Abstract
Fluorescence resonance energy transfer (FRET)-PCR is widely recognized for its high sensitivity and specificity in pathogen detection. However, there are some gaps in probe design when it is applied for simultaneous detection and differentiation of similar targets. This study aims to investigate the effects of the numbers and position of nucleotide mismatches (NM) in probe on PCR efficiency and melting temperature (Tm). The results indicated that NM at the center reduces amplification efficiency and Tm more significantly than NM at the 5′-terminal or 3′-terminal of the probe.
PCR is widely utilized for its high sensitivity and specificity in amplifying targets, particularly in pathogen detection [1]. However, discriminating closely related organisms poses significant challenges. It is important to differentiate the cross-species (e.g., five Ebola virus species) and cross-genus (e.g., Babesia and Theileria) pathogens as they significantly differ in pathogenicity and multiple species/genus can be present in a geographical area [2,3]. Addressing this challenge, researchers have developed a cost-effective and convenient method utilizing the melting temperature (Tm) from fluorescence resonance energy transfer (FRET)-PCR. The FRET-qPCR system includes specific primers (similar to conventional PCR) and a pair of probes (1–5 bp apart) located internally to the primers. A donor fluorophore (e.g., 6-Carboxyfluorescein (6-FAM)) was labeled at the 3′-terminal of the upstream probe and an acceptor fluorophore (e.g., LC®Red640) was labeled at the 5′-terminal of the downstream probe. During annealing, both probes bind to the template, positioning the fluorophores close together. Therefore, the acceptor fluorophore can absorb the energy of the donor fluorophore, leading to its excitation. On the opposite one, during denaturation, the probes dissociate, preventing the excitation of the acceptor fluorophore. FRET probe signals are real-time signals during annealing, directly corresponding to the amount of template. Base mismatches introduced in the probe-binding region will decrease the Tm, enabling the differentiation of pathogen genotypes. However, the optimal design of probes remains uncertain.
To tackle this uncertainty, we deliberately designed varying numbers of nucleotide mismatches (NM) at the 5′-terminal (1-6 consecutive NM), center (4–6 consecutive NM), or 3′-terminal (1-6 consecutive NM) of the 6-FAM-labeled probes, respectively, in a well-evaluated Chlamydia pneumoniae 23S rRNA FRET-PCR assay [4]. Subsequently, 16 6-FAM-labeled probes (1 perfect match and 15 with NM, emitted at 510 nm), one LC®Red640-labeled probe (excited at 498 nm), and the primers were synthesized using Integrated DNA Technologies (Figure 1) (Coralville, IA, USA).
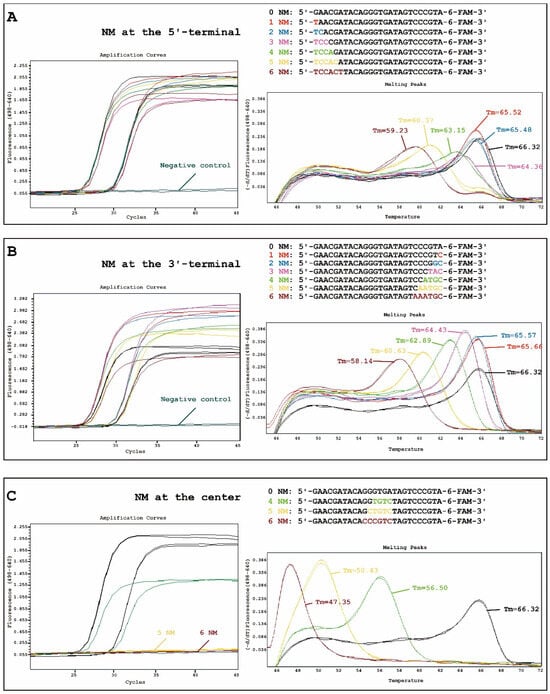
Figure 1.
Amplification (left) and melting (right) curves of the fluorescence resonance energy transfer (FRET)-qPCR when amplifying 100 (~25 cycles) and 10 (~29 cycles) copy numbers of Chlamydia pneumoniae 23S rRNA with the introduction of 1–6 consecutive nucleotide mismatches (NM) at the 5′-terminal (A), 1–6 consecutive NM at the 3′-terminal (B), or 4–6 consecutive NM at the center (C) of the 6-Carboxyfluorescein (6-FAM)-labeled probes. The y-axis denotes the log scale of the ratios for the negative first derivative of the fluorescent signal in two channels (F4 as LC®Red640 /F1 as 6-FAM).
