Locomotory Effect of Reversibly Restraining the Pectines of Scorpions
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Care of Scorpions
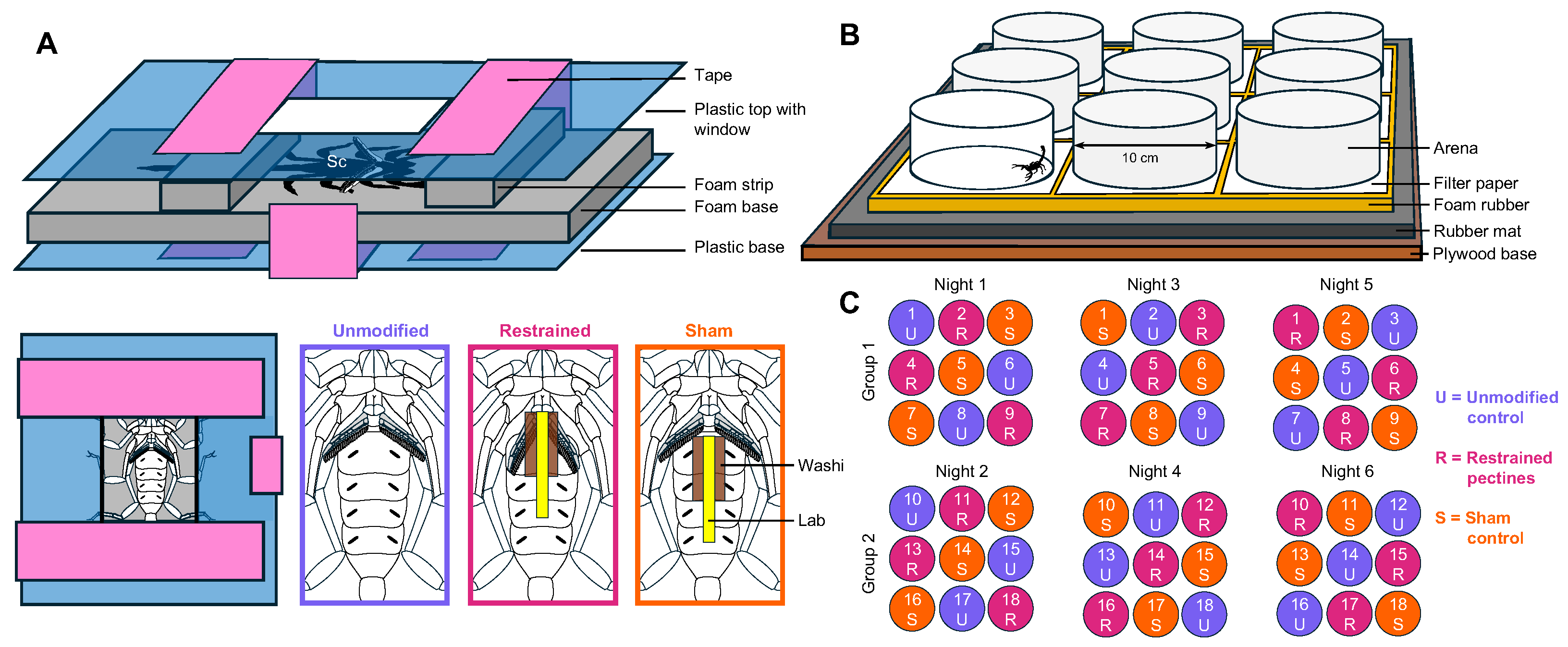
2.2. Immobilizing the Pectines
2.3. Behavioral Testing Setup
2.4. Trial Protocol
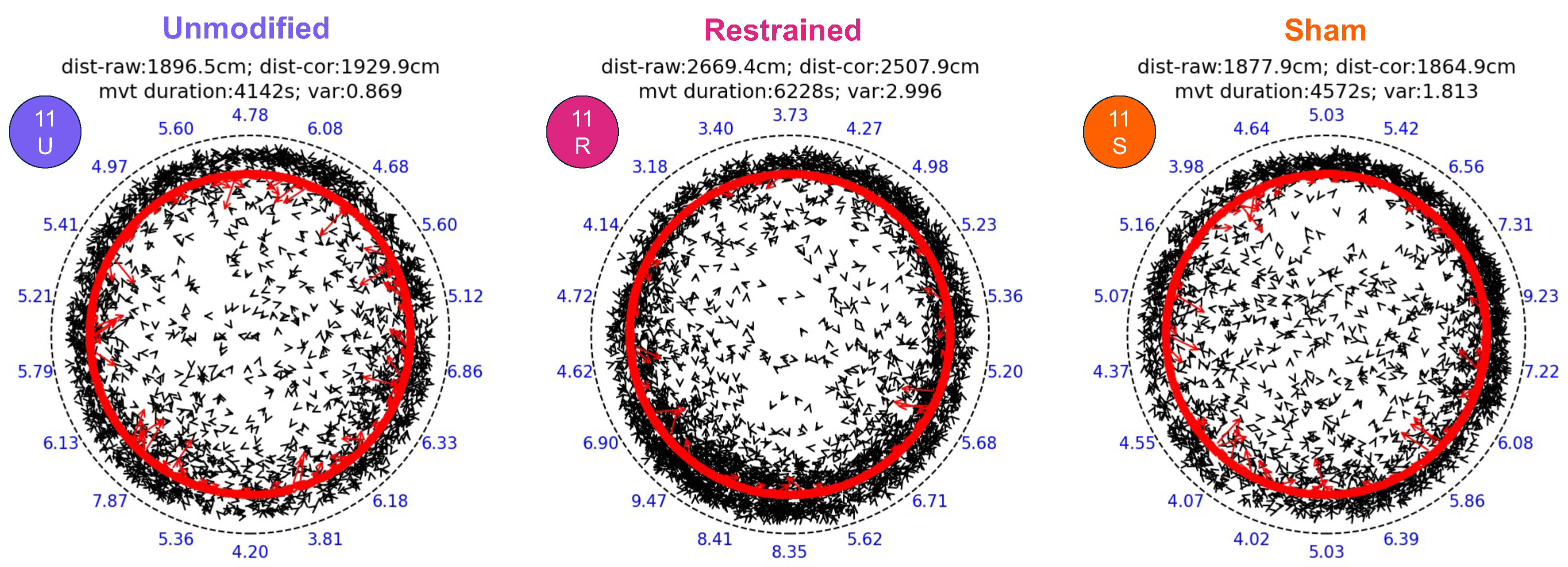
2.5. Video Processing and Statistical Analysis
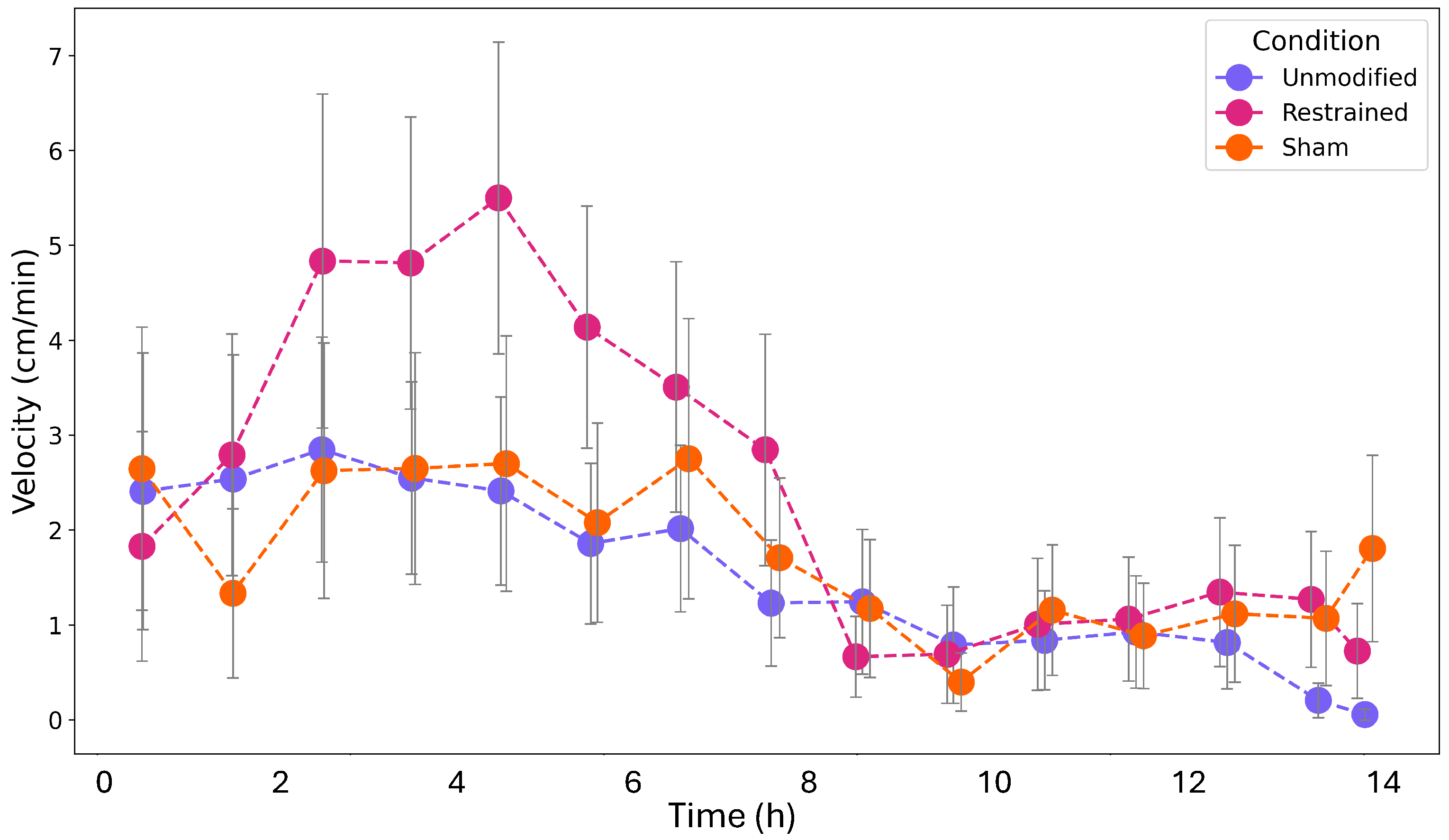
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abushama, F.T. On the behaviour and sensory physiology of the scorpion Leiurus quinquestriatus (H. & E.). Anim. Behav. 1964, 12, 140–153. [Google Scholar] [CrossRef]
- Brownell, P.H. Compressional and surface waves in sand: Used by desert scorpions to locate prey. Science 1977, 197, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Foelix, R.F.; Schabronath, J. Fine structure of scorpion sensory organs. I. Tarsal sensilla. Bull.-Br. Arachnol. Soc. 1983, 6, 53–67. [Google Scholar]
- Foelix, R.F.; Müller-Vorholt, G. The fine structure of scorpion sensory organs. II.Pecten sensilla. Bull. Br. Arachnol. Soc. 1983, 6, 68–74. [Google Scholar]
- Fleissner, G. Intracellular recordings of light responses from spiking and nonspiking cells in the median and lateral eyes of the scorpion. Naturwissenschaften 1985, 72, 46–48. [Google Scholar] [CrossRef]
- Meßlinger, K. Fine structure of scorpion trichobothria (Arachnida, Scorpiones). Zoomorphology 1987, 107, 49–57. [Google Scholar] [CrossRef]
- Ashford, K.; Blankenship, R.; Carpenter, W.; Wheeler, I.; Gaffin, D. Response of the eastern sand scorpion, Paruroctonus utahensis, to air movement from a moth analog. J. Arachnol. 2018, 46, 226–230. [Google Scholar] [CrossRef]
- Fet, V.; Brewer, M.S.; Soleglad, M.E.; Neff, D.P.A. Constellation array: A new sensory structure in scorpions (Arachnida: Scorpiones). Boletín Soc. Entomológica Aragonesa 2006, 1, 269–278. [Google Scholar]
