Spatio-Temporal Distribution and Population Dynamics of Two Sympatric Species: The Rock Shrimps Sicyonia dorsalis Kingsley, 1878 and Sicyonia typica (Boeck, 1864) (Penaeoidea: Sicyoniidae) on the Coast of Ilhéus, Bahia, Northeastern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Data Analyses
3. Results
3.1. Environmental Factors
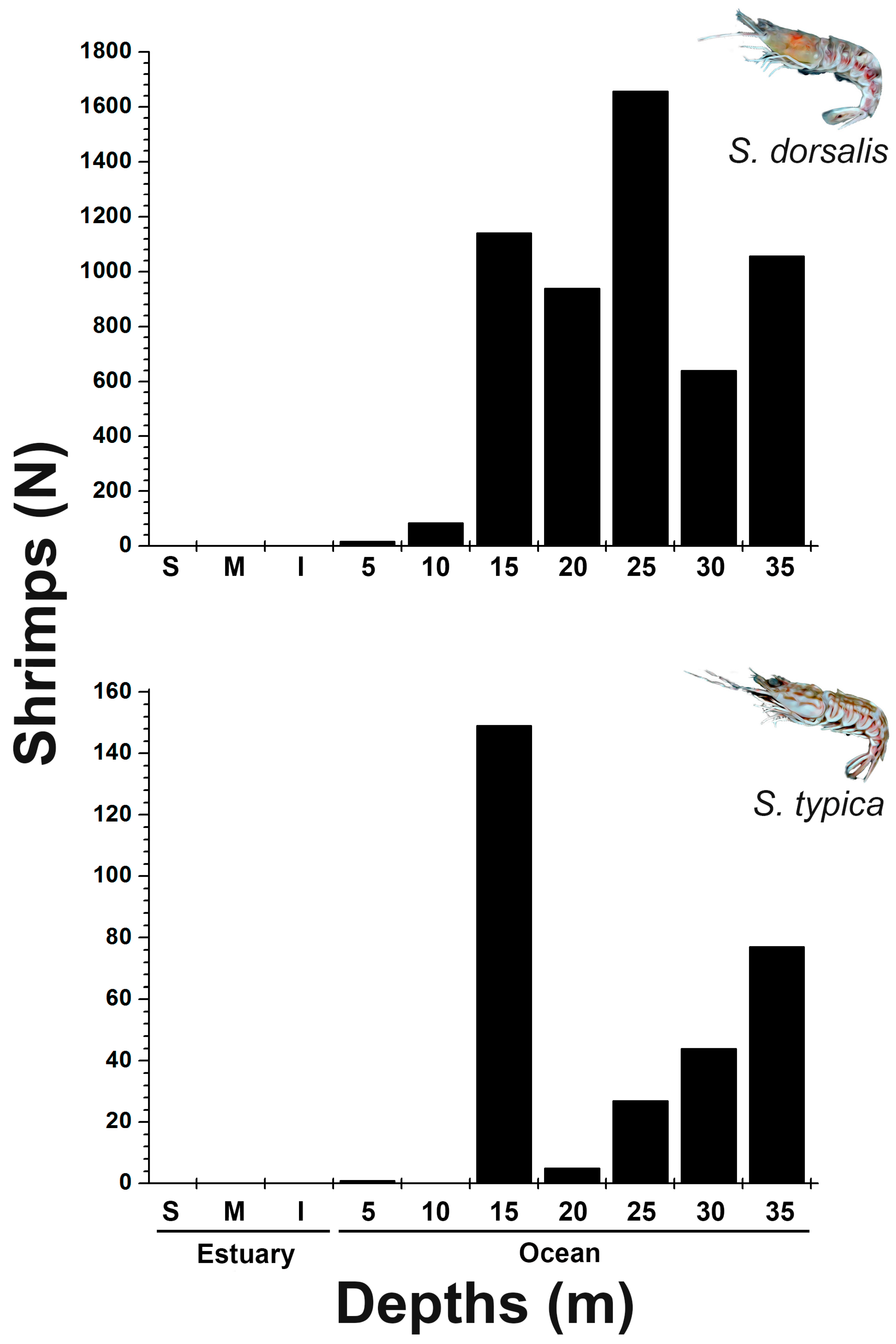
