Testing for Causal Association Between Serum Urate, Gout, and Prostatic Cancer in European Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure Instrumental Variable and Outcome GWAS Summary Statistics for Two-Sample MR Study
2.2. Statistical Analysis
3. Results
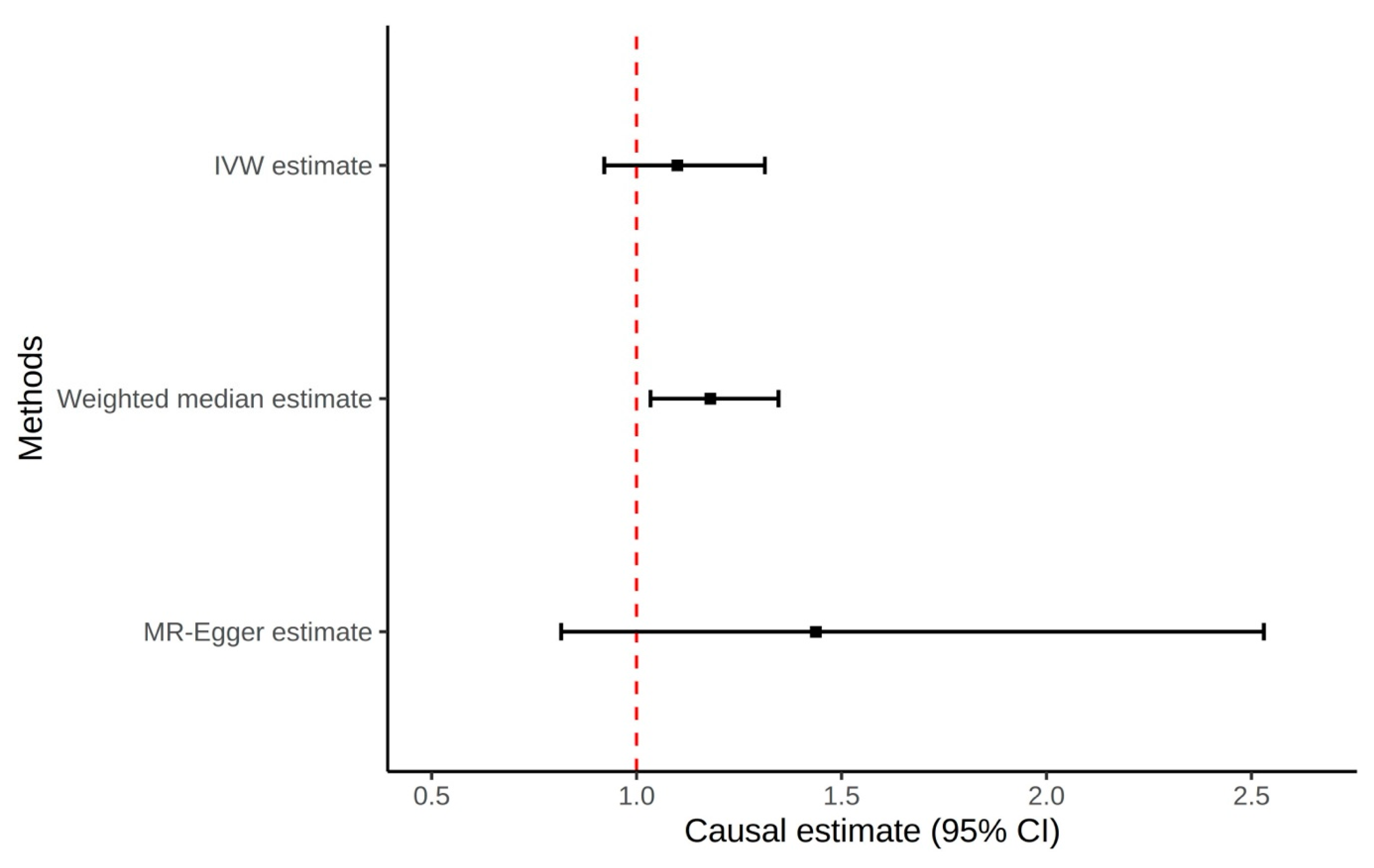
3.1. Gout to Prostate Cancer Mendelian Randomization
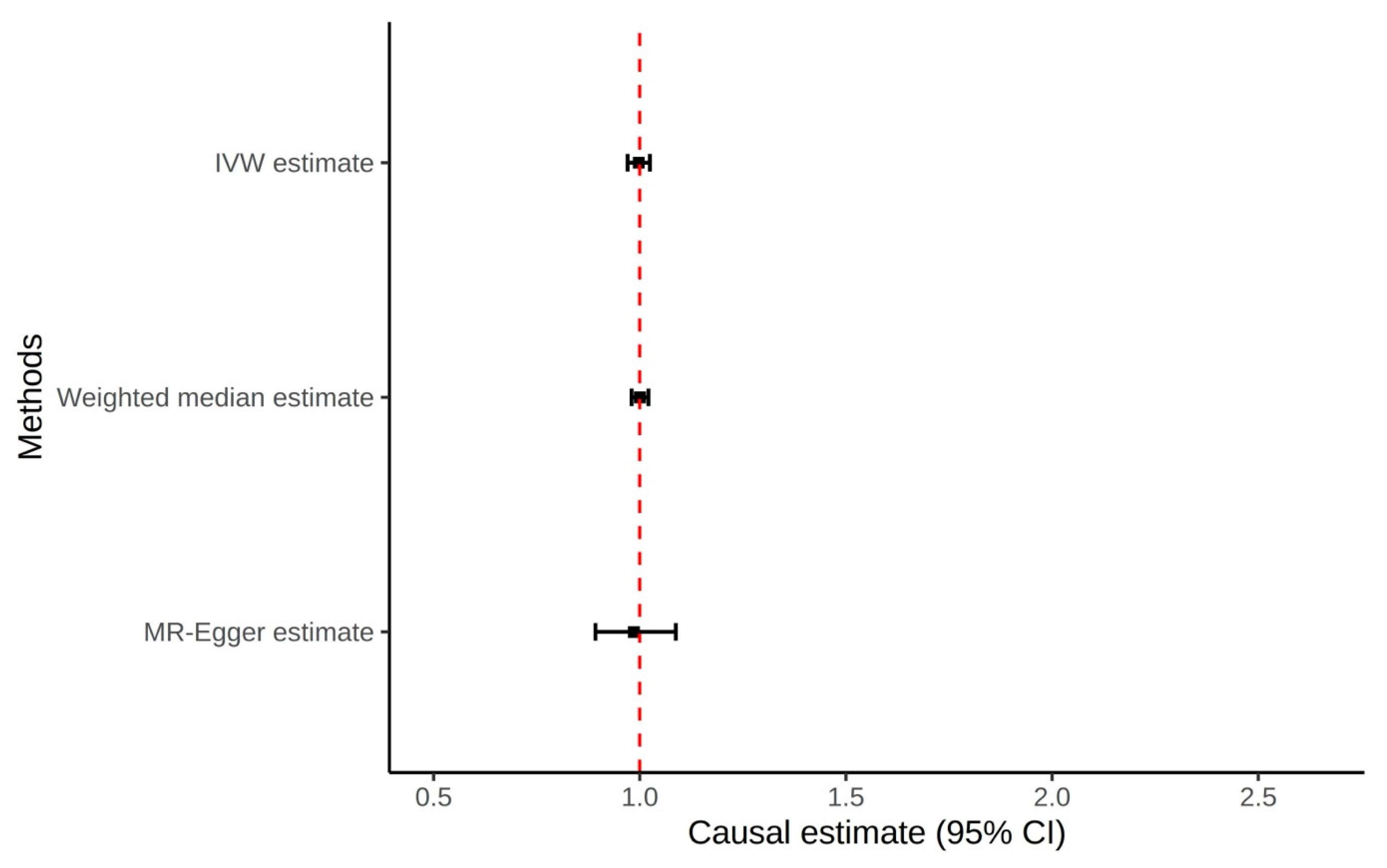
3.2. Serum Urate to Prostate Cancer Mendelian Randomization
3.3. Prostate Cancer to Serum Urate and Gout Mendelian Randomization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Colantonio, L.D.; Saag, K.G.; Singh, J.A.; Chen, L.; Reynolds, R.J.; Gaffo, A.; Plante, T.B.; Curtis, J.R.; Bridges, S.L., Jr.; Levitan, E.B.; et al. Gout is associated with an increased risk for incident heart failure among older adults: The REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Arthritis Res. Ther. 2020, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am. J. Med. 2012, 125, 679–687.e1. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Lu, N.; Tanikella, S.; Lin, K.; Joshi, A.D.; Raffield, L.M.; Warner, E.; Merriman, T.; Hsu, J.; et al. Trends in Prevalence of Gout Among US Asian Adults, 2011-2018. JAMA Netw. Open 2023, 6, e239501. [Google Scholar] [CrossRef]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The National Health and Nutrition Examination Survey, 2007-2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Halperin Kuhns, V.L.; Woodward, O.M. Sex differences in urate handling. Int. J. Mol. Sci. 2020, 21, 4269. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Guo, T.; Lee, J.; Terkeltaub, R. Atherogenic activation of human vascular smooth muscle cells by monosodium urate crystals. Gout Urate Cryst. Depos. Dis. 2023, 1, 192–207. [Google Scholar]
- Srivastava, A.; Chopra, S.K.; Dasgupta, P.R. Biochemical analysis of human seminal plasma. II. Protein, non-protein nitrogen, urea, uric acid and creatine. Andrologia 1984, 16, 265–268. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Lu, J.C.; Zhang, R.S.; Xia, Y.X.; Huang, Y.F. Determination of uric acid in seminal plasma and correlation between seminal uric acid and semen parameters. Zhonghua Nan Ke Xue 2007, 13, 1016–1019. [Google Scholar]
- Park, J.J.; Roudier, M.P.; Soman, D.; Mokadam, N.A.; Simkin, P.A. Prevalence of birefringent crystals in cardiac and prostatic tissues, an observational study. BMJ Open 2014, 4, e005308. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Pasaribu, N.; Lee, S.S.; Tsai, W.C.; Li, C.Y.; Lin, G.T.; Chuang, H.Y.; Tung, Y.C.; Tu, H.P. Risk of incident benign prostatic hyperplasia in patients with gout: A retrospective cohort study. Prostate Cancer Prostatic Dis. 2018, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kukko, V.; Kaipia, A.; Talala, K.; Taari, K.; Tammela, T.L.J.; Auvinen, A.; Murtola, T.J. Allopurinol and risk of benign prostatic hyperplasia in a Finnish population-based cohort. Prostate Cancer Prostatic Dis. 2018, 21, 373–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Ou, G.; Li, R.; Peng, L.; Shi, J. Causal relationship between benign prostatic hyperplasia and prostate cancer: A bidirectional Mendelian randomization analysis. Postgrad. Med. J. 2024, 101, 427–434. [Google Scholar] [CrossRef]
- Orsted, D.D.; Bojesen, S.E. The link between benign prostatic hyperplasia and prostate cancer. Nat. Rev. Urol. 2013, 10, 49–54. [Google Scholar] [CrossRef]
- De Leeuw, C.; Savage, J.; Bucur, I.G.; Heskes, T.; Posthuma, D. Understanding the assumptions underlying Mendelian randomization. Eur. J. Hum. Genet. 2022, 30, 653–660. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, J.; Wong, M.C.S. Association between serum uric acid and prostate cancer risk in East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2023, 62, 1323–1329. [Google Scholar] [CrossRef]
- Jiang, M.; Ren, L.; Chen, S.; Li, G. Serum uric acid levels and risk of eight site-specific cancers: A Mendelian randomization study. Front. Genet. 2021, 12, 608311. [Google Scholar] [CrossRef] [PubMed]
- Major, T.J.; Takei, R.; Matsuo, H.; Leask, M.P.; Sumpter, N.A.; Topless, R.K.; Shirai, Y.; Wang, W.; Cadzow, M.J.; Phipps-Green, A.J.; et al. A genome-wide association analysis reveals new pathogenic pathways in gout. Nat. Genet. 2024, 56, 2392–2406. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Jansen, T.L.; Dalbeth, N.; Fransen, J.; Schumacher, H.R.; Berendsen, D.; Brown, M.; Choi, H.; Edwards, N.L.; Janssens, H.J.; et al. 2015 Gout Classification Criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015, 67, 2557–2568. [Google Scholar] [CrossRef]
- Wang, A.; Shen, J.; Rodriguez, A.A.; Saunders, E.J.; Chen, F.; Janivara, R.; Darst, B.F.; Sheng, X.; Xu, Y.; Chou, A.J.; et al. Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants. Nat. Genet. 2023, 55, 2065–2074. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Zhao, Q.; Lawlor, D.A.; Sheehan, N.A.; Thompson, J.; Davey Smith, G. Improving the accuracy of two-sample summary-data Mendelian randomization: Moving beyond the NOME assumption. Int. J. Epidemiol. 2019, 48, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Yavorska, O. MendelianRandomization: Mendelian Randomization Package, R package version 0.10.0; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Lokki, M.L.; Paakkanen, R. The complexity and diversity of major histocompatibility complex challenge disease association studies. HLA 2019, 93, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Barber, J.R.; Tin, A.; De Marzo, A.M.; Kottgen, A.; Joshu, C.E.; Platz, E.A. Serum Urate, Genetic Variation, and Prostate Cancer Risk: Atherosclerosis Risk in Communities (ARIC) Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1259–1261. [Google Scholar] [CrossRef]
- Singh, S.; Jaiswal, S.; Faujdar, G.; Priyadarshi, S. Comparison of serum uric acid levels between localised prostate cancer patients and a control group. Urologia 2024, 91, 320–325. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.H.; Cho, H.J.; Ha, Y.J.; Kang, E.H.; Shin, K.; Byun, S.S.; Lee, E.Y.; Song, Y.W.; Lee, Y.J. Influence of androgen deprivation therapy on serum urate levels in patients with prostate cancer: A retrospective observational study. PLoS ONE 2018, 13, e0209049. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Yen, J.H.; Chang, S.J. Gout patients have an increased risk of developing most cancers, especially urological cancers. Scand. J. Rheumatol. 2014, 43, 385–390. [Google Scholar] [CrossRef]
- Kuo, C.F.; Luo, S.F.; See, L.C.; Chou, I.J.; Fang, Y.F.; Yu, K.H. Increased risk of cancer among gout patients: A nationwide population study. Jt. Bone Spine 2012, 79, 375–378. [Google Scholar] [CrossRef]
- Oh, Y.J.; Lee, Y.J.; Lee, E.; Park, B.; Kwon, J.W.; Heo, J.; Moon, K.W. Cancer risk in Korean patients with gout. Korean J. Intern. Med. 2022, 37, 460–467. [Google Scholar] [CrossRef]
- Boffetta, P.; Nordenvall, C.; Nyren, O.; Ye, W. A prospective study of gout and cancer. Eur. J. Cancer Prev. 2009, 18, 127–132. [Google Scholar] [CrossRef]
- Dahl, H.C.; Kanchwala, M.; Thomas-Jardin, S.E.; Sandhu, A.; Kanumuri, P.; Nawas, A.F.; Xing, C.; Lin, C.; Frigo, D.E.; Delk, N.A. Chronic IL-1 exposure drives LNCaP cells to evolve androgen and AR independence. PLoS ONE 2020, 15, e0242970. [Google Scholar] [CrossRef]
- Fan, Y.C.; Lee, K.D.; Tsai, Y.C. Roles of interleukin-1 receptor antagonist in prostate cancer progression. Biomedicines 2020, 8, 602. [Google Scholar] [CrossRef]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of zinc-exporters expression in prostate cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef]
- McNaughton, C.O.; Wilt, T. Allopurinol for chronic prostatitis. Cochrane Database Syst. Rev. 2002, CD001041. [Google Scholar] [CrossRef]
- Persson, B.E.; Ronquist, G.; Ekblom, M. Ameliorative effect of allopurinol on nonbacterial prostatitis: A parallel double-blind controlled study. J. Urol. 1996, 155, 961–964. [Google Scholar] [CrossRef]
- Kambe, T.; Matsunaga, M.; Takeda, T.A. Understanding the contribution of zinc transporters in the function of the early secretory pathway. Int. J. Mol. Sci. 2017, 18, 2179. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef] [PubMed]

| OR (95% CI) 1 | p Value | MR Egger Intercept (SE) | p Value of Pleiotropy | |
|---|---|---|---|---|
| Gout (Non-hyperuricemia Compartment) to Prostate Cancer—Gout GWAS | ||||
| IVW | 1.10 (0.92–1.31) | 0.29 | ||
| MR Egger | 1.44 (0.82–2.53) | 0.21 | −0.010 (0.010) | 0.33 |
| Weighted median | 1.18 (1.03–1.35) | 0.01 | ||
| Gout (Non-hyperuricemia Compartment) to Prostate Cancer outliers excluded—Gout GWAS | ||||
| IVW | 1.15 (1.01–1.30) | 0.03 | ||
| MR Egger | 1.11 (0.72–1.71) | 0.64 | 0.001 (0.008) | 0.87 |
| Weighted median | 1.18 (1.04–1.35) | 0.01 | ||
| Urate Transporter Genes to Prostate Cancer—Urate GWAS | ||||
| IVW | 1.00 (0.97–1.02) | 0.83 | ||
| MR Egger | 0.99 (0.89–1.09) | 0.77 | 0.004 (0.017) | 0.80 |
| Weighted median | 1.00 (0.98–1.02) | 0.97 | ||
| Prostate Cancer to Gout—Prostate Cancer GWAS | ||||
| IVW | 0.99 (0.96–1.02) | 0.61 | ||
| MR Egger | 0.96 (0.89–1.05) | 0.39 | 0.005 (0.007) | 0.47 |
| Weighted median | 0.99 (0.96–1.02) | 0.55 | ||
| Prostate Cancer to Serum Urate—Prostate Cancer GWAS | ||||
| IVW | 1.01 (0.93–1.09) | 0.89 | ||
| MR Egger | 1.00 (0.80–1.25) | 0.99 | 0.001 (0.020) | 0.97 |
| Weighted median | 1.02 (0.95–1.10) | 0.53 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Gout, Hyperuricemia and Crystal Associated Disease Network. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrupatla, S.; Sumpter, N.; Takei, R.; Merriman, T.R.; Singh, J. Testing for Causal Association Between Serum Urate, Gout, and Prostatic Cancer in European Males. Gout Urate Cryst. Depos. Dis. 2025, 3, 20. https://doi.org/10.3390/gucdd3040020
Chandrupatla S, Sumpter N, Takei R, Merriman TR, Singh J. Testing for Causal Association Between Serum Urate, Gout, and Prostatic Cancer in European Males. Gout, Urate, and Crystal Deposition Disease. 2025; 3(4):20. https://doi.org/10.3390/gucdd3040020
Chicago/Turabian StyleChandrupatla, Sumanth, Nicholas Sumpter, Riku Takei, Tony R. Merriman, and Jasvinder Singh. 2025. "Testing for Causal Association Between Serum Urate, Gout, and Prostatic Cancer in European Males" Gout, Urate, and Crystal Deposition Disease 3, no. 4: 20. https://doi.org/10.3390/gucdd3040020
APA StyleChandrupatla, S., Sumpter, N., Takei, R., Merriman, T. R., & Singh, J. (2025). Testing for Causal Association Between Serum Urate, Gout, and Prostatic Cancer in European Males. Gout, Urate, and Crystal Deposition Disease, 3(4), 20. https://doi.org/10.3390/gucdd3040020