- Cloudsley-Thompson, J. LXVII.—On the function of the pectines of scorpions. Ann. Mag. Nat. Hist. 1955, 8, 556–560. [Google Scholar] [CrossRef]
- Ivanov, V.; Balashov, Y. The structural and functional organization of the pectine in a scorpion Buthus eupeus Koch (Scorpiones, Buthidae) studied by electron microscopy. In The Fauna and Ecology of Arachnida; Trudy Zoological Institute: Leningrad, Russia, 1979; Volume 85, pp. 73–87. [Google Scholar]
- Gaffin, D.D.; Brownell, P.H. Response properties of chemosensory peg sensilla on the pectines of scorpions. J. Comp. Physiol. 1997, 181, 291–300. [Google Scholar] [CrossRef]
- Knowlton, E.D.; Gaffin, D.D. Functionally redundant peg sensilla on the scorpion pecten. J. Comp. Physiol. 2011, 197, 895. [Google Scholar] [CrossRef] [PubMed]
- Gaffin, D.D.; Brownell, P.H. Evidence of chemical signaling in the sand scorpion, Paruroctonus mesaensis (Scorpionida: Vaejovidae). Ethology 1992, 91, 59–69. [Google Scholar] [CrossRef]
- Gaffin, D.; Brownell, P. Chemosensory behavior and physiology. In Scorpion Biology and Research; Oxford University Press: Oxford, UK, 2001; pp. 184–203. [Google Scholar]
- Melville, J.M.; Tallarovic, S.K.; Brownell, P.H. Evidence of mate trailing in the giant hairy desert scorpion, Hadrurus arizonensis (Scorpionida, Iuridae). J. Insect Behav. 2003, 16, 97–115. [Google Scholar] [CrossRef]
- Taylor, M.S.; Cosper, C.R.; Gaffin, D.D. Behavioral evidence of pheromonal signaling in desert grassland scorpions Paruroctonus utahensis. J. Arachnol. 2012, 40, 240–244. [Google Scholar] [CrossRef]
- Krapf, D. Contact chemoreception of prey in hunting scorpions (Arachnida: Scorpiones). Zool. Anz. 1986, 217, 119–129. [Google Scholar]