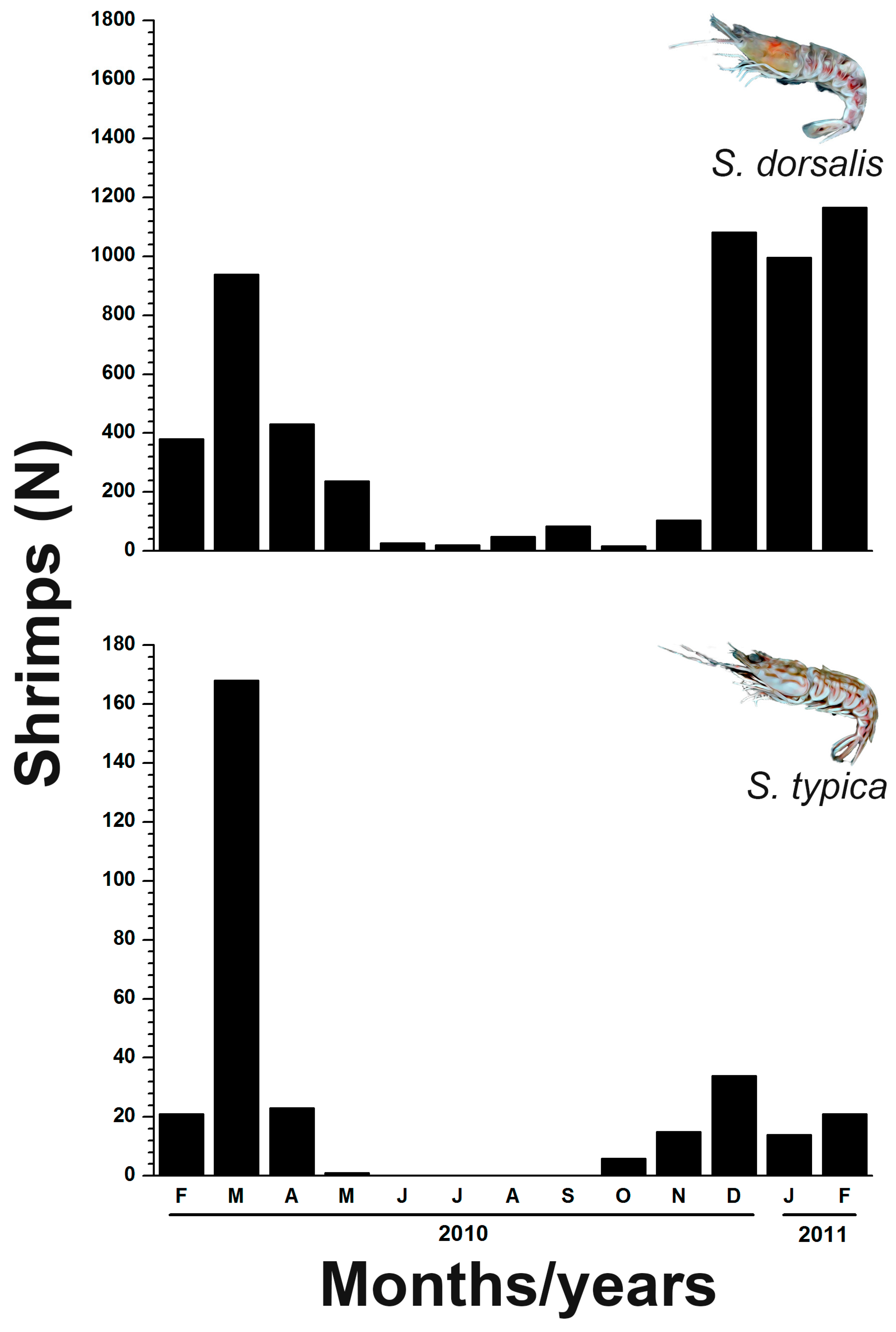
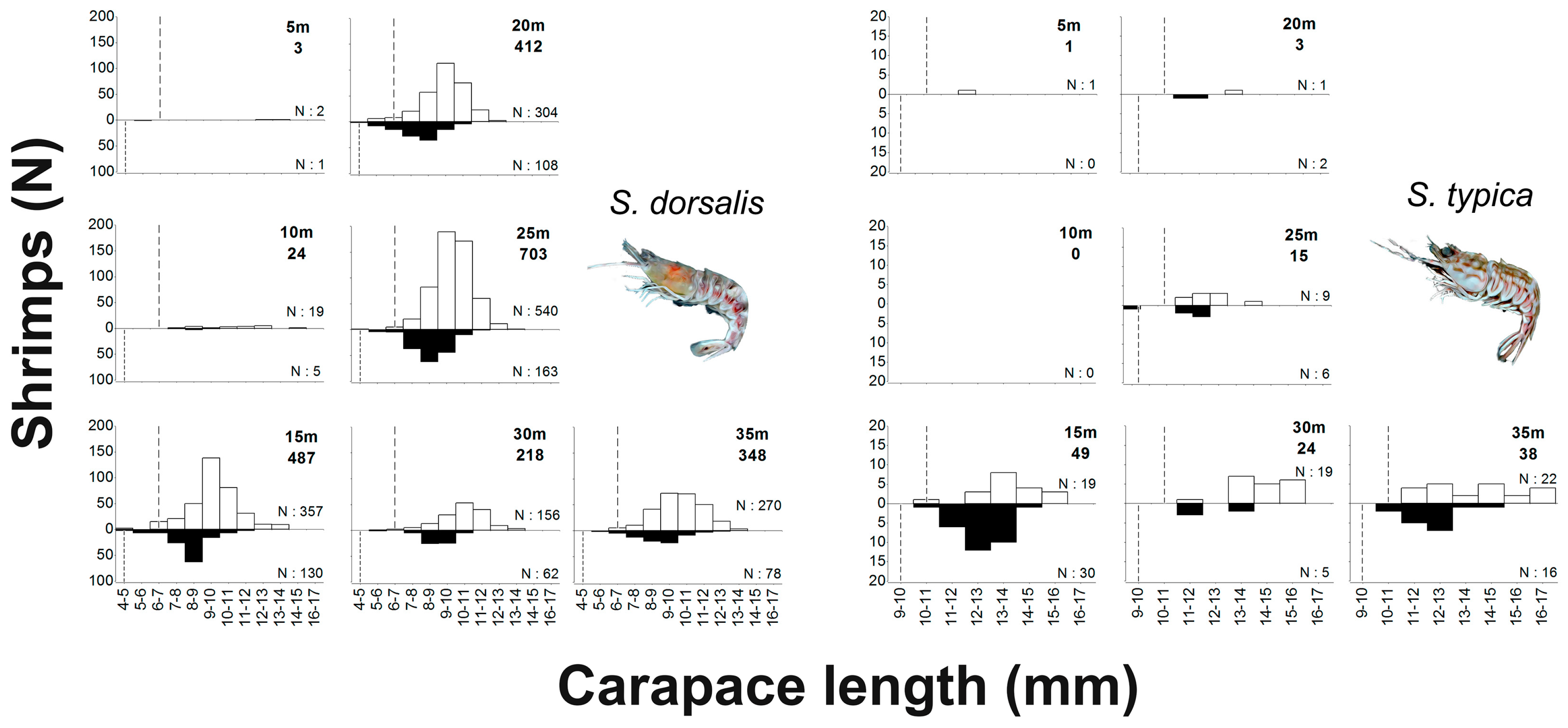
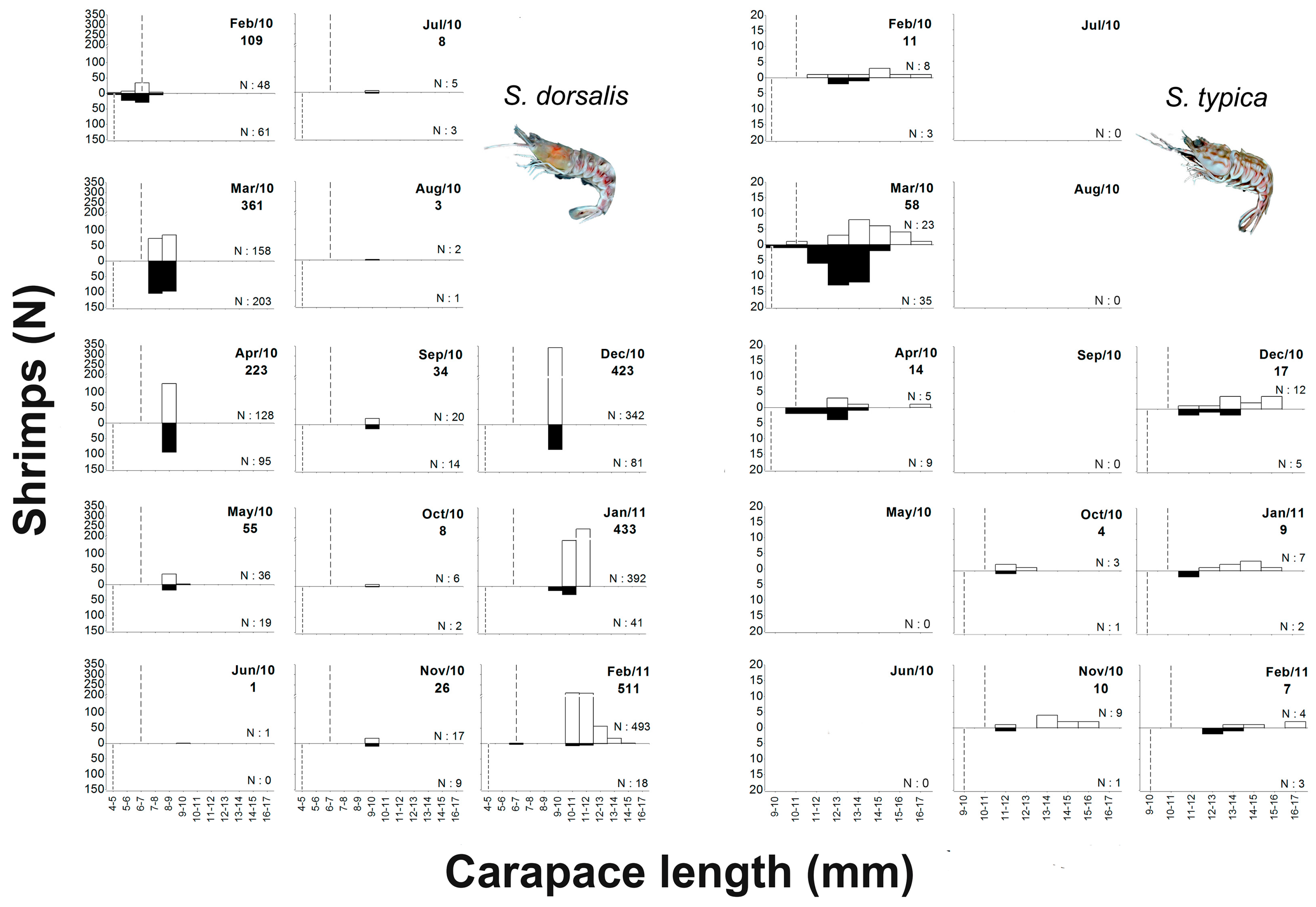
3.2. Spatio-Temporal Distribution of Sicyonia dorsalis and Sicyonia typica
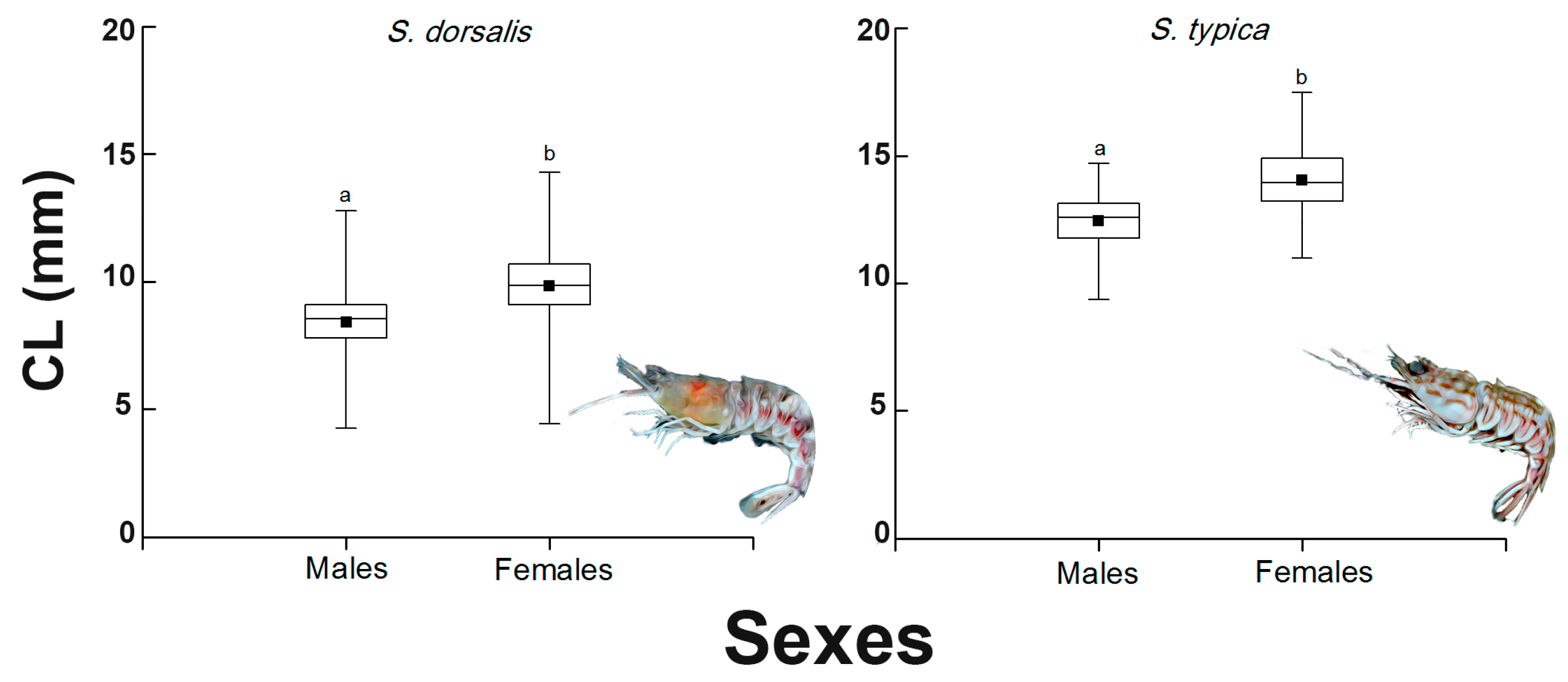
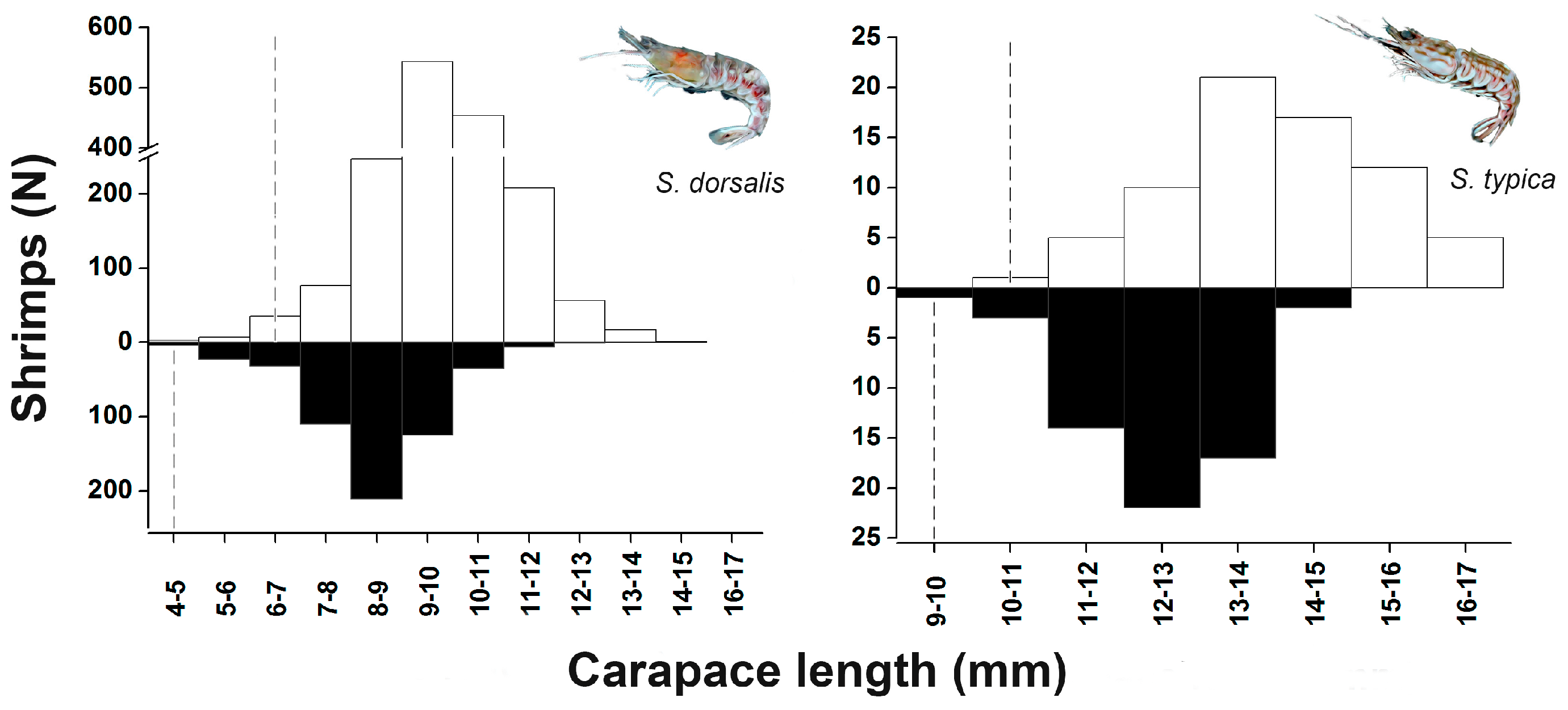
3.3. Frequency Distribution by Size Classes
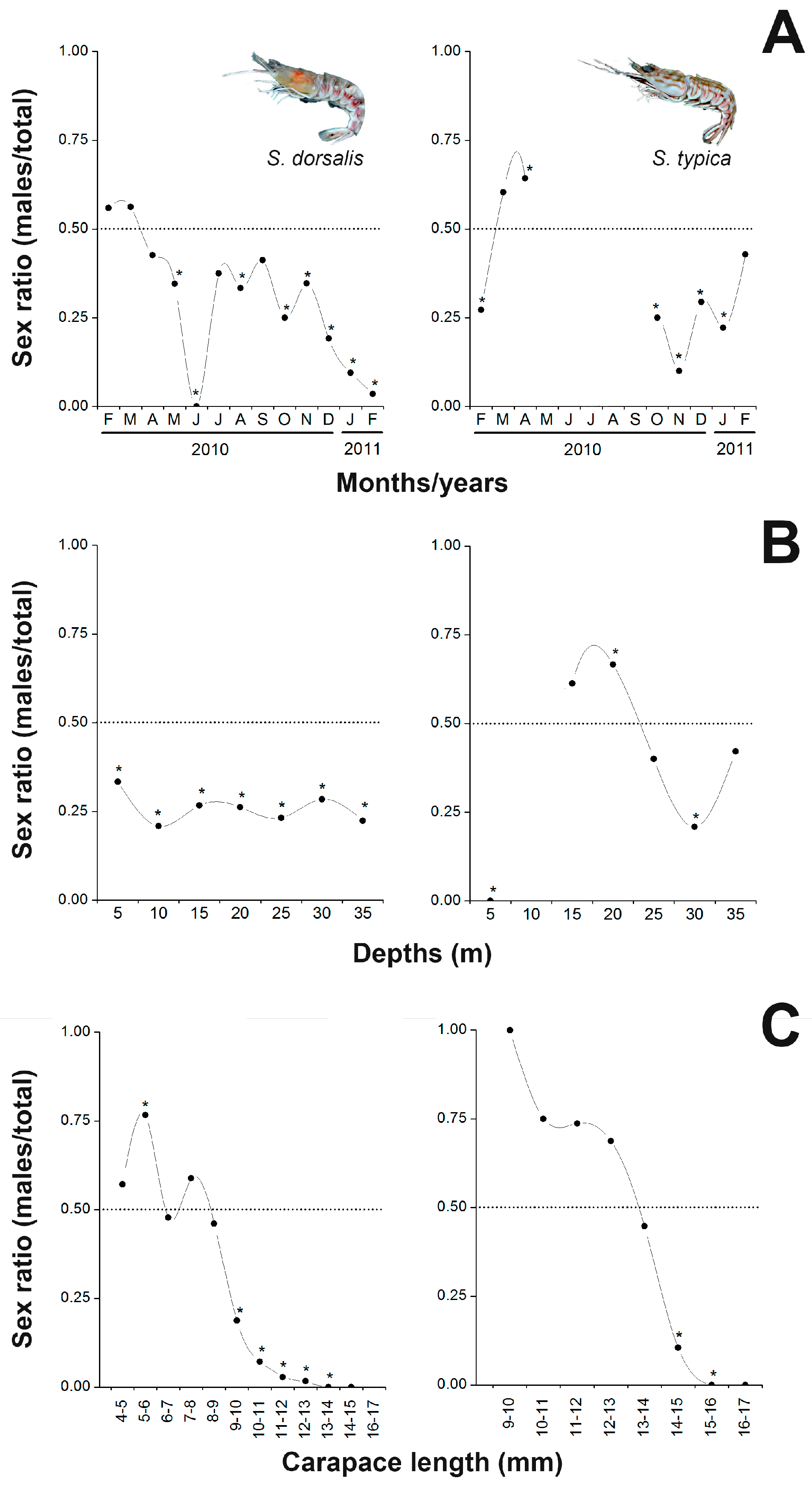
3.4. Sex Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Incao, F. Brazilian rock shrimps of the genus Sicyonia (Decapoda: Sicyoniidae). Nauplius 1995, 3, 101–125. [Google Scholar]
- Perez-Farfante, I.; Kensley, B.F. Penaeids and Sergestoid Shrimps and Prawns of the World: Keys and Diagnoses for the Families and Genera; Editions du Muséum National d’Histoire Naturelle: Paris, France, 1997; pp. 1–233. [Google Scholar]
- De Grave, S.; Fransen, C.H.J.M. Carideorum catalogus: The recent species of the Dendrobranchiate, Stenopodidean, Procarididean and Caridean shrimps (Crustacea: Decapoda). Zool. Med. Leiden 2011, 85, 195–589. [Google Scholar]
- World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=106824 (accessed on 30 May 2024).
