Updated Checklist of the Freshwater Shrimps (Decapoda: Caridea: Atyidae) of Mindoro Island, the Philippines, with a Description of a New Species of Caridina †
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Molecular Study
2.3. Morphological Study
3. Results
3.1. Molecular Study
3.2. Morphological Study
| 1.1 First two pairs of pereiopods identical | 2 (Atya group) |
| 1.2 First pair distinctly shorter than second | 3 |
| 2.1 Carapace with an acute projection on the pterygostomian margin, rostrum with more than two teeth on the ventral margin | Atyopsis spinipes |
| 2.2 Carapace with rounded pterygostomian margin, rostrum with fewer than two teeth on the ventral margin | Atyoida pilipes |
| 3.1 More than six post-orbital teeth situated on the dorsal margin of the rostrum. A long stylocerite, reaching at least to the beginning of the second segment of the antennular peduncle | 4 (C. serratirostris group) |
| 3.2 Fewer than six dorsal rostrum teeth situated on the carapace behind the orbital margin. A long or short stylocerite | 5 |
| 4.1 Rostrum with 22–26 dorsal teeth and P2 carpus 8.2–10.9 times as long as it is wide | Caridina serratirostris |
| 4.2 Rostrum with 17–22 dorsal teeth and P2 carpus 11.9–12.0 times as long as it is wide. | C. celebensis |
| 5.1 Number of spiniform setae on the uropodal diaeresis 6–10 and a very long and upcurved rostrum with 5–9 dorsal teeth, widely spaced | C. leptopoda sp. nov. |
| 5.2 Number of spiniform setae on the uropodal diaeresis 8–22 and a long or short rostrum with 8–28 dorsal teeth, closely set | 6 |
| 6.1 Uropodal diaeresis with 14–22 spinules, short rostrum, armed or not, and high unarmed pre-anal carina | 7 |
| 6.2 Uropodal diaeresis with 8–17 spinules, rostrum variable in shape and length, and low pre-anal carina with or without a spine | 13 |
| 7.1 Telson with numerous and very long plumose intermediate setae | 8 (C. weberi group) |
| 7.2 Telson with few long intermediate setae | 11 (C. typus group) |
| 8.1 Rostrum without dorsal teeth | Atydina atyoides |
| 8.2 Teeth present on the dorsal margin of the rostrum | 9 |
| 9.1 Rostrum straight, 9–17 teeth on the dorsal margin | C. laoagensis |
| 9.2 Rostrum bent downwards, 8–10 teeth on the dorsal margin | 10 |
| 10.1 Outer terminal projection of uropodal diaeresis shorter than the first movable spine | C. parvirostris |
| 10.2 Outer terminal projection of uropodal diaeresis longer than the first movable spine | C. papuana |
| 11.1 Rostrum reaching to or longer than the antennular peduncle | C. villadolidi |
| 11.2 Rostrum shorter than the antennular peduncle | 12 |
| 12.1 Rostrum reaching the end of the second segment of the antennular peduncle | C. typus |
| 12.2 Rostrum reaching the end of the first segment of the antennular peduncle | C. zhujiangensis |
| 13.1 No subapical teeth on the rostrum, tip of the rostrum rounded, and P1 carpus deeply excavated | 14 (C. brevicarpalis group) |
| 13.2 Subapical teeth often present on the rostrum and tip of the rostrum pointed P1 carpus not deeply excavated | 15 |
| 14.1 Rostrum overreaching the scaphocerite | C. endehensis |
| 14.2 Rostrum reaching the end of the antennular peduncle | C. brevicarpalis |
| 15.1 Presence of one or few subapical teeth on the rostrum, fewer than two post-orbital teeth | 16 (C. nilotica group) |
| 15.2 No subapical teeth on the rostrum, more than three post-orbital teeth | C. bruneiana |
| 16.1 Distinct projection on the pre-anal carina | C. gracilipes |
| 16.2 Absence of projection, sometimes the presence of a cluster of setae | C. elongapoda |
3.3. Taxonomy: Updated Checklist of the Species of Atyid Shrimps from Mindoro Island
- Atya pilipes Newport, 1847 [43]: 160 (Type locality: Apia, Upolu, Samoa).
- Atyoida pilipes—Chace, 1983 [44]: 13, figs. 3, 4 (part); 1997: 4.
- Caridina acuminata Stimpson, 1860 [45]: 29 (Type locality: Ogasawara (Bonin) Islands, Japan).
- Caridina brevirostris Stimpson, 1860 [45]: 29 (Type locality: Okinawa (Loo Choo) Island, Ryukyu Islands, Japan).