- Alexander, A.J. The Courtship and Mating of the Scorpion, Opisthophthalmus latimanus. Proc. Zool. Soc. Lond. 1957, 128, 529–544. [Google Scholar] [CrossRef]
- Alexander, A.J. Courtship and mating in the buthid scorpions. Proc. Zool. Soc. Lond. 1959, 133, 145–169. [Google Scholar] [CrossRef]
- Drozd, D.; Wolf, H.; Stemme, T. Mechanosensory pathways of scorpion pecten hair sensillae—adjustment of body height and pecten position. J. Comp. Neurol. 2022, 530, 2918–2937. [Google Scholar] [CrossRef]
- Peeples, H.M.; Gaffin, D.D. An assessment of the mechanosensory responses of peg sensilla on scorpion pectines. J. Arachnol. 2024, 52, 1–8. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Curry, C.M. Arachnid navigation—A review of classic and emerging models. J. Arachnol. 2020, 48, 1–25. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Muñoz, M.G.; Hoefnagels, M.H. Evidence of learning walks related to scorpion home burrow navigation. J. Exp. Biol. 2022, 225, jeb243947. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Escobar, J.; Hebets, E.A.; Bingman, V.P.; Wiegmann, D.D.; Gaffin, D.D. Comparative biology of spatial navigation in three arachnid orders (Amblypygi, Araneae, and Scorpiones). J. Comp. Physiol. 2023, 209, 747–779. [Google Scholar] [CrossRef] [PubMed]
- Hladik, J.; Bailer, Y.; Wolf, H.; Stemme, T. Shelter selection in females of two scorpion species depends on shelter size and scent. J. Comp. Physiol. 2024, 211, 163–183. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Walvoord, M.E. Scorpion peg sensilla: Are they the same or are they different? Euscorpius 2004, 17, 7–15. [Google Scholar]
- Prévost, E.D.; Stemme, T. Non-visual homing and the current status of navigation in scorpions. Anim. Cogn. 2020, 23, 1215–1234. [Google Scholar] [CrossRef]

