH Mediates Cellular Efflux of ATP, Not Pyrophosphate. J. Bone Miner. Res. 2022, 37, 1024–1031. [Google Scholar] [CrossRef]
- Cheng, P.T.; Pritzker, K.P. The effect of calcium and magnesium ions on calcium pyrophosphate crystal formation in aqueous solutions. J. Rheumatol. 1981, 8, 772–782. [Google Scholar]
- Zhang, Y.; Brown, M.A. Genetic studies of chondrocalcinosis. Curr. Opin. Rheumatol. 2005, 17, 330–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Johnson, K.; Russell, R.G.; Wordsworth, B.P.; Carr, A.J.; Terkeltaub, R.A.; Brown, M.A. Association of sporadic chondrocalcinosis with a -4-basepair G-to-A transition in the 5′-untranslated region of ANKH that promotes enhanced expression of ANKH protein and excess generation of extracellular inorganic pyrophosphate. Arthritis Rheum. 2005, 52, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Szeri, F.; Lundkvist, S.; Donnelly, S.; Engelke, U.F.H.; Rhee, K.; Williams, C.J.; Sundberg, J.P.; Wevers, R.A.; Tomlinson, R.E.; Jansen, R.S.; et al. The membrane protein ANKH is crucial for bone mechanical performance by mediating cellular export of citrate and ATP. PLoS Genet. 2020, 16, e1008884. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Qazi, U.; Bernstein, M.; Charniak, A.; Gohr, C.; Mitton-Fitzgerald, E.; Ortiz, A.; Cardinal, L.; Kaell, A.T.; Rosenthal, A.K. Mutations in osteoprotegerin account for the CCAL1 locus in calcium pyrophosphate deposition disease. Osteoarthr. Cartil. 2018, 26, 797–806. [Google Scholar] [CrossRef]
- Abhishek, A.; Doherty, S.; Maciewicz, R.; Muir, K.; Zhang, W.; Doherty, M.; Valdes, A.M. The association between ANKH promoter polymorphism and chondrocalcinosis is independent of age and osteoarthritis: Results of a case-control study. Arthritis Res. Ther. 2014, 16, R25. [Google Scholar] [CrossRef]
- Bartels, C.M.; Singh, J.A.; Parperis, K.; Huber, K.; Rosenthal, A.K. Validation of Administrative Codes for Calcium Pyrophosphate Deposition: A Veterans Administration Study. J. Clin. Rheumatol. 2015, 21, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Pritzker, K.; Firestein, G.S.; Terkeltaub, R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J. Immunol. 2005, 174, 5016–5023. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Renaudin, F.; Orliaguet, L.; Castelli, F.; Fenaille, F.; Prignon, A.; Alzaid, F.; Combes, C.; Delvaux, A.; Adimy, Y.; Cohen-Solal, M.; et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann. Rheum. Dis. 2020, 79, 1506–1514. [Google Scholar] [CrossRef]
- Campillo-Gimenez, L.; Renaudin, F.; Jalabert, M.; Gras, P.; Gosset, M.; Rey, C.; Sarda, S.; Collet, C.; Cohen-Solal, M.; Combes, C.; et al. Inflammatory Potential of Four Different Phases of Calcium Pyrophosphate Relies on NF-kappaB Activation and MAPK Pathways. Front. Immunol. 2018, 9, 2248. [Google Scholar] [CrossRef]
- Voulgari, P.V.; Venetsanopoulou, A.I.; Drosos, A.A. Recent advances in the therapeutic management of calcium pyrophosphate deposition disease. Front. Med. 2024, 11, 1327715. [Google Scholar] [CrossRef]
- Dumusc, A.; Pazar Maldonado, B.; Benaim, C.; Zufferey, P.; Aubry-Rozier, B.; So, A. Anakinra compared to prednisone in the treatment of acute CPPD crystal arthritis: A randomized controlled double-blinded pilot study. Jt. Bone Spine 2021, 88, 105088. [Google Scholar] [CrossRef]
- Cipolletta, E.; Di Matteo, A.; Grassi, W.; Filippucci, E. Treatment of acute CPP crystal arthritis: What are we missing? Comment on: “Anakinra compared to prednisone in the treatment of acute CPPD crystal arthritis: A randomized controlled double-blinded pilot study” by Dumusc A. et al. Joint Bone Spine. 2020;88:105088. Jt. Bone Spine 2021, 88, 105217. [Google Scholar] [CrossRef]
- Antoniadou, C.; Fytanidis, N.; Devetzis, V.; Kantartzi, K.; Papagoras, C. Anakinra for Refractory Pseudogout in Patients with End-stage Renal Disease on Haemodialysis. Mediterr. J. Rheumatol. 2024, 35, 58–62. [Google Scholar] [CrossRef]