A FRET-PCR was performed to amplify varying copy numbers (100 and 10) of C. pneumoniae 23S rRNA using a LightCycler 480-II platform (Roche, Basel, Switzerland). The findings revealed that 1–6 consecutive NM at the 5′-terminal or 3′-terminal did not significantly reduce the amplification efficiency (Figure 1A,B). Interestingly, the introduction of 5–6 consecutive NM at the center of the probe resulted in the absence of an amplification curve (Figure 1C). These results differed significantly from the impact of NM in primers, where the NM at the 3′-terminal primarily reduce the amplification efficiency [5].
In addition, we found that 1–3 consecutive NM at the 5′-terminal and 1–2 NM at the 3′-terminal had little impact on the change in Tm. When the number of NM increases, the Tm decreases gradually. Importantly, NM introduced at the center (ΔTm (0–4 NMs) = 9.82 °C, ΔTm (4–5 NMs) = 6.07 °C, ΔTm (5–6 NMs) = 3.08 °C) had the most pronounced effect on the change in Tm compared to those at the 5′-terminal (ΔTm (0–4 NM) = 3.17 °C, ΔTm (4–5 NM) = 2.78 °C, ΔTm (5–6 NM) = 1.14 °C) and 3′-terminal (ΔTm (0–4 NM) = 3.43 °C, ΔTm (4–5 NM) = 2.26 °C, ΔTm (5–6 NM) = 2.49 °C) (Figure 1).
In cases where NM introduction is unavoidable, it is advisable to design them at either the 5′-terminal or 3′-terminal of the probe. For applications in differential diagnosis or molecular typing, the presence of ≥4 consecutive NM at the 5′-terminal or ≥3 consecutive NM at the 3′-terminal of the probe is required to significantly reduce the Tm. Although the NM at the center of the probe has a greater impact on the Tm, it is not recommended to introduce them in order to avoid the disappearance of amplification curves. The findings presented herein significantly contribute to the rational design of FRET-PCR probes, facilitating the convenient differentiation of similar targets without the necessity of DNA sequencing.
Author Contributions
Conceptualization, C.W. and K.H.; methodology, K.H., J.L., C.Y. and Y.Y.; formal analysis, K.H. and C.W.; investigation, K.H., J.L. and C.W.; writing—original draft preparation, K.H. and Y.Y.; writing—review and editing, C.W. and Y.Y.; supervision, C.W. and Y.Y.; project administration, C.W. and Y.Y.; funding acquisition, J.L., C.Y. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key Research and Development Program of China (2023YFD1800503), Key R&D projects of Jiangxi Academy of Sciences (2023YSBG21002), College Students’ Innovative Entrepreneurial Training Plan Program of Yangzhou University (XCX20230774), Young Elite Scientists Sponsorship Program by CAST (2022QNRC001), and Young Elite Scientists Sponsorship Program of Jiangsu Province (TJ-2022-031).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
No authors declared any potential conflicts of interest.
Abbreviations
PCR, Polymerase chain reaction; Tm, melting temperature; FRET, fluorescence resonance energy transfer; FAM, Carboxyfluorescein; rRNA, ribosomal RNA; ΔTm, the change in melting temperature; NM, nucleotide mismatches; 6-FAM, 6-Carboxyfluorescein.
References
- Parida, M.; Shukla, J.; Sharma, S.; Lakshmana Rao, P.V. Rapid and Real-time Detection of Human Viral Infections: Current Trends and Future Perspectives. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2012, 82, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Malvy, D.; McElroy, A.K.; de Clerck, H.; Gunther, S.; van Griensven, J. Ebola virus disease. Lancet 2019, 393, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda, J.; Olvera-Ramirez, A.; Aguilar-Tipacamu, G.; Canto, G.J. Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 2012, 19, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhang, J.; Li, J.; Qiu, H.; Wei, L.; Yang, Y.; Wang, C. Exploring the Impact of Primer-Template Mismatches on PCR Performance of DNA Polymerases Varying in Proofreading Activity. Genes 2024, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Lefever, S.; Pattyn, F.; Hellemans, J.; Vandesompele, J. Single-nucleotide polymorphisms and other mismatches reduce performance of quantitative PCR assays. Clin. Chem. 2013, 59, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).