Optimizing Automated Detection for Cytoplasmic TDP25 Aggregates in Fluorescence Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Transfection, and Image Acquisition
2.2. Nematode Culture and Image Acquisition
2.3. Image Analysis
2.4. Plots and Statistics
3. Results and Discussion
3.1. Identification of the Weighting Factor for TDP25 Aggregates in PunctaFinder Algorithm
3.2. Increase in the Detection Efficiency of the Aggregate-Positive Cells by Improving the Signal-to-Noise Ratio of the Fluorescence Images
3.3. Further Improvement in the Detection Efficiency of Aggregate-Positive Cells by Reducing the Number of Target Aggregates per Analyzed Image
3.4. Issues and Perspectives for More Efficient Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TDP-43 | Transactive response element DNA/RNA-binding protein 43 kDa |
| 2D | Two-dimension |
| LSM | Laser scanning microscope |
References
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kikuchi, S.; Katada, S.; Nagai, Y.; Nishizawa, M.; Onodera, O. Soluble polyglutamine oligomers formed prior to inclusion body formation are cytotoxic. Hum. Mol. Genet. 2008, 17, 345–356. [Google Scholar] [CrossRef]
- Harel, I.; Chen, Y.R.; Ziv, I.; Singh, P.P.; Heinzer, D.; Navarro Negredo, P.; Goshtchevsky, U.; Wang, W.; Astre, G.; Moses, E.; et al. Identification of protein aggregates in the aging vertebrate brain with prion-like and phase-separation properties. Cell Rep. 2024, 43, 112787. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Lee, J.H.; Kwon, D.Y.; Genovesio, A.; Fenistein, D.; Ogier, A.; Brondani, V.; Grailhe, R. Dimerization, oligomerization, and aggregation of human amyotrophic lateral sclerosis copper/zinc superoxide dismutase 1 protein mutant forms in live cells. J. Biol. Chem. 2014, 289, 15094–15103. [Google Scholar] [CrossRef] [PubMed]
- Perni, M.; Mannini, B.; Xu, C.K.; Kumita, J.R.; Dobson, C.M.; Chiti, F.; Vendruscolo, M. Exogenous misfolded protein oligomers can cross the intestinal barrier and cause a disease phenotype in C. elegans. Sci. Rep. 2021, 11, 14391. [Google Scholar] [CrossRef]
- Bigi, A.; Cascella, R.; Chiti, F.; Cecchi, C. Amyloid fibrils act as a reservoir of soluble oligomers, the main culprits in protein deposition diseases. BioEssays News Rev. Mol. Cell. Dev. Biol. 2022, 44, e2200086. [Google Scholar] [CrossRef]
- Miangolarra, A.M.; Duperray-Susini, A.; Coppey, M.; Castellana, M. Two timescales control the creation of large protein aggregates in cells. Biophys. J. 2021, 120, 2394–2399. [Google Scholar] [CrossRef]
- Safren, N.; Sharkey, L.M.; Barmada, S.J. Neuronal Puncta/Aggregate Formation by WT and Mutant UBQLN2. Methods Mol. Biol. 2023, 2551, 561–573. [Google Scholar] [CrossRef]