- Cintra, I.H.A.; Paiva, K.D.S.; Herrmann, M.; Barbosa, J.M.; Klautau, A.G.M.; Silva, K.C.A. Carcinofauna acompanhante do camarão-rosa em pescarias industriais na plataforma continental amazônica. ActaFish 2017, 5, 69–77. [Google Scholar] [CrossRef]
- Nóbrega, P.S.V.; Santos, C.R.M.; Cordeiro, A.P.B.; Martinelli-Lemos, J.M. Invertebrates assemblage captured by a pink shrimp’s fishery on Amazon continental shelf. Lat. Am. J. Aquat. Res. 2021, 49, 227–241. [Google Scholar] [CrossRef]
- Severino-Rodrigues, E.; Guerra, D.; Graça-Lopes, R. Carcinofauna acompanhante da pesca dirigida ao camarão-sete-barbas (Xiphopenaeus kroyeri) desembarcada na praia do Perequê, Estado de São Paulo, Brasil. Bol. Inst. Pesca 2002, 28, 33–48. [Google Scholar]
- Vasques, R.O.R.; Almeida, A.O.; Coelho, P.A.; Cuevas, J.M.; Couto, E.C.G. A previous list of Dendrobranchiata from shrimp trawlings in Ilhéus, Bahia, Brazil. Nauplius 2003, 11, 115–121. [Google Scholar]
- Mantelatto, F.L.; Bernardo, C.H.; Silva, T.E.; Bernardes, V.P.; Fransozo, A. Composição e distribuição de crustáceos decápodes associados à pesca do camarão-sete-barbas Xiphopenaeus kroyeri (Heller, 1862) no litoral norte do Estado de São Paulo. Bol. Inst. Pesca 2016, 42, 307–326. [Google Scholar] [CrossRef]
- Santos, M.D.C.F.; Silva, K.D.A.; Cintra, I.H.A. Carcinofauna acompanhante da pesca artesanal do camarão-sete-barbas ao largo da foz do Rio São Francisco (Alagoas e Sergipe, Brasil). ActaFish 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Costa, R.C.; Fransozo, A.; Melo, G.A.S.; Freire, F.A.M. Chave ilustrada para identificação dos camarões Dendrobranchiata do litoral norte do estado de São Paulo, Brasil. Biota Neotrop. 2003, 3, 1–12. [Google Scholar] [CrossRef]
- Graça-Lopes, R.; Tomás, A.R.G.; Tutui, S.L.S.; Severino-Rodrigues, E.; Puzzi, A. Fauna acompanhante da pesca camaroeira no litoral do Estado de São Paulo, Brasil. Bol. Inst. Pesca 2002, 28, 173–188. [Google Scholar]
- Graça-Lopes, R.; Puzzi, A.; Severino-Rodrigues, E.; Bartolotto, A.S.; Guerra, D.S.F.; Figueiredo, K.T.B. Comparação entre a produção de camarão-sete-barbas e de fauna acompanhante pela frota de pequeno porte sediada na praia de Perequê, Estado de São Paulo, Brasil. Bol. Inst. Pesca 2002, 28, 189–194. [Google Scholar]
- Costa, R.C.; Carvalho-Batista, A.; Herrera, D.R.; Pantaleão, J.A.F.; Teodoro, S.d.S.A.; Davanso, T.M. Carcino-bycatch of the seabob shrimp fishery (Xiphopenaeus kroyeri) in Macaé, Rio de Janeiro, Brazilian Southeast. Bol. Inst. Pesca 2016, 42, 611–624. [Google Scholar] [CrossRef]
- Castilho, A.L.; Furlan, M.; Costa, R.C.; Fransozo, V. Abundance and temporal-spatial distribution of the rock shrimp Sicyonia dorsalis Kingsley, 1878 (Decapoda, Penaeoidea) from the northern coast of São Paulo State, Brazil. Senckenb. Marit. 2008, 38, 75–82. [Google Scholar] [CrossRef]
- Castilho, A.L.; Furlan, M.; Costa, R.C.; Fransozo, V. Reproductive biology of the rock shrimp Sicyonia dorsalis (Decapoda: Penaeoidea) from the southeastern coast of Brazil. Invertebr. Reprod. Dev. 2008, 52, 59–68. [Google Scholar] [CrossRef]
- Costa, R.C.; Simões, S.M. Avaliação dos Camarões Sicyonideos (Decapoda: Sicyoniidae). In Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010–2014; Pinheiro, M.A.A., Boos, H., Eds.; Sociedade Brasileira de Carcinologia—SBC: Porto Alegre, Rio Grande do Sul, Brazil, 2016; pp. 366–376. [Google Scholar]
- Castilho, A.L.; Pie, M.; Fransozo, A.; Pinheiro, A.; Costa, R.C. The relationship between environmental variation and species abundance in shrimp community (Crustacea: Decapoda: Penaeoidea) in south-eastern Brazil. J. Mar. Biol. Assoc. UK 2008, 88, 119–123. [Google Scholar] [CrossRef]
- Furlan, M.; Castilho, A.L.; Fernandes-Góes, L.C.; Fransozo, V.; Bertini, G.; Costa, R.C. Effect of environmental factors on the abundance of decapod crustaceans from soft bottoms off southeastern Brazil. An. Acad. Bras. Ciênc. 2012, 85, 1345–1356. [Google Scholar] [CrossRef]
- Silva, E.R.; Sancinetti, G.S.; Fransozo, A.; Azevedo, A.; Costa, R.C. Biodiversity, distribution and abundance of shrimps Penaeoidea and Caridea communities in a region the vicinity of upwelling in Southeastern of Brazil. Nauplius 2014, 22, 1–11. [Google Scholar] [CrossRef]
- Dall, W.; Hill, B.J.; Rothilsberg, P.C.; Staples, D.J. The biology of the Penaeidae. In Advances in Marine Biology; Blaxter, J.H.S., Southward, A.J., Eds.; Academic Press: San Diego, CA, USA, 1990; Volume 27, p. 489. [Google Scholar]
- Bauer, R.T. Shrimps: Their Diversity, Intriguing Adaptations and Varied Lifestyles. In Fish & Fisheries Series; Lorezen, K., Ed.; Springer Nature: Cham, Switzerland, 2023; Volume 42, 720p. [Google Scholar] [CrossRef]