| Normality and Sphericity Assumptions Met? | Degrees of Freedom | Repeated Measures ANOVA (Parametric) F Value | ANOVA Effect Size Measure (2) | Friedman Test (Nonparametric) W Value | p Value | |
|---|---|---|---|---|---|---|
| Total distance | yes | 2 | 5.4792 | 0.1324 | --- | 0.0094 *** |
| restrained vs. sham | 15 | 0.0474 ** | ||||
| restrained vs. unmodified | 15 | 0.0160 ** | ||||
| sham vs. unmodified | 15 | 1.0000 | ||||
| Movement duration | no | 2 | --- | 0.5430 | 0.0002 **** | |
| restrained vs. sham | 15 | 0.0369 ** | ||||
| restrained vs. unmodified | 15 | 0.0051 *** | ||||
| sham vs. unmodified | 15 | 1.0000 | ||||
| Sector occupancy variance | no | 2 | --- | 0.0039 | 0.9394 | |
| Mean velocity | yes | 2 | 2.9167 | 0.0476 | --- | 0.0696 * |
| Median velocity | yes | 2 | 1.2430 | 0.0217 | --- | 0.3029 |
| Mode velocity | no | 2 | --- | 0.0273 | 0.6456 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaffin, D.D.; Gálvez Falcón, S.E.; Hoefnagels, M.H. Locomotory Effect of Reversibly Restraining the Pectines of Scorpions. Arthropoda 2025, 3, 12. https://doi.org/10.3390/arthropoda3030012
Gaffin DD, Gálvez Falcón SE, Hoefnagels MH. Locomotory Effect of Reversibly Restraining the Pectines of Scorpions. Arthropoda. 2025; 3(3):12. https://doi.org/10.3390/arthropoda3030012
Chicago/Turabian StyleGaffin, Douglas D., Sofía E. Gálvez Falcón, and Mariëlle H. Hoefnagels. 2025. "Locomotory Effect of Reversibly Restraining the Pectines of Scorpions" Arthropoda 3, no. 3: 12. https://doi.org/10.3390/arthropoda3030012
APA StyleGaffin, D. D., Gálvez Falcón, S. E., & Hoefnagels, M. H. (2025). Locomotory Effect of Reversibly Restraining the Pectines of Scorpions. Arthropoda, 3(3), 12. https://doi.org/10.3390/arthropoda3030012