- Guo, J.L.; Buist, A.; Soares, A.; Callaerts, K.; Calafate, S.; Stevenaert, F.; Daniels, J.P.; Zoll, B.E.; Crowe, A.; Brunden, K.R.; et al. The Dynamics and Turnover of Tau Aggregates in Cultured Cells: INSIGHTS INTO THERAPIES FOR TAUOPATHIES. J. Biol. Chem. 2016, 291, 13175–13193. [Google Scholar] [CrossRef]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- Jawerth, L.; Fischer-Friedrich, E.; Saha, S.; Wang, J.; Franzmann, T.; Zhang, X.; Sachweh, J.; Ruer, M.; Ijavi, M.; Saha, S.; et al. Protein condensates as aging Maxwell fluids. Science 2020, 370, 1317–1323. [Google Scholar] [CrossRef]
- Mondal, S.; Narayan, K.; Botterbusch, S.; Powers, I.; Zheng, J.; James, H.P.; Jin, R.; Baumgart, T. Multivalent interactions between molecular components involved in fast endophilin mediated endocytosis drive protein phase separation. Nat. Commun. 2022, 13, 5017. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U. Protein Misfolding Diseases. Annu. Rev. Biochem. 2017, 86, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Jaqaman, K.; Loerke, D.; Mettlen, M.; Kuwata, H.; Grinstein, S.; Schmid, S.L.; Danuser, G. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 2008, 5, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Tinevez, J.Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- Shah, S.I.; Ong, H.L.; Demuro, A.; Ullah, G. PunctaSpecks: A tool for automated detection, tracking, and analysis of multiple types of fluorescently labeled biomolecules. Cell Calcium 2020, 89, 102224. [Google Scholar] [CrossRef]
