How the Inclusion of Pigeon Pea in Beef Cattle Diets Affects CH4 Intensity: An In Vitro Fermentation Assessment
Abstract
1. Introduction
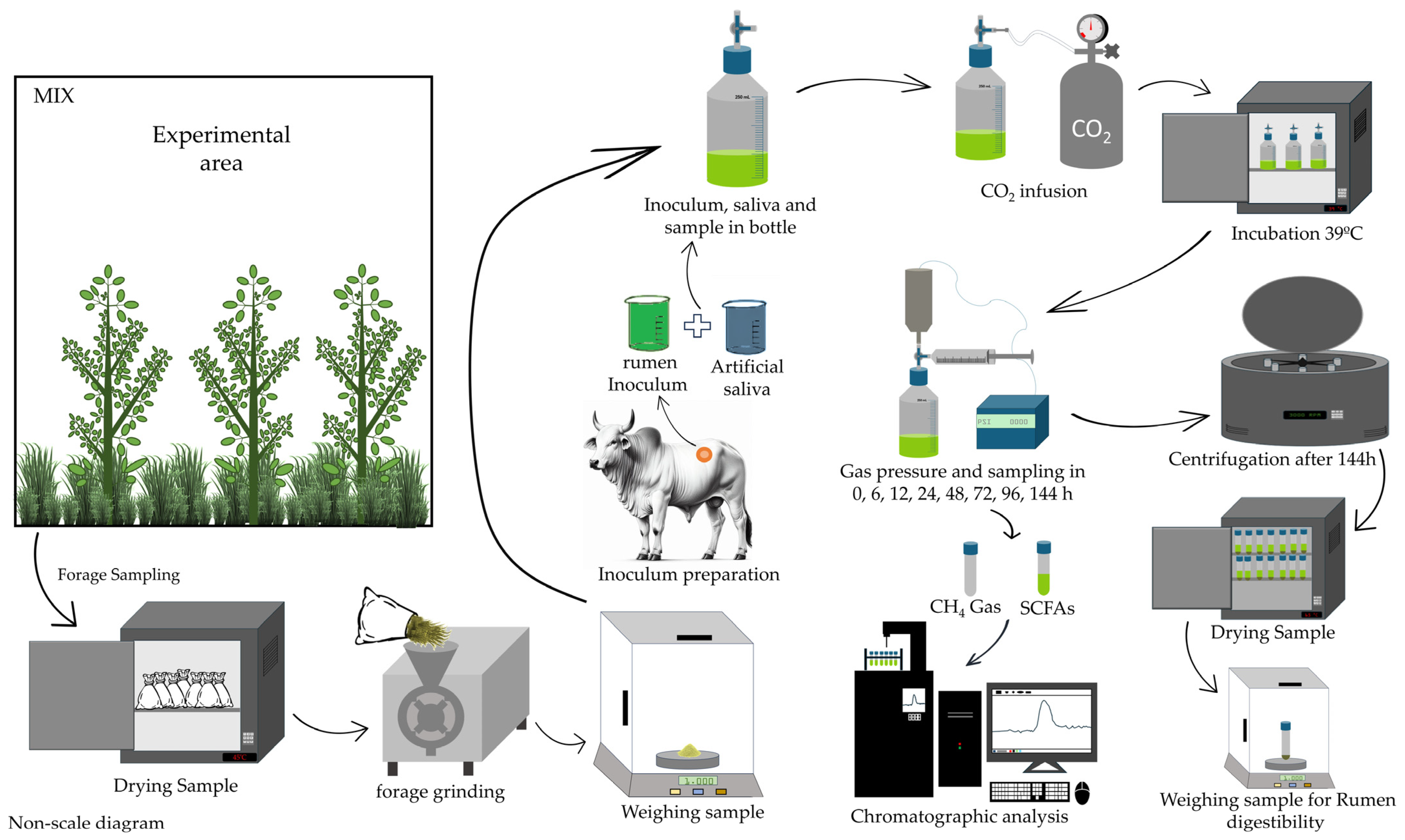
2. Materials and Methods
2.1. Planning, Location, and Pastures
2.2. In Vitro Fermentation
2.3. In Vitro Digestibility
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feltran-Barbieri, R.; Féres, J.G. Degraded Pastures in Brazil: Improving Livestock Production and Forest Restoration. R. Soc. Open Sci. 2021, 8, 201854. [Google Scholar] [CrossRef]
- Homem, B.G.C.; Borges, L.P.C.; de Lima, I.B.G.; Guimarães, B.C.; Spasiani, P.P.; Ferreira, I.M.; Meo-Filho, P.; Berndt, A.; Alves, B.J.R.; Urquiaga, S.; et al. Forage Peanut Legume as a Strategy for Improving Beef Production without Increasing Livestock Greenhouse Gas Emissions. Animal 2024, 18, 101158. [Google Scholar] [CrossRef]
- Furtado, A.J.; Abdalla Filho, A.L.; Bruno, J.F.; Neto, R.P.; Lobo, A.A.G.; da Silva, G.V.; Junior, F.P.; Alves, T.C.; Berndt, A.; de Faria Pedroso, A.; et al. Pigeon Pea Intercropped with Tropical Pasture as a Mitigation Strategy for Enteric Methane Emissions of Nellore Steers. Animals 2023, 13, 1323. [Google Scholar] [CrossRef]
- Boddey, R.M.; Casagrande, D.R.; Homem, B.G.; Alves, B.J. Forage legumes in grass pastures in tropical Brazil and likely impacts on greenhouse gas emissions: A review. Grass Forage Sci. 2020, 75, 357–371. [Google Scholar] [CrossRef]
- Vastolo, A.; Serrapica, F.; Cavallini, D.; Fusaro, I.; Atzori, A.S.; Todaro, M. Alternative and novel livestock feed: Reducing environmental impact. Front. Vet. Sci. 2024, 11, 1441905. [Google Scholar] [CrossRef]
- Pasquini Neto, R.; Furtado, A.J.; da Silva, G.V.; Lobo, A.A.G.; Abdalla Filho, A.L.; Brunetti, H.B.; Bosi, C.; Pedroso, A.D.F.; Pezzopane, J.R.M.; Oliveira, P.P.A.; et al. Forage Accumulation and Nutritive Value in Extensive, Intensive, and Integrated Pasture-Based Beef Cattle Production Systems. Crop Pasture Sci. 2024, 75, CP24043. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; de Oliveira, P.P.A.; Pedroso, A.d.F.; Bonani, W.L.; Bosi, C.; Brunetti, H.B.; Neto, R.P.; Furtado, A.J.; Rodrigues, P.H.M. Intercropping of Tropical Grassland and Pigeon Pea: Impact on Microclimate, Soil Water, and Forage Production. Rangel. Ecol. Manag. 2024, 95, 1–10. [Google Scholar] [CrossRef]