- Costa, R.C.; Fransozo, A.; Pinheiro, A.P. Ecological distribution of the shrimp Pleoticus muelleri (Bate, 1888) (Decapoda: Penaeoidea) in southeastern Brazil. Hydrobiologia 2004, 529, 195–203. [Google Scholar] [CrossRef]
- Costa, R.C.; Fransozo, A. Abundance and ecologic distribution of the shrimp Rimapenaeus constrictus (Crustacea: Penaeidae) in the northern coast of São Paulo State, Brazil. J. Nat. Hist. 2004, 38, 901–912. [Google Scholar] [CrossRef]
- Hiroki, K.A.N.; Fransozo, A.; Costa, R.C.; Castilho, A.L.; Shimizu, R.M.; Almeida, A.C.; Furlan, M. Bathymetric distribution of the shrimp Rimapenaeus constrictus (Stimpson, 1874) (Decapoda, Penaeidae) in two locations off the southeastern Brazilian coast. Mar. Biol. Res. 2011, 7, 176–185. [Google Scholar] [CrossRef]
- Bernardes, V.P.; Sousa, A.N.; Bernardo, C.H.; Teixeira, G.M.; Costa, R.C.; Mantelatto, F.L.; Fransozo, A. Comparison of the spatio-temporal distribution of the roughneck shrimp Rimapenaeus constrictus (Stimpson, 1874) (Crustacea, Penaeoidea) from monthly samples collected 20 years apart: Effects of a marine protected area in southeastern Brazil. Mar. Ecol. 2020, 41, e12605. [Google Scholar] [CrossRef]
- Díaz, H.; Conde, J.E. Population dynamics and life history of the mangrove crab Aratus pisonii (Brachyura, Grapsidae) in a marine environment. Bull. Mar. Sci. 1989, 45, 14–163. [Google Scholar]
- Boschi, E.E. Biología Pesquera del Langostino del Litoral Patagonico de Argentina (Pleoticus muelleri); FAO: Rome, Italy, 1989; 71p. [Google Scholar]
- Wenner, A.M. Sex ratio as a function of size in marine crustacea. Am. Nat. 1972, 106, 321–350. [Google Scholar] [CrossRef]
- Fischer, R.A. The Genetical Theory of Natural Selection, 2nd ed.; Doven: New York, NY, USA, 1958; 291p. [Google Scholar]
- Wilson, M.F.; Pianka, E.R. Sexual selection, sex ratio and mating system. Am. Nat. 1963, 97, 405–407. [Google Scholar] [CrossRef]
- Camargo, T.R.; Rossi, N.; Castilho, A.L.; Costa, R.C.; Mantelatto, F.L.; Zara, F.J. Integrative analysis of sperm ultrastructure and molecular genetics supports the phylogenetic positioning of the sympatric rock shrimps Sicyonia dorsalis and Sicyonia typica (Decapoda, Sicyoniidae). Zoomorphology 2016, 135, 67–81. [Google Scholar] [CrossRef]
- Pantaleão, J.A.F.; Pescinelli, R.A.; Mantelatto, F.L.; Costa, R.C. Early larval development of the rock shrimps Sicyonia dorsalis Kingsley, 1878 and S. typica (Boeck, 1864) (Dendrobranchiata) with remarks of larval morphology of Sicyoniidae Ortmann, 1898. Biota Neotrop. 2022, 22, e20221404. [Google Scholar] [CrossRef]
- Piantkoski, E.L.; Costa, R.C.; Davanso, T.M.; Herrera, D.R.; Simões, S.M. Which environmental factors are most relevant to the distribution of Sicyonia dorsalis (Penaeoidea: Sicyoniidae) in an upwelling region? Biologia 2021, 76, 1753–1762. [Google Scholar] [CrossRef]
- Costa, R.C.; Fransozo, A.; Negreiros-Fransozo, M.L. Ecology of the rock shrimp Sicyonia dorsalis Kingsley, 1878 (Crustacea: Sicyoniidae) in a subtropical region of Brazil. Gulf Caribb. Res. 2005, 17, 49–56. [Google Scholar] [CrossRef][Green Version]
- Pralon, B.G.N. Dinâmica Populacional do Camarão Pedra Sicyonia typica (Boeck, 1864) (Penaeoidea: Sicyoniidae) No Litoral norte do Estado de São Paulo. Ph.D. Thesis, São Paulo State University (UNESP), Botucatu, São Paulo, Brazil, 2012. [Google Scholar]
- Bittencourt, A.C.D.S.P.; Dominguez, J.M.L.; Martin, L.; Silva, I.R. Patterns of sediment dispersion coastwise the State of Bahia—Brazil. An. Acad. Bras. Ciênc. 2000, 72, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Ekau, W.; Knoppers, B. An introduction to the pelagic system of the North-East and East Brazilian shelf. Arch. Fish Mar. Res. 1999, 47, 113–125. [Google Scholar]
- Eça, G.F. Biogeoquímica de Nutrientes e Clorofila-a na Plataforma Continental Rasa Entre Itacaré e Canavieiras—Bahia. Master’s Thesis, Santa Cruz State University (UESC), Ilhéus, Bahia, Brazil, 2009. [Google Scholar]
- Faria-Filho, A.F.; Araújo, Q.R. Zoneamento do meio físico do município de Ilhéus, Bahia, Brasil, utilizando a técnica de geoprocessamento. Bol. Técnico CEPLAC/CEPEC 2003, 187, 1–20. [Google Scholar]
- Parsons, T.R.; Takahashi, M.; Hargrave, B. Biological Oceanographic Processes, 3rd ed.; Pergamon Press: Oxford, UK, 1984; p. 330. [Google Scholar]
- Agência Nacional De Águas. Available online: https://www.snirh.gov.br/hidroweb/serieshistoricas (accessed on 13 May 2023).
- Instituto Nacional De Pesquisas Espaciais. Available online: https://www.gov.br/inpe/pt-br (accessed on 13 May 2023).
- Williams, A.B. Shrimps, Lobsters, and Crabs of the Atlantic Coast of the Eastern United States, Maine to Florida; Smithsonian Institution Press: Washington, DC, USA, 1984; p. 550. [Google Scholar]
- Perez-Farfante, I. The rock shrimp genus Sicyonia (Crustacea: Decapoda: Penaeoidea) in the eastern Pacific. Fish. Bull. 1985, 83, 1–79. [Google Scholar]
- Bauer, R.T.; Rivera-Vega, L.W. Pattern of reproduction and recruitment in two sicyoniid shrimp species (Decapoda: Penaeoidea) from a tropical seagrass habitat. J. Exp. Mar. Biol. Ecol. 1992, 161, 223–240. [Google Scholar] [CrossRef]
- Suguio, K. Introdução à Sedimentologia; EDUSP: São Paulo, Brazil, 1973. [Google Scholar]
- Krumbein, W.C. Size Frequency Distributions of Sediments. J. Sediment. Petrol. 1934, 4, 65–77. [Google Scholar] [CrossRef]
- McManus, D.A. A criticism of certain usage of the phi notation. J. Sediment. Petrol. 1963, 33, 670–674. [Google Scholar] [CrossRef]
- Dean, W.E. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. J. Sediment. Res. 1974, 44, 242–248. [Google Scholar] [CrossRef]
- Couto, E.C.G. Comparação entre métodos para obtenção do teor de matéria orgânica em sedimentos estuarinos e costeiros através de ignição. Braz. Arch. Biol. Technol. 1994, 37, 443–448. [Google Scholar]
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics; Sinauer Associates, Inc. Sunderland: Sunderland, MA, USA, 2004. [Google Scholar]
- R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 8 September 2023).
- The Vegan Package: Community Ecology Package. Available online: http://CRAN.R-project.org/package=vegan (accessed on 8 September 2023).
- Sturges, H.A. The choice of a class interval. J. Am. Stat. Assoc. 1926, 21, 65–66. [Google Scholar] [CrossRef]
- Keunecke, K.A.; Vianna, M.; Fonseca, D.B.; D’Incao, F. The pink-shrimp trawling bycatch in the northern coast of São Paulo, Brazil, with emphasis on crustaceans. Nauplius 2007, 15, 49–55. [Google Scholar]
- Robert, R.; Borzone, C.A.; da Natividade, C.D. Os camarões da fauna acompanhante na pesca dirigida ao camarão-sete-barbas (Xiphopenaeus kroyeri) no litoral do Paraná. Bol. Inst. Pesca 2007, 33, 237–246. [Google Scholar]
- Pantaleão, J.A.F.; Batista, A.C.; Fransozo, A.; Costa, R.C. The influence of the upwelling on the diversity and distribution of marine shrimp (Penaeoidea and Caridea) in two tropical coastal areas of southeastern Brazil. Hydrobiologia 2016, 763, 381–395. [Google Scholar] [CrossRef]
- Rodrigues-Filho, J.L.; Couto, E.C.G.; Barbieri, E.; Branco, J.O. Ciclos sazonais da carcinofauna capturada na pesca do camarão-sete-barbas, Xiphopenaeus kroyeri no litoral de Santa Catarina. Bol. Inst. Pesca 2016, 42, 648–661. [Google Scholar] [CrossRef]
- Bochini, G.L.; Stanski, G.; Castilho, A.L.; Costa, R.C. The crustacean bycatch of seabob shrimp Xiphopenaeus kroyeri (Heller, 1862) fisheries in the Cananéia region, southern coast of São Paulo, Brazil. Reg. Stud. Mar. Sci. 2019, 31, 100799. [Google Scholar] [CrossRef]
- Couto, E.C.G.; Guimarães, F.J.; Oliveira, C.A.M.; Vasques, R.O.; Lopes, J.B.B.S. O camarão sete-barbas na Bahia: Aspectos da sua pesca e biologia. Bol. Inst. Pesca 2013, 39, 263–282. [Google Scholar] [CrossRef]
- Gab-Alla, A.A.F.A.; Hartnoll, R.G.; Ghobashy, A.F.; Mohammed, S.Z. Biology of penaeid prawns in the Suez Canal Lakes. Mar. Biol. 1990, 107, 417–426. [Google Scholar] [CrossRef]
- Ren, S.; Mather, P.B.; Tang, B.; Hurwood, D.A. Comparison of reproductive performance of domesticated Litopenaeus vannamei females reared in recirculating tanks and earthen ponds: An evaluation of reproductive quality of spawns in relation to female body size and spawning order. Front. Mar. Sci. 2020, 7, 560. [Google Scholar] [CrossRef]
- Mayr, E. Geographical character gradients and climatic adaptation. Evolution 1956, 10, 105–108. [Google Scholar] [CrossRef]
- Bauer, R.T. Testing generalizations about latitudinal variation in reproduction and recruitment patterns with sicyoniid and caridean shrimp species. Invertebr. Reprod. Dev. 1992, 22, 193–202. [Google Scholar] [CrossRef]
- Bauer, R.T. Repetitive copulation and variable success of insemination in the marine shrimp Sicyonia dorsalis (Decapoda: Penaeoidea). J. Crustac. Biol. 1992, 12, 153–160. [Google Scholar] [CrossRef]
- Baeza, J.A.; Bauer, R.T. Experimental test of socially mediated sex change in a protandric simultaneous hermaphrodite, the marine shrimp Lysmata wurdemanni (Caridea: Hippolytidae). Behav. Ecol. Sociobiol. 2004, 55, 544–550. [Google Scholar] [CrossRef]
- Braga, A.A.; López Greco, L.S.; Santos, D.C.; Fransozo, A. Morphological evidence for protandric simultaneous hermaphroditism in the caridean Exhippolysmata oplophoroides. J. Crustac. Biol. 2009, 29, 34–41. [Google Scholar] [CrossRef]
- Baeza, J.A.; Braga, A.A.; López-Greco, L.S.; Perez, E.; Negreiros-Fransozo, M.L.; Fransozo, A. Population dynamics, sex ratio and size at sex change in a protandric simultaneous hermaphrodite, the spiny shrimp Exhippolysmata oplophoroides. Mar. Biol. 2010, 157, 2643–2653. [Google Scholar] [CrossRef]
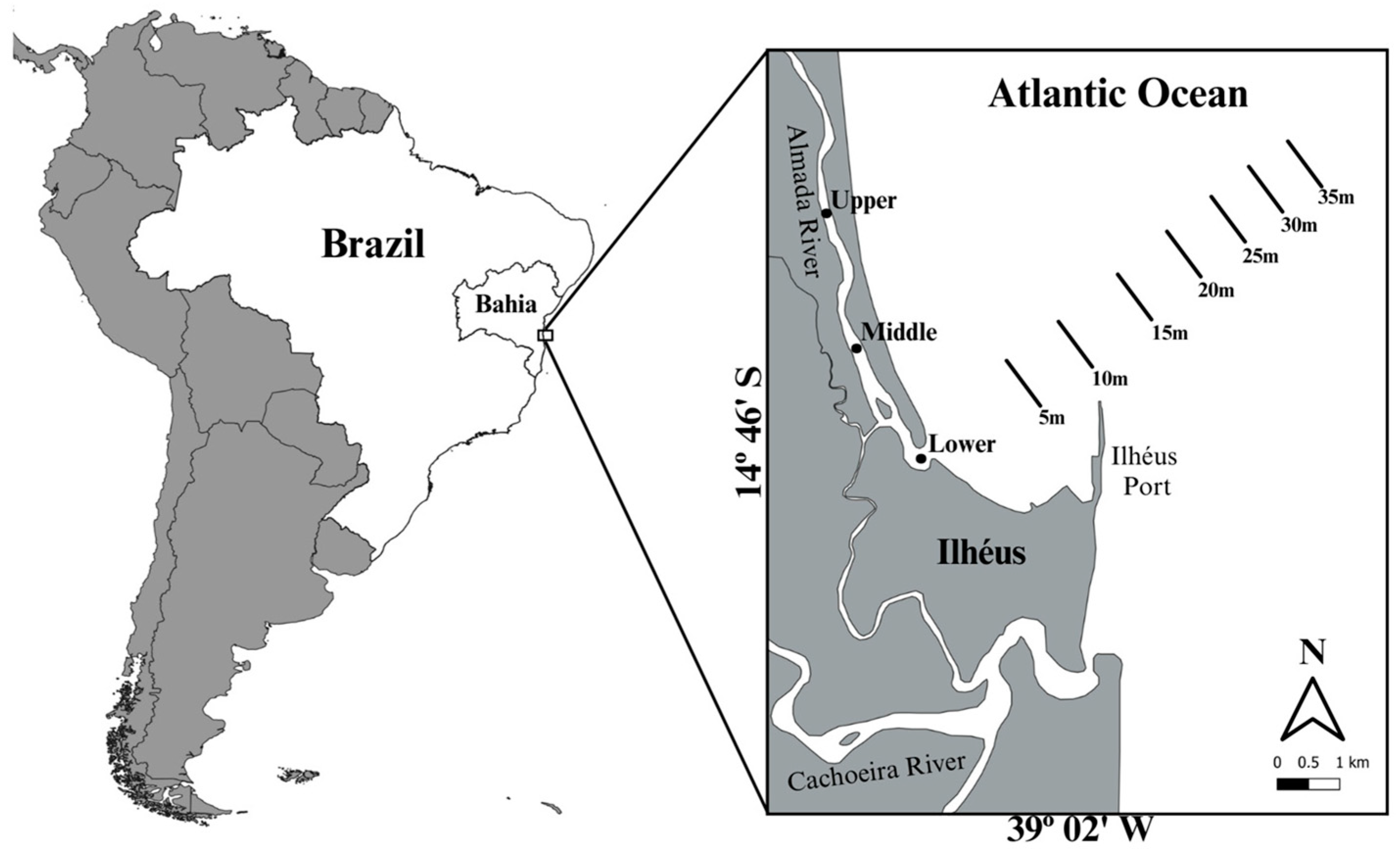

| Stations | Start | End | |
|---|---|---|---|
| Estuary | Upper | 14°43′40.1′′ S 39°03′591′′ W | 14°43′44.5′′ S 39°04′013′′ W |
| Middle | 14°44′54.7′′ S 39°03′476′′ W | 14°44′53.0′′ S 39°03′481′′ W | |
| Lower | 14°46′30.8′′ S 39°03′173′′ W | 14°46′26.7′′ S 39°03′187′′ W | |
| Ocean | 5 m | 14°45′56.8′′ S 39°02′845′′ W | 14°46′28.5′′ S 39°02′699′′ W |
| 10 m | 14°45′14.8′′ S 39°01′677′′ W | 14°45′68.9′′ S 39°01′759′′ W | |
| 15 m | 14°45′00.8′′ S 39°00′888′′ W | 14°45′10.0′′ S 39°00′848′′ W | |
| 20 m | 14°45′73.7′′ S 39°00′407′′ W | 14°45′18.0′′ S 39°00′465′′ W | |
| 25 m | 14°44′87.7′′ S 39°00′177′′ W | 14°45′01.0′′ S 39°00′137′′ W | |
| 30 m | 14°44′55.1′′ S 38°59′743′′ W | 14°43′80.0′′ S 39°00′235′′ W | |
| 35 m | 14°42′26.4′′ S 39°00′087′′ W | 14°42′22.2′′ S 39°00′107′′ W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, R.G.; Paschoal, L.R.P.; Guimarães, F.J.; Brandão, S.N.; Couto, E.d.C.G. Spatio-Temporal Distribution and Population Dynamics of Two Sympatric Species: The Rock Shrimps Sicyonia dorsalis Kingsley, 1878 and Sicyonia typica (Boeck, 1864) (Penaeoidea: Sicyoniidae) on the Coast of Ilhéus, Bahia, Northeastern Brazil. Arthropoda 2025, 3, 1. https://doi.org/10.3390/arthropoda3010001
Tavares RG, Paschoal LRP, Guimarães FJ, Brandão SN, Couto EdCG. Spatio-Temporal Distribution and Population Dynamics of Two Sympatric Species: The Rock Shrimps Sicyonia dorsalis Kingsley, 1878 and Sicyonia typica (Boeck, 1864) (Penaeoidea: Sicyoniidae) on the Coast of Ilhéus, Bahia, Northeastern Brazil. Arthropoda. 2025; 3(1):1. https://doi.org/10.3390/arthropoda3010001
Chicago/Turabian StyleTavares, Renzo Gonçalves, Lucas Rezende Penido Paschoal, Fernanda Jordão Guimarães, Simone Nunes Brandão, and Erminda da Conceição Guerreiro Couto. 2025. "Spatio-Temporal Distribution and Population Dynamics of Two Sympatric Species: The Rock Shrimps Sicyonia dorsalis Kingsley, 1878 and Sicyonia typica (Boeck, 1864) (Penaeoidea: Sicyoniidae) on the Coast of Ilhéus, Bahia, Northeastern Brazil" Arthropoda 3, no. 1: 1. https://doi.org/10.3390/arthropoda3010001
APA StyleTavares, R. G., Paschoal, L. R. P., Guimarães, F. J., Brandão, S. N., & Couto, E. d. C. G. (2025). Spatio-Temporal Distribution and Population Dynamics of Two Sympatric Species: The Rock Shrimps Sicyonia dorsalis Kingsley, 1878 and Sicyonia typica (Boeck, 1864) (Penaeoidea: Sicyoniidae) on the Coast of Ilhéus, Bahia, Northeastern Brazil. Arthropoda, 3(1), 1. https://doi.org/10.3390/arthropoda3010001