- Klickstein, J.A.; Mukkavalli, S.; Raman, M. AggreCount: An unbiased image analysis tool for identifying and quantifying cellular aggregates in a spatially defined manner. J. Biol. Chem. 2020, 295, 17672–17683. [Google Scholar] [CrossRef]
- Ershov, D.; Phan, M.S.; Pylvanainen, J.W.; Rigaud, S.U.; Le Blanc, L.; Charles-Orszag, A.; Conway, J.R.W.; Laine, R.F.; Roy, N.H.; Bonazzi, D.; et al. TrackMate 7: Integrating state-of-the-art segmentation algorithms into tracking pipelines. Nat. Methods 2022, 19, 829–832. [Google Scholar] [CrossRef]
- Mantes, A.D.; Herrera, A.; Khven, I.; Schlaeppi, A.; Kyriacou, E.; Tsissios, G.; Skoufa, E.; Santangeli, L.; Buglakova, E.; Durmus, E.B.; et al. Spotiflow: Accurate and efficient spot detection for fluorescence microscopy with deep stereographic flow regression. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Terpstra, H.M.; Gomez-Sanchez, R.; Veldsink, A.C.; Otto, T.A.; Veenhoff, L.M.; Heinemann, M. PunctaFinder: An algorithm for automated spot detection in fluorescence microscopy images. Mol. Biol. Cell 2024, 35, mr9. [Google Scholar] [CrossRef]
- Kitamura, A.; Nakayama, Y.; Shibasaki, A.; Taki, A.; Yuno, S.; Takeda, K.; Yahara, M.; Tanabe, N.; Kinjo, M. Interaction of RNA with a C-terminal fragment of the amyotrophic lateral sclerosis-associated TDP43 reduces cytotoxicity. Sci. Rep. 2016, 6, 19230. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Kinjo, M.; Kitamura, A. Short Repeat Ribonucleic Acid Reduces Cytotoxicity by Preventing the Aggregation of TDP-43 and Its 25 KDa Carboxy-Terminal Fragment. JACS Au 2024, 4, 3896–3909. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Fujimoto, A.; Kawashima, R.; Lyu, Y.; Sasaki, K.; Hamada, Y.; Moriya, K.; Kurata, A.; Takahashi, K.; Brielmann, R.; et al. Hetero-oligomerization of TDP-43 carboxy-terminal fragments with cellular proteins contributes to proteotoxicity. Commun. Biol. 2024, 7, 743. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of behaviour. Br. Med. Bull. 1973, 29, 269–271. [Google Scholar] [CrossRef]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Sonawat, D.; Mandal, S.; Khare, K.; Rathore, A.S. Novel semi-automated fluorescence microscope imaging algorithm for monitoring IgG aggregates in serum. Sci. Rep. 2021, 11, 11375. [Google Scholar] [CrossRef]
- Hebert, B.; Costantino, S.; Wiseman, P.W. Spatiotemporal image correlation spectroscopy (STICS) theory, verification, and application to protein velocity mapping in living CHO cells. Biophys. J. 2005, 88, 3601–3614. [Google Scholar] [CrossRef]
- Kitamura, A.; Shimizu, H.; Kinjo, M. Determination of cytoplasmic optineurin foci sizes using image correlation spectroscopy. J. Biochem. 2018, 164, 223–229. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhou, Y.; Wu, J.; Zhao, Z.; Fan, J.; Deng, F.; Wu, Z.; Xiao, G.; He, J.; et al. Real-time denoising enables high-sensitivity fluorescence time-lapse imaging beyond the shot-noise limit. Nat. Biotechnol. 2023, 41, 282–292. [Google Scholar] [CrossRef]