- Takahashi, L.S.; da Costa, R.L.D.; Pérez-Marquez, S.; Niderkorn, V.; Lugo, F.C.; Abdalla, A.L. Assessing Nutritional Quality and Gas Production Kinetics: Incorporating Tithonia Diversifolia into Sugarcane Silage. Agrofor. Syst. 2024, 1, 1–12. [Google Scholar] [CrossRef]
- Kelln, B.M.; Penner, G.B.; Acharya, S.N.; McAllister, T.A.; McKinnon, J.J.; Saleem, A.M.; Biligetu, B.; Lardner, H.A. Effect of Mixtures of Legume Species on Ruminal Fermentation, Methane, and Microbial Nitrogen Production in Batch and Continuous Culture (RUSITEC) Systems. Can. J. Anim. Sci. 2023, 103, 326–337. [Google Scholar] [CrossRef]
- Ligoski, B.; Gonçalves, L.F.; Claudio, F.L.; Alves, E.M.; Krüger, A.M.; Bizzuti, B.E.; Lima, P.d.M.T.; Abdalla, A.L.; Paim, T.d.P. Silage of Intercropping Corn, Palisade Grass, and Pigeon Pea Increases Protein Content and Reduces In Vitro Methane Production. Agronomy 2020, 10, 1784. [Google Scholar] [CrossRef]
- Suassuna, J.M.A.; de Medeiros, A.N.; Oliveira, B.D.; Rufino, O.A. Métodos in situ e in vitro utilizados para avaliação de alimentos e dietas de ruminantes. Pubvet 2021, 15, 188. [Google Scholar] [CrossRef]
- Mott, G.O.; Lucas, H.L. The design, conduct and interpretation of grazing trials on cultivated and improved pastures. In Proceedings of the 6th International Grassland Congress, Philadelphia, PA, USA, 17–23 August 1952; pp. 1380–1395. [Google Scholar]
- Sollenberger, L.E.; Cherney, D. Evaluating forage production and quality. In Forages: The Science of Grassland Agriculture, 5th ed.; Barnes, R.F., Nelson, C.J., Miller, D., Eds.; Iowa State University Press: Ames, IA, USA, 1995; Volume 2, pp. 97–110. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC International: Arlington, TX, USA, 1990. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage fiber analysis. (Apparatus, reagents, procedures and some applications). In Agriculture Handbook, United States Department of Agriculture; U.S. Agricultural Research Service: Washington, DC, USA, 1970; p. 20. [Google Scholar]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Medeiros, S.R.; Gomes, R.C.; Bungenstab, D.J. Nutrição de Bovinos de Corte: Fundamentos e Aplicações; Embrapa: Brasília, Brazil, 2015; p. 176. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1010951 (accessed on 24 September 2024).
- Bueno, I.C.S.; Cabral Filho, S.L.S.; Gobbo, S.P.; Louvandini, H.; Vitti, D.M.S.S.; Abdalla, A.L. Influence of Inoculum Source in a Gas Production Method. Anim. Feed Sci. Technol. 2005, 123–124, 95–105. [Google Scholar] [CrossRef]
- McDougall, E. Studies on Ruminant Saliva. The Composition and Output of Sheep’s Saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef]
- Kamiński, M.; Kartanowicz, R.; Jastrzębski, D.; Kamiński, M.M. Determination of carbon monoxide, methane and carbon dioxide in refinery hydrogen gases and air by gas chromatography. J. Chromatogr. A 2003, 989, 277–283. [Google Scholar] [CrossRef]
- Perna Junior, F.; Cassiano, E.C.O.; Martins, M.F.; Romero, L.A.; Zapata, D.C.V.; Pinedo, L.A.; Marino, C.T.; Rodrigues, P.H.M. Effect of Tannins-Rich Extract from Acacia Mearnsii or Monensin as Feed Additives on Ruminal Fermentation Efficiency in Cattle. Livest. Sci. 2017, 203, 21–29. [Google Scholar] [CrossRef]
- France, J.; Kebreab, E. Mathematical Modelling in Animal Nutrition; CABI: Wallingford, UK, 2008; ISBN 9781845933548. [Google Scholar]
- Szegö, G. Orthogonal Polynomials, 4th ed.; American Mathematical Society: Providence, RI, USA, 1975; ISBN 0821810235. [Google Scholar]
- Aung, M.; Thein, S.M.; Phaw, N.C.; Phyo, T.H.; Phyo, Y.P.; Phyo, N.Y.; Van Bik, L.; Bo, A.B.; Maw, H.; Maw, A.M.; et al. In Vitro Fermentation of Grass Based Diet Supplemented with Two Different Tree Legume Forages in Ruminant. Adv. Anim. Vet. Sci. 2019, 7, 272–279. [Google Scholar] [CrossRef]
- Holguín, V.A.; Cuchillo-Hilario, M.; Mazabel, J.; Quintero, S.; Mora-Delgado, J. Efecto de La Mezcla Ensilada de Penisetum Purpureum y Tithonia Diversifolia Sobre La Fermentación Ruminal in Vitro y Su Emisión de Metano En El Sistema RUSITEC. Rev. Mex. Cienc. Pecu. 2020, 11, 19–37. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S. Methane Mitigation from Ruminants Using Tannins and Saponins. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Dhanasekaran, D.K.; Dias-Silva, T.P.; Filho, A.L.A.; Sakita, G.Z.; Abdalla, A.L.; Louvandini, H.; Elghandour, M.M. Plants extract and bioactive compounds on rumen methanogenesis. Agrofor. Syst. 2020, 94, 1541–1553. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and Ruminant Nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. Comparison of fermentation of diets of variable composition and microbial populations in the rumen of sheep and Rusitec fermenters. I. Digestibility, fermentation parameters, and microbial growth. J. Dairy Sci. 2010, 93, 3684–3698. [Google Scholar] [CrossRef]
- Moore, k.J.; Jung, H.J.G. Lignin and fiber digestion. J. Range Manag. 2001, 54, 420–430. [Google Scholar] [CrossRef]
- Perna Junior, F.; Galbiatti Sandoval Nogueira, R.; Ferreira Carvalho, R.; Cuellar Orlandi Cassiano, E.; Mazza Rodrigues, P.H. Use of Tannin Extract as a Strategy to Reduce Methane in Nellore and Holstein Cattle and Its Effect on Intake, Digestibility, Microbial Efficiency and Ruminal Fermentation. J. Anim. Physiol. Anim. Nutr. 2023, 107, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Melesse, A.; Steingass, H.; Schollenberger, M.; Rodehutscord, M. Screening of common tropical grass and legume forages in Ethiopia for their nutrient composition and methane production profile in vitro. Trop. Grassl Forrajes Trop. 2017, 5, 163–175. [Google Scholar] [CrossRef]
- Quintero-Anzueta, S.; Molina-Botero, I.C.; Ramirez-Navas, J.S.; Rao, I.; Chirinda, N.; Barahona-Rosales, R.; Moorby, J.; Arango, J. Nutritional Evaluation of Tropical Forage Grass Alone and Grass-Legume Diets to Reduce in Vitro Methane Production. Front. Sustain. Food Syst. 2021, 5, 663003. [Google Scholar] [CrossRef]
- Tupy, O.; Oliveira, P.P.A.; Esteves, S.N.; Godoy, R. Análise de Risco Do Investimento Em Guandu Como Suplemento Volumoso Para Bovinos de Corte a Pasto. Inform. Econôm. 2023, 53, eie092020. [Google Scholar] [CrossRef]


| Inclusion of Pigeon Pea | Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CP (%) | NDF (%) | ADF (%) | Lig (%) | EE (%) | Ash (%) | NFC (%) | GE (cal/g) | CT * | |
| 0% | 8.8 | 69.1 | 38.5 | 3.5 | 2.0 | 8.7 | 11.5 | 3687.2 | 0.6 |
| 25% | 11.6 | 63.1 | 35.7 | 6.9 | 2.8 | 7.8 | 14.6 | 3875.9 | 12.7 |
| 50% | 14.4 | 57.2 | 32.9 | 10.3 | 3.6 | 7.0 | 17.8 | 4064.5 | 24.7 |
| 75% | 17.2 | 51.2 | 30.1 | 13.7 | 4.4 | 6.2 | 21.0 | 4253.1 | 36.7 |
| 100% | 20.0 | 45.3 | 27.3 | 17.1 | 5.3 | 5.4 | 24.2 | 4441.7 | 48.8 |
| Variables * | Pigeon Pea | Average | SEM | Statistical Probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | Linear | Quadratic | Cubic | Cubic Deviation | |||
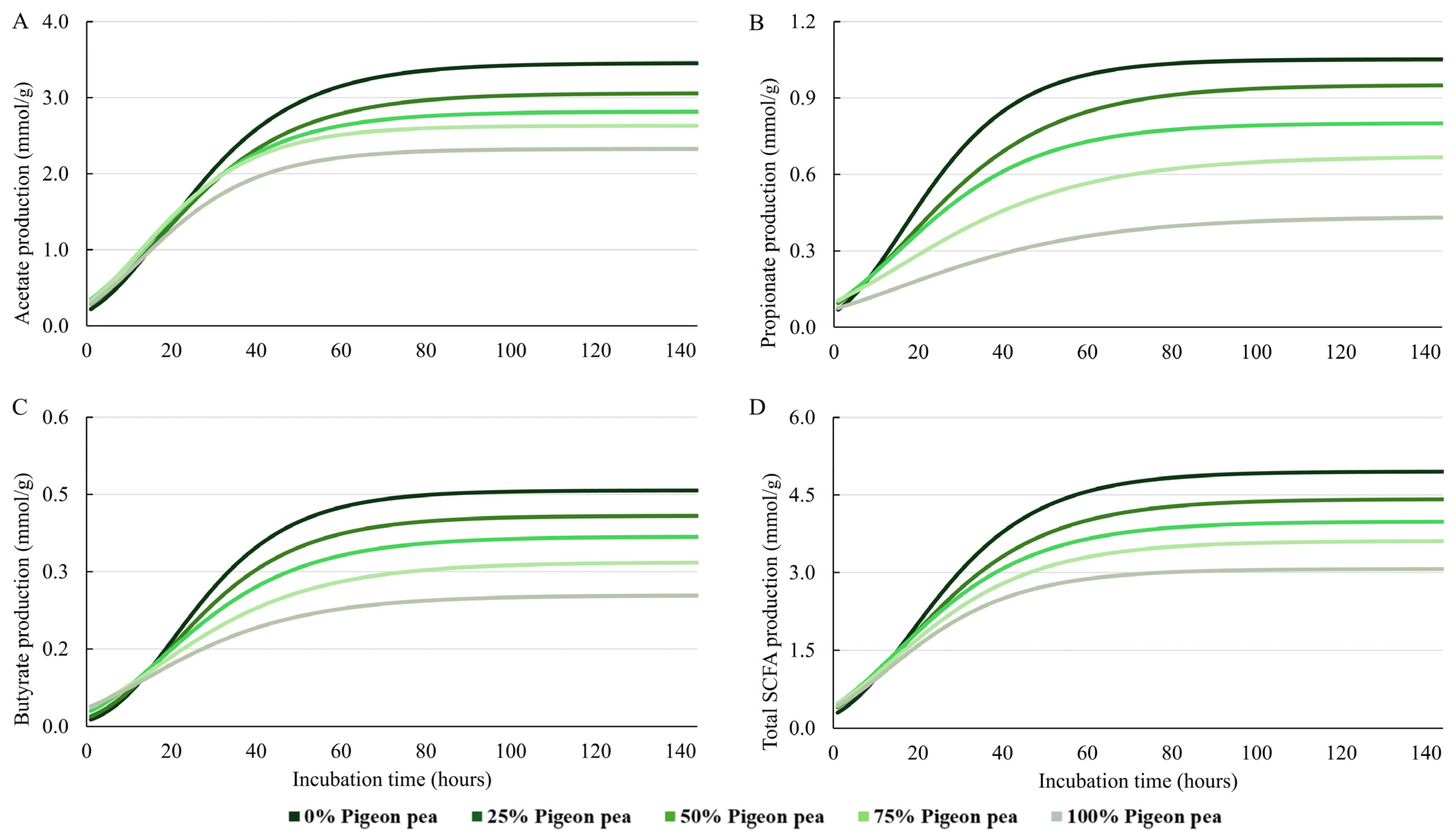
| Acetate | |||||||||||
| A | 3.46 | 3.06 | 2.82 | 2.63 | 2.33 | 2.86 | 0.10 | <0.0001 | 0.3037 | 0.1896 | 0.8685 |
| K | 0.058 | 0.055 | 0.058 | 0.064 | 0.063 | 0.060 | 0.001 | 0.0226 | 0.5154 | 0.1294 | 0.7382 |
| Ti | 18.58 | 16.68 | 13.63 | 13.05 | 12.50 | 14.89 | 0.67 | <0.0001 | 0.0515 | 0.5658 | 0.2714 |
| Yi | 1.27 | 1.13 | 1.04 | 0.97 | 0.86 | 1.05 | 0.04 | <0.0001 | 0.3037 | 0.1896 | 0.8685 |
| Propionate | |||||||||||
| A | 1.05 | 0.95 | 0.80 | 0.67 | 0.43 | 0.78 | 0.06 | <0.0001 | 0.1916 | 0.7199 | 0.6409 |
| K | 0.065 | 0.051 | 0.052 | 0.040 | 0.037 | 0.045 | 0.003 | 0.0002 | 0.5162 | 0.5510 | 0.1243 |
| Ti | 17.97 | 17.57 | 16.42 | 16.32 | 15.76 | 16.81 | 0.42 | 0.0914 | 0.8622 | 0.9126 | 0.7496 |
| Yi | 0.39 | 0.35 | 0.29 | 0.25 | 0.16 | 0.29 | 0.02 | <0.0001 | 0.1916 | 0.7199 | 0.6409 |
| Butyrate | |||||||||||
| A | 0.46 | 0.41 | 0.37 | 0.32 | 0.25 | 0.36 | 0.02 | <0.0001 | 0.2926 | 0.4416 | 0.8691 |
| K | 0.065 | 0.060 | 0.054 | 0.048 | 0.048 | 0.055 | 0.002 | 0.0029 | 0.3981 | 0.6034 | 0.8647 |
| Ti | 20.34 | 19.76 | 18.11 | 16.81 | 13.90 | 17.78 | 0.70 | 0.0002 | 0.2153 | 0.8495 | 0.6580 |
| Yi | 0.17 | 0.15 | 0.14 | 0.12 | 0.09 | 0.13 | 0.01 | <0.0001 | 0.2926 | 0.4416 | 0.8691 |
| Total SCFA | |||||||||||
| A | 4.95 | 4.42 | 3.99 | 3.61 | 3.07 | 4.01 | 0.18 | <0.0001 | 0.8639 | 0.2813 | 0.7696 |
| K | 0.060 | 0.054 | 0.054 | 0.053 | 0.058 | 0.056 | 0.001 | 0.4544 | 0.0574 | 0.9270 | 0.5670 |
| Ti | 18.24 | 17.15 | 14.27 | 13.79 | 12.73 | 15.24 | 0.61 | <0.0001 | 0.3357 | 0.5699 | 0.2133 |
| Yi | 1.82 | 1.63 | 1.47 | 1.33 | 1.13 | 1.47 | 0.06 | <0.0001 | 0.8639 | 0.2813 | 0.7696 |
| Variables * | Pigeon Pea | Average | SEM | Statistical Probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | Linear | Quadratic | Cubic | Cubic Deviation | |||
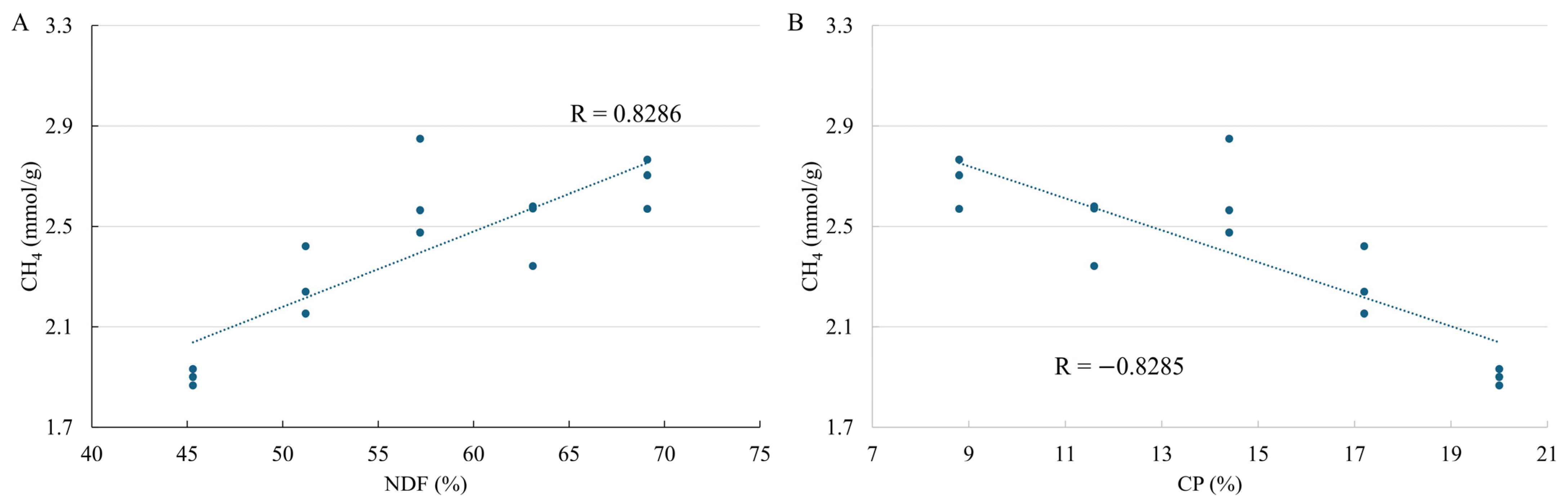
| CH4 (mmol/g) | |||||||||||
| A | 2.66 | 2.52 | 2.48 | 2.20 | 1.86 | 2.34 | 0.09 | <0.0001 | 0.0115 | 0.3993 | 0.3470 |
| K | 0.049 | 0.044 | 0.042 | 0.041 | 0.033 | 0.041 | 0.002 | 0.0008 | 0.6305 | 0.2012 | 0.8351 |
| Ti | 27.45 | 27.70 | 29.30 | 25.64 | 29.01 | 27.82 | 0.57 | 0.7841 | 0.8328 | 0.1689 | 0.0916 |
| Yi | 0.98 | 0.89 | 0.95 | 0.81 | 0.69 | 0.86 | 0.03 | <0.0001 | 0.0328 | 0.1834 | 0.4450 |
| Total gas production (mL/g) | |||||||||||
| A | 256.97 | 213.07 | 186.93 | 169.70 | 108.57 | 187.05 | 13.56 | <0.0001 | 0.4764 | 0.0609 | 0.5822 |
| K | 0.044 | 0.046 | 0.052 | 0.053 | 0.054 | 0.050 | 0.001 | 0.0026 | 0.3332 | 0.7515 | 0.3613 |
| Ti | 24.53 | 23.80 | 20.76 | 15.78 | 13.68 | 19.71 | 1.19 | <0.0001 | 0.1488 | 0.0665 | 0.5205 |
| Yi | 94.53 | 78.38 | 68.77 | 62.43 | 39.94 | 68.81 | 4.99 | <0.0001 | 0.4764 | 0.0609 | 0.5822 |