- Atya brevirostris De Man, 1892 [46]: 360, 520, pl. 21: figs. 21, 21a–d (Type locality: Indonesia, Flores, several localities, and Timor, Koinino (=Kuanino) River near Kupang).
- Pseudatya beauforti Roux, 1928 [47]: 209, figs. 1–9 (Type locality: Indonesia, Bacan Island).
- Atya spinipes Newport, 1847 [43]: 159 (Type locality: Philippine Islands).
- Atyopsis spinipes—Chace, 1983 [44]: 35, figs. 20–22.
- Atya dentirostris Thallwitz, 1891 [50]: 101 (Type locality: North Celebes).
- Atya brevirostris var. De Mani Nobili, 1900 [51]: 475, fig. 2. (Type locality: Indonesia, Mentawei Islands, Sipora Island, Sereinu (=Saurenu?) river).
- Restricted synonymy:
- Caridina Wyckii var. gracilipes De Man, 1892 [46]: 387, pl. 24, figs. 29e–k (Type locality: River near Maros, Sulawesi, Indonesia).
- Caridina nilotica var. bengalensis De Man, 1908 [53]: 265, pl. 20, figs. 6–6b (Type locality: Port Canning, Lower Bengal, India).
- Caridina acuticaudata Dang, 1975 [56]: 70, fig. 4 (Type locality: Boi River, Hoa Binh Province, Vietnam).
- Non-Caridina longirostris—Chace, 1997 [20]: 14 (part), fig. 7.
- Caridina nilotica elongapoda Liang and Yan, 1977 [58]: 220, figs. 5–8.—Liang and Zheng, 1988: 15 (Type locality: Xinzai, Gulei village, Zhangpu County, Fujian, southern China).
- Non Caridina longirostris—Chace, 1997 [20]: fig. 6 (part?).
- Caridina bruneiana Choy, 1992 [64]: 49, Figs. 1–4 (Type locality: Negara Brunei Darussalam, on the upper reaches of Temburong River at Batang Duri, 04°36′05″ N 115°06′45″ E, altitude approx. 33 m).
- Caridina brevicarpalis De Man, 1892 [46]: 365 (key), 397–399, pl. 24, fig. 30a–e (Type locality: River near Palopo, Luwu, Sulawesi, Indonesia).
- Caridina brevicarpalis—Ortmann, 1894 [67]: 11; 1894 [68]: 402 (key), 404.—Roux, 1904 [69]: 553.—Bouvier, 1912 [70]: 919; 1913 [71]: 463; 1925 [72]: 178–180, figs. 372–374.—Roux, 1928 [47]: 200–201.—Cai and Shokita, 2006 [12]: 248.—Page et al., 2007 [73]: 649, 653, fig. 2 (part, Borneo specimen).—de Mazancourt et al., 2017 [74]: 226, fig. 4 (part, Indonesian specimen).
- Caridina brevicarpalis brevicarpalis—Chace, 1997 [20]: 8.
- Caridina brevicarpalis var. endehensis De Man, 1892 [46]: 399, pl. 24, fig. 30e (Type locality: River Ba near Endeh, Flores, Indonesia).
- Caridina laoagensis Blanco, 1939 [79]: 390, pl. 2 (Type locality: Laoag River, Laoag, Ilocos Norte Province, Luzon, the Philippines).
- Caridina Weberi var. papuana Nobili, 1905 [84]: 481, Pl. 12, fig. 1a,b (Type locality: Small forest stream, Stephansort, Madang Province, Papua New Guinea).
- Caridina weberi papuana—Roux, 1934 [86]: 221.
- Caridina parvirostris De Man, 1892 [46]: 375, pl. 22, fig. 24 (Type locality: River near Bombang (=Boba?), Flores Island, Indonesia).
- Atydina atyoides—Cai, 2010 [91]: 76, figs. 1, 2.
- Caridina serratirostris var. celebensis De Man, 1892 [46]: 385, pl. 23, figs. 28f–h (Type locality: River near Palopo, Luwu, Sulawesi, Indonesia).
- Caridina serratirostris koterai Kamita, 1951 [93]: 75, pl. 5, figs. A–G.
- Caridina celebensis—Hayashi, 1989 [97]: 376, figs. e, g.—Shokita, 2003 [98]: 250, fig. 19K.—Cai and Shokita, 2006 [12]: 247; 2006 [80]: 2140.—Karge and Klotz, 2007 [76]: 90.—Page et al., 2007 [73]: fig. 2.—von Rintelen et al., 2008 [99]: 2244, fig. 4.—de Mazancourt et al., 2020 [65]: 71, figs, 2B, 24.
- Caridina leptocarpa Liang and Zheng, 1988 [100]: 15, figs. 1–91 (type locality: Fuzhou, 26°N 119°E, Min River, Fujian Province, China).
- Caridