Ultrasound Findings of Monosodium Urate Aggregates in Patients with Gout
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
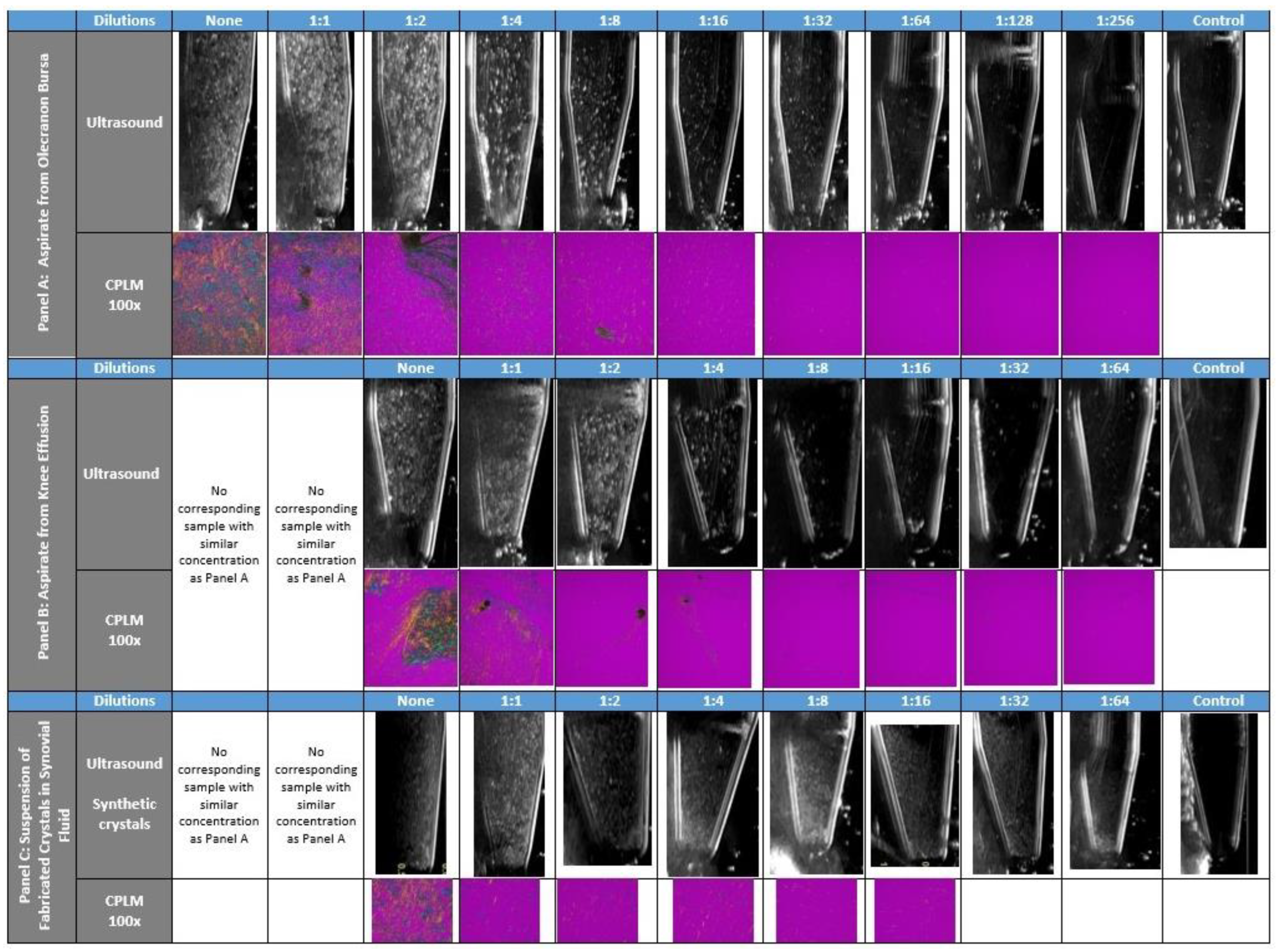
2.2. Ultrasound & CPLM Imaging
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda, J.; Coyfish, M.; Guillo, S.; Jansen, T.; Janssens, H.; et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann. Rheum. Dis. 2020, 79, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Jansen, T.L.; Dalbeth, N.; Fransen, J.; Schumacher, H.R.; Berendsen, D.; Brown, M.; Choi, H.; Edwards, N.L.; Janssens, H.J.; et al. 2015 Gout Classification Criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015, 67, 2557–2568. [Google Scholar] [CrossRef] [PubMed]
- Terslev, L.; Gutierrez, M.; Schmidt, W.A.; Keen, H.I.; Filippucci, E.; Kane, D.; Thiele, R.; Kaeley, G.; Balint, P.; Mandl, P.; et al. Ultrasound as an Outcome Measure in Gout. A Validation Process by the OMERACT Ultrasound Working Group. J. Rheumatol. 2015, 42, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Cazenave, T.; Martire, V.; Reginato, A.M.; Gutierrez, M.; Waimann, C.A.; Pineda, C.; Rosa, J.E.; Ruta, S.; Sedano-Santiago, O.; Bertoli, A.M.; et al. Reliability of OMERACT ultrasound elementary lesions in gout: Results from a multicenter exercise. Rheumatol. Int. 2019, 39, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Chowalloor, P.V.; Keen, H.I. A systematic review of ultrasonography in gout and asymptomatic hyperuricaemia. Ann. Rheum. Dis. 2013, 72, 638–645. [Google Scholar] [CrossRef]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef]
- Pineda, C.; Fuentes-Gómez, A.J.; Hernández-Díaz, C.; Zamudio-Cuevas, Y.; Fernández-Torres, J.; López-Macay, A.; Alba-Sánchez, I.; Camacho-Galindo, J.; Ventura, L.; Gómez-Quiróz, L.E.; et al. Animal model of acute gout reproduces the inflammatory and ultrasonographic joint changes of human gout. Arthritis Res. 2015, 17, 37. [Google Scholar] [CrossRef]
- Denko, C.W.; Whitehouse, M.W. Experimental inflammation induced by naturally occurring microcrystalline calcium salts. J. Rheumatol. 1976, 3, 54–62. [Google Scholar]
- Newberry, S.J.; FitzGerald, J.D.; Motala, A.; Booth, M.; Maglione, M.A.; Han, D.; Tariq, A.; O’Hanlon, C.E.; Shanman, R.; Dudley, W.; et al. Diagnosis of Gout: A Systematic Review in Support of an American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 2017, 166, 27–36. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, S.; Wang, W.; Zhou, Q.; Wu, H. Combining Hyperechoic Aggregates and the Double-Contour Sign Increases the Sensitivity of Sonography for Detection of Monosodium Urate Deposits in Gout. J. Ultrasound Med. 2017, 36, 935–940. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhöfer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, E.; Jeremic, I.; Czegley, C.; Weidner, D.; Biermann, M.H.; Veissi, S.; Maueröder, C.; Schauer, C.; Bilyy, R.; Dumych, T.; et al. Blood-borne phagocytes internalize urate microaggregates and prevent intravascular NETosis by urate crystals. Sci. Rep. 2016, 6, 38229. [Google Scholar] [CrossRef]
- Chatfield, S.M.; Grebe, K.; Whitehead, L.W.; Rogers, K.L.; Nebl, T.; Murphy, J.M.; Wicks, I.P. Monosodium Urate Crystals Generate Nuclease-Resistant Neutrophil Extracellular Traps via a Distinct Molecular Pathway. J. Immunol. 2018, 200, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Zell, M.; Aung, T.; Kaldas, M.; Rosenthal, A.K.; Bai, B.; Liu, T.; Ozcan, A.; FitzGerald, J.D. Calcium pyrophosphate crystal size and characteristics. Osteoarthr. Cart. Open 2021, 3, 100133. [Google Scholar] [CrossRef] [PubMed]
- Filippucci, E.; Di Geso, L.; Grassi, W. Tips and tricks to recognize microcrystalline arthritis. Rheumatology 2012, 51 (Suppl. 7), vii18–vii21. [Google Scholar] [CrossRef]
- Grassi, W.; Meenagh, G.; Pascual, E.; Filippucci, E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin. Arthritis Rheum. 2006, 36, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Frediani, B.; Filippou, G.; Falsetti, P.; Lorenzini, S.; Baldi, F.; Acciai, C.; Siagkri, C.; Marotto, D.; Galeazzi, M.; Marcolongo, R. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: Ultrasonographic criteria proposed. Ann. Rheum. Dis. 2005, 64, 638–640. [Google Scholar] [CrossRef]
- Filippou, G.; Pacini, G.; Sirotti, S.; Zadory, M.; Carboni, D.; Damiani, A.; Fiorentini, E.; Cipolletta, E.; Filippucci, E.; Froehlich, J.M.; et al. Comparison of ultrasound attenuation by calcium pyrophosphate, hydroxyapatite and monosodium urate crystals: A proof-of-concept study. Ann. Rheum. Dis. 2022, 81, 1199–1201. [Google Scholar] [CrossRef]
- Christiansen, S.N.; Filippou, G.; Scirè, C.A.; Balint, P.V.; Bruyn, G.A.; Dalbeth, N.; Dejaco, C.; Sedie, A.D.; Filippucci, E.; Hammer, H.B.; et al. Consensus-based semi-quantitative ultrasound scoring system for gout lesions: Results of an OMERACT Delphi process and web-reliability exercise. Semin. Arthritis Rheum. 2021, 51, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Zhang, D.; Levine, B.D.; Dalbeth, N.; Pool, B.; Ranganath, V.K.; Benhaim, P.; Nelson, S.D.; Hsieh, S.S.; FitzGerald, J.D. Limitations of dual-energy CT in the detection of monosodium urate deposition in dense liquid tophi and calcified tophi. Skelet. Radiol. 2021, 50, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Zell, M.; Zhang, D.; FitzGerald, J. Diagnostic advances in synovial fluid analysis and radiographic identification for crystalline arthritis. Curr. Opin Rheumatol. 2019, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ogdie, A.; Taylor, W.J.; Weatherall, M.; Fransen, J.; Jansen, T.L.; Neogi, T.; Schumacher, H.R.; Dalbeth, N. Imaging modalities for the classification of gout: Systematic literature review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Anderson, N.G.; Becce, F.; Rajeswari, M.; Polson, M.; Guyen, O.; Viry, A.; Choi, C.; Kirkbride, T.E.; Raja, A.Y. Clinical Utility of Multi-Energy Spectral Photon-Counting Computed Tomography in Crystal Arthritis. Arthritis Rheumatol. 2019, 71, 1158–1162. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, E.; Dalbeth, N.; Pool, B.; Ramirez Cazares, A.; Ranganath, V.K.; FitzGerald, J.D. Ultrasound Findings of Monosodium Urate Aggregates in Patients with Gout. Gout Urate Cryst. Depos. Dis. 2023, 1, 83-88. https://doi.org/10.3390/gucdd1020008
Liu E, Dalbeth N, Pool B, Ramirez Cazares A, Ranganath VK, FitzGerald JD. Ultrasound Findings of Monosodium Urate Aggregates in Patients with Gout. Gout, Urate, and Crystal Deposition Disease. 2023; 1(2):83-88. https://doi.org/10.3390/gucdd1020008
Chicago/Turabian StyleLiu, Eric, Nicola Dalbeth, Bregina Pool, Andrea Ramirez Cazares, Veena K. Ranganath, and John D. FitzGerald. 2023. "Ultrasound Findings of Monosodium Urate Aggregates in Patients with Gout" Gout, Urate, and Crystal Deposition Disease 1, no. 2: 83-88. https://doi.org/10.3390/gucdd1020008
APA StyleLiu, E., Dalbeth, N., Pool, B., Ramirez Cazares, A., Ranganath, V. K., & FitzGerald, J. D. (2023). Ultrasound Findings of Monosodium Urate Aggregates in Patients with Gout. Gout, Urate, and Crystal Deposition Disease, 1(2), 83-88. https://doi.org/10.3390/gucdd1020008