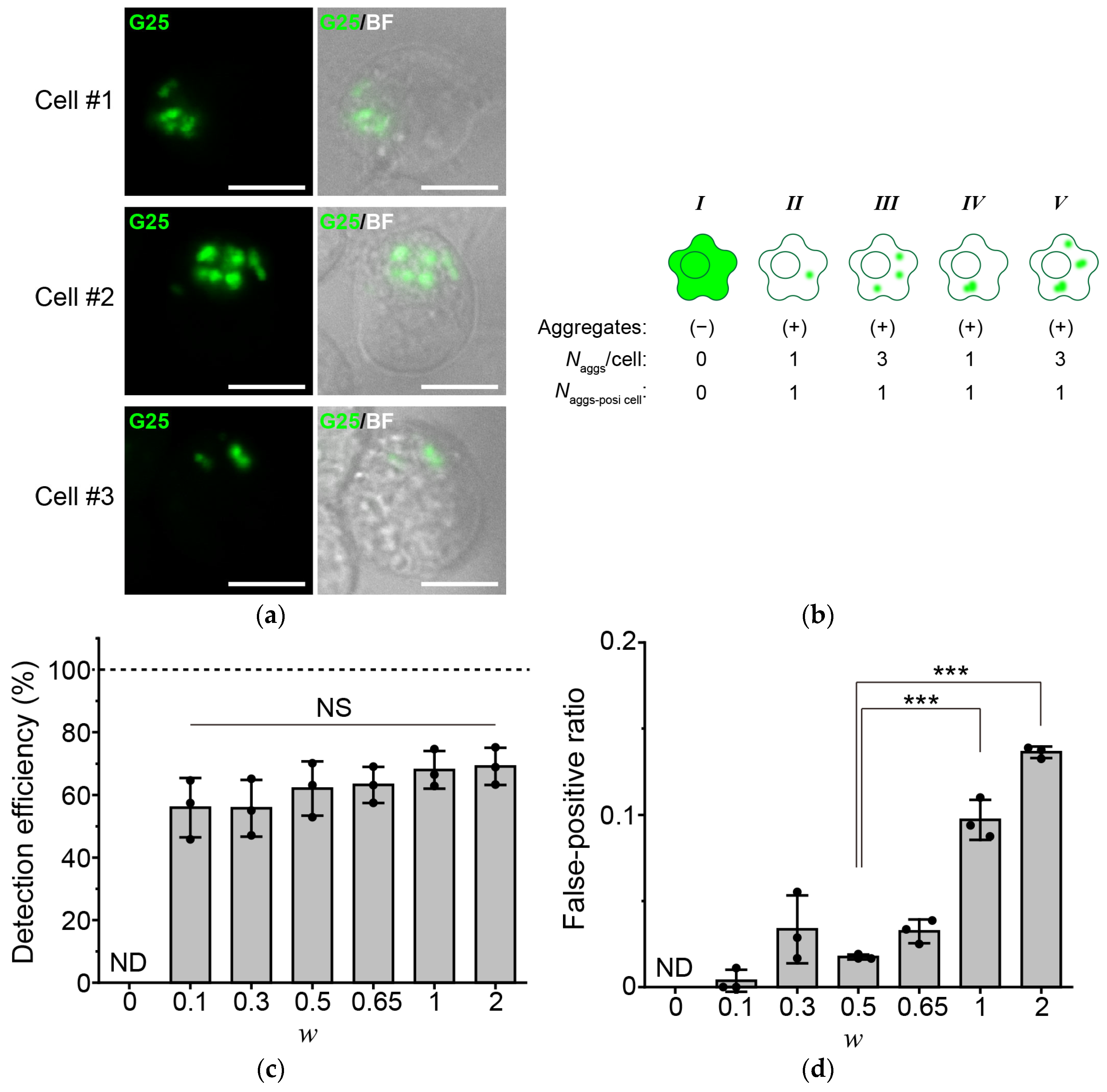
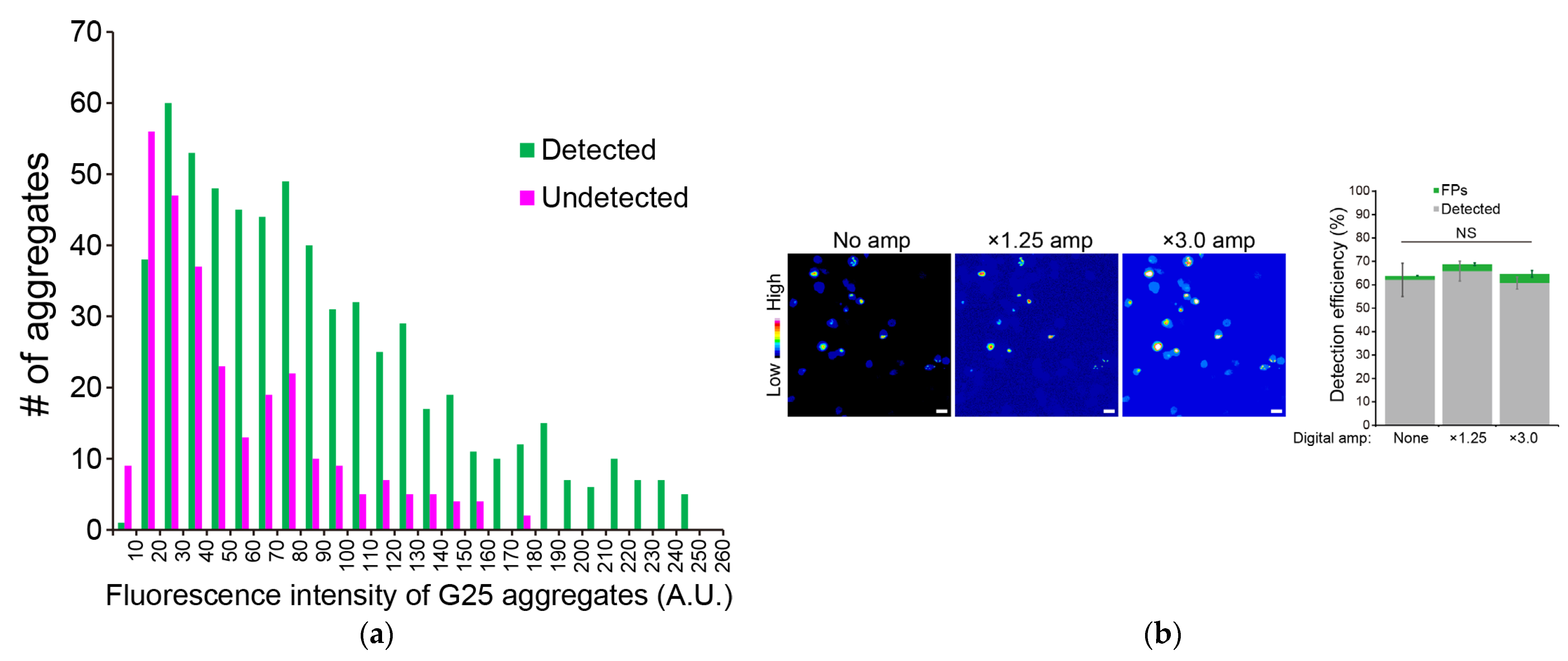
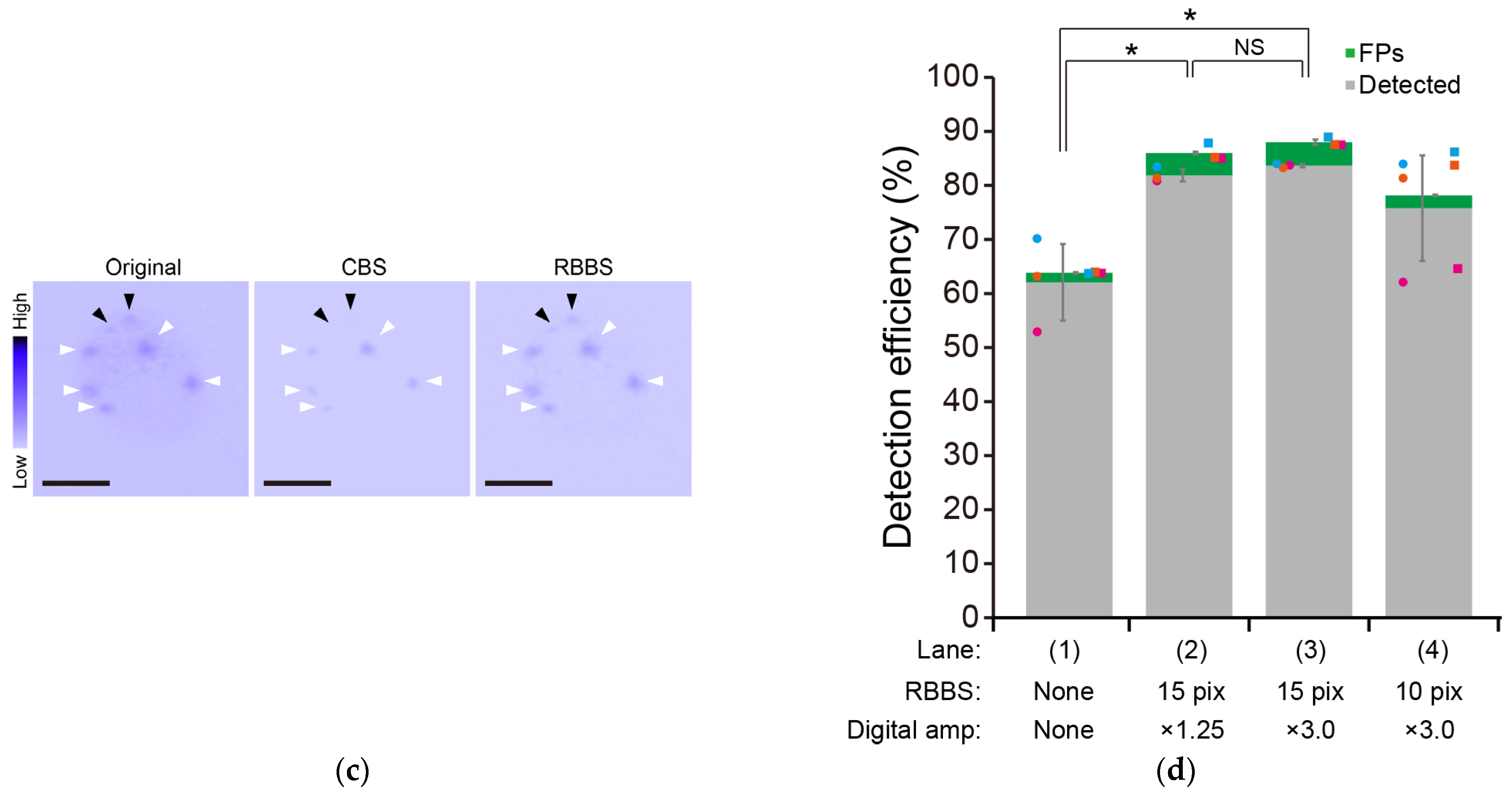
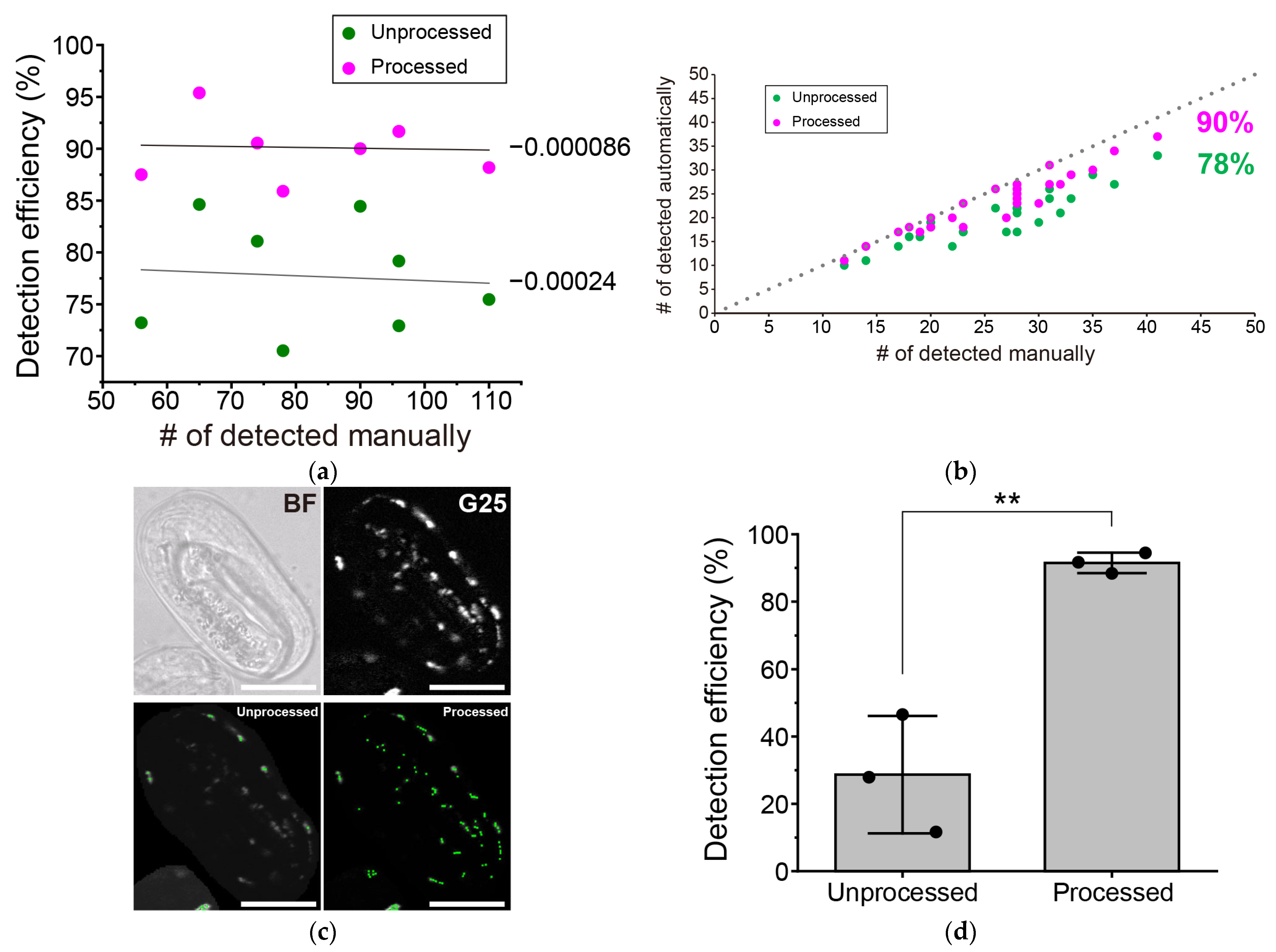
| w | Tratio local | Tratio global | TCV |
|---|---|---|---|
| 0 | 2.38 | 9.99 | 0.02 |
| 0.1 | 1.00 | 2.79 | 0.35 |
| 0.3 | 1.15 | 1.56 | 0.17 |
| 0.5 | 1.00 | 2.22 | 0.32 |
| 0.65 | 1.06 | 2.22 | 0.32 |
| 1.0 | 1.06 | 1.80 | 0.32 |
| 2.0 | 1.03 | 1.80 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sogawa, S.; Sasaki, K.; Kitamura, A. Optimizing Automated Detection for Cytoplasmic TDP25 Aggregates in Fluorescence Imaging. Spectrosc. J. 2025, 3, 18. https://doi.org/10.3390/spectroscj3020018
Sogawa S, Sasaki K, Kitamura A. Optimizing Automated Detection for Cytoplasmic TDP25 Aggregates in Fluorescence Imaging. Spectroscopy Journal. 2025; 3(2):18. https://doi.org/10.3390/spectroscj3020018
Chicago/Turabian StyleSogawa, Sumire, Kotetsu Sasaki, and Akira Kitamura. 2025. "Optimizing Automated Detection for Cytoplasmic TDP25 Aggregates in Fluorescence Imaging" Spectroscopy Journal 3, no. 2: 18. https://doi.org/10.3390/spectroscj3020018
APA StyleSogawa, S., Sasaki, K., & Kitamura, A. (2025). Optimizing Automated Detection for Cytoplasmic TDP25 Aggregates in Fluorescence Imaging. Spectroscopy Journal, 3(2), 18. https://doi.org/10.3390/spectroscj3020018