- Cipolletta, E.; Di Matteo, A.; Scanu, A.; Isidori, M.; Di Battista, J.; Punzi, L.; Grassi, W.; Filippucci, E. Biologics in the treatment of calcium pyrophosphate deposition disease: A systematic literature review. Clin. Exp. Rheumatol. 2020, 38, 1001–1007. [Google Scholar] [CrossRef]
- Verhoeven, F.; Prati, C.; Godfrin-Valnet, M.; Guillot, X.; Wendling, D. IL1 blockade in crystal-induced arthritis: Impact of disease duration and the inflammatory syndrome. Comments on the article by Couderc M. et al. “Efficacy of anakinra in articular chondrocalcinosis”. Jt. Bone Spine 2013, 80, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Pascart, T.; Richette, P. IL-6: A new target in crystal-induced arthritides—A narrative review. Jt. Bone Spine 2025, 92, 105935. [Google Scholar] [CrossRef] [PubMed]
- Quilis, N.; Andres, M.; Vela, P.; Pascual, E. Interleukin-6 pathway blockade as an option for managing refractory cases of crystal arthritis: Two cases report. Jt. Bone Spine 2018, 85, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Ea, H.K.; Frazier, A.; Blanchard, A.; Liote, F.; Marotte, H.; Bardin, T.; Richette, P. Tocilizumab in symptomatic calcium pyrophosphate deposition disease: A pilot study. Ann. Rheum. Dis. 2020, 79, 1126–1128. [Google Scholar] [CrossRef]
- Carrabin, S.; Houze, M.; Jauffret, C.; Bardin, T.; Ea, H.; Lioté, F.; Richette, P.; Pascart, T.; Latourte, A. Efficacy and Safety of Tocilizumab in the Treatment of Chronic Inflammatory Forms of CPPD: Retrospective Study of 55 Cases. Arthritis Rheumatol. 2024, 76, 5202–5203. [Google Scholar]
- Tian, Y.; He, X.; Li, R.; Wu, Y.; Ren, Q.; Hou, Y. Recent advances in the treatment of gout with NLRP3 inflammasome inhibitors. Bioorg. Med. Chem. 2024, 112, 117874. [Google Scholar] [CrossRef]
- Marchetti, C.; Swartzwelter, B.; Gamboni, F.; Neff, C.P.; Richter, K.; Azam, T.; Carta, S.; Tengesdal, I.; Nemkov, T.; D’Alessandro, A.; et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, E1530–E1539. [Google Scholar] [CrossRef]
- Kluck, V.; Jansen, T.; Janssen, M.; Comarniceanu, A.; Efde, M.; Tengesdal, I.W.; Schraa, K.; Cleophas, M.C.P.; Scribner, C.L.; Skouras, D.B.; et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: An open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020, 2, e270–e280. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; Braverman, G.; Yue, L.; Fields, T. Pipeline Therapies for Gout. Curr. Rheumatol. Rep. 2024, 26, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.E.; Wu, A.; Zhou, H.; Pham, M.A.; Lin, S.; McNulty, R. Targeting the NLRP3 inflammasome for inflammatory disease therapy. Trends Pharmacol. Sci. 2025, 46, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Stack, J.; McCarthy, G. Calcium pyrophosphate deposition (CPPD) disease-Treatment options. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101720. [Google Scholar] [CrossRef]
- Hojen, J.F.; Kristensen, M.L.V.; McKee, A.S.; Wade, M.T.; Azam, T.; Lunding, L.P.; de Graaf, D.M.; Swartzwelter, B.J.; Wegmann, M.; Tolstrup, M.; et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat. Immunol. 2019, 20, 1138–1149. [Google Scholar] [CrossRef]
- Gronberg, C.; Rattik, S.; Tran-Manh, C.; Zhou, X.; Rius Rigau, A.; Li, Y.N.; Gyorfi, A.H.; Dickel, N.; Kunz, M.; Kreuter, A.; et al. Combined inhibition of IL-1, IL-33 and IL-36 signalling by targeting IL1RAP ameliorates skin and lung fibrosis in preclinical models of systemic sclerosis. Ann. Rheum. Dis. 2024, 83, 1156–1168. [Google Scholar] [CrossRef]
- Lema, D.A.; Jakobsson, G.; Daoud, A.; Elias, D.; Talor, M.V.; Rattik, S.; Gronberg, C.; Kalinoski, H.; Jaensson Gyllenback, E.; Wang, N.; et al. IL1RAP Blockade with a Monoclonal Antibody Reduces Cardiac Inflammation and Preserves Heart Function in Viral and Autoimmune Myocarditis. Circ. Heart Fail. 2024, 17, e011729. [Google Scholar] [CrossRef] [PubMed]