| Variables | Pigeon Pea | Average | SEM | Statistical Probabilities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 25% | 50% | 75% | 100% | Linear | Quadratic | Cubic | Cubic Deviation | |||
| DMD (%) | 61.7 | 54.8 | 48.4 | 45.2 | 36.6 | 46.2 | 0.86 | <0.0001 | 0.9766 | 0.0539 | 0.1511 |
| REL (%) | 30.3 | 29.9 | 32.9 | 32.7 | 32.1 | 31.5 | 0.43 | 0.0141 | 0.2110 | 0.0877 | 0.1626 |
| CH4/digestibility (mmol/g DMD) | 4.34 | 4.56 | 5.04 | 5.03 | 5.20 | 4.8 | 0.10 | 0.0001 | 0.1964 | 0.8013 | 0.1860 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtado, A.J.; Perna Junior, F.; Pasquini Neto, R.; Abdalla Filho, A.L.; Chamilete, S.A.M.; Oliveira, P.P.A.; Rodrigues, P.H.M. How the Inclusion of Pigeon Pea in Beef Cattle Diets Affects CH4 Intensity: An In Vitro Fermentation Assessment. Grasses 2024, 3, 253-263. https://doi.org/10.3390/grasses3040018
Furtado AJ, Perna Junior F, Pasquini Neto R, Abdalla Filho AL, Chamilete SAM, Oliveira PPA, Rodrigues PHM. How the Inclusion of Pigeon Pea in Beef Cattle Diets Affects CH4 Intensity: An In Vitro Fermentation Assessment. Grasses. 2024; 3(4):253-263. https://doi.org/10.3390/grasses3040018
Chicago/Turabian StyleFurtado, Althieres José, Flavio Perna Junior, Rolando Pasquini Neto, Adibe Luiz Abdalla Filho, Sophia Aparecida Morro Chamilete, Patrícia Perondi Anchão Oliveira, and Paulo Henrique Mazza Rodrigues. 2024. "How the Inclusion of Pigeon Pea in Beef Cattle Diets Affects CH4 Intensity: An In Vitro Fermentation Assessment" Grasses 3, no. 4: 253-263. https://doi.org/10.3390/grasses3040018
APA StyleFurtado, A. J., Perna Junior, F., Pasquini Neto, R., Abdalla Filho, A. L., Chamilete, S. A. M., Oliveira, P. P. A., & Rodrigues, P. H. M. (2024). How the Inclusion of Pigeon Pea in Beef Cattle Diets Affects CH4 Intensity: An In Vitro Fermentation Assessment. Grasses, 3(4), 253-263. https://doi.org/10.3390/grasses3040018