- Grassot, J.; Marzouki, F.; Hervé, R.; Beckler, M.; Semerano, L.; Bessis, N.; Rivière, E.; Sanson, C.; Pouliquen, G.; Pouletty, P.; et al. Intra-articular Treatment Combining Sustained Release Colchicine Encapsulated in Microspheres, and Ropivacaine, Is Effective in Inflammatory Arthritis in Rats. Arthritis Rheumatol. 2024, 76, 547–548. [Google Scholar]
- Tedeschi, S.K.; Becce, F.; Pascart, T.; Guermazi, A.; Budzik, J.F.; Dalbeth, N.; Filippou, G.; Iagnocco, A.; Kohler, M.J.; Laredo, J.D.; et al. Imaging Features of Calcium Pyrophosphate Deposition Disease: Consensus Definitions from an International Multidisciplinary Working Group. Arthritis Care Res. 2023, 75, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Sirotti, S.; Becce, F.; Sconfienza, L.M.; Terslev, L.; Naredo, E.; Zufferey, P.; Pineda, C.; Gutierrez, M.; Adinolfi, A.; Serban, T.; et al. Reliability and Diagnostic Accuracy of Radiography for the Diagnosis of Calcium Pyrophosphate Deposition: Performance of the Novel Definitions Developed by an International Multidisciplinary Working Group. Arthritis Rheumatol. 2023, 75, 630–638. [Google Scholar] [CrossRef]
- Filippou, G.; Sirotti, S.; Cipolletta, E.; Filippucci, E. Optimizing the Use of Ultrasound in Calcium Pyrophosphate Deposition (CPPD): A Review from the Ground Up. Gout Urate Cryst. Depos. Dis. 2024, 2, 17–33. [Google Scholar] [CrossRef]
- Laurent, V.; Filippou, G.; Sirotti, S.; Pascart, T. Advanced imaging techniques in crystal arthritis. Ther. Adv. Musculoskelet. Dis. 2025, 17, 1759720X251316097. [Google Scholar] [CrossRef]
- Cipolletta, E.; Rozza, D.; Andres, M.; Ottaviani, S.; Pascart, T.; Calvo-Aranda, E.; Chiarvetto Peralta, M.V.; Muto, P.; Calabuig, I.; Gómez-Sabater, S.; et al. Development and internal-external cross-validation of a patient-reported definition for acute calcium pyrophosphate crystal arthritis. Rheumatology 2025, 64, 2609–2617. [Google Scholar] [CrossRef]
- Cai, K.; Fuller, A.; Hensey, O.; Grossberg, D.; Christensen, R.; Shea, B.; Singh, J.A.; Abhishek, A.; Tedeschi, S.; Dalbeth, N. Outcome domains reported in calcium pyrophosphate deposition studies: A scoping review by the OMERACT CPPD working group. Semin. Arthritis Rheum. 2020, 50, 719–727. [Google Scholar] [CrossRef]
- Fuller, A.; Cai, K.; Diaz-Torne, C.; Filippou, G.; Pascart, T.; Hensey, O.; Grossberg, D.; Christensen, R.; Shea, B.; Singh, J.A.; et al. Outcome domains reported by patients, caregivers, healthcare professionals and stakeholders for calcium pyrophosphate deposition (CPPD): A content analysis based on semi-structured qualitative interviews from the OMERACT CPPD working group. Semin. Arthritis Rheum. 2021, 51, 650–654. [Google Scholar] [CrossRef]
- Cai, K.; Fuller, A.; Zhang, Y.; Hensey, O.; Grossberg, D.; Christensen, R.; Shea, B.; Singh, J.A.; McCarthy, G.M.; Rosenthal, A.K.; et al. Towards development of core domain sets for short term and long term studies of calcium pyrophosphate crystal deposition (CPPD) disease: A framework paper by the OMERACT CPPD working group. Semin. Arthritis Rheum. 2021, 51, 946–950. [Google Scholar] [CrossRef]
- Zhang, Y.; Tedeschi, S.K.; Abhishek, A.; Hensey, O.; Grossberg, D.; Cai, K.; Shea, B.; Singh, J.A.; Christensen, R.; Serban, T.; et al. Core domain set for chronic and/or recurrent manifestations of calcium pyrophosphate deposition disease: OMERACT delphi survey to establish consensus. Semin. Arthritis Rheum. 2025, 72, 152669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tedeschi, S.K.; Abhishek, A.; Hensey, O.; Grossberg, D.; Cai, K.; Shea, B.; Singh, J.A.; Christensen, R.; Serban, T.; et al. Core domain set for studies of acute calcium pyrophosphate crystal arthritis: OMERACT delphi survey to establish consensus. Semin. Arthritis Rheum. 2025, 72, 152670. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).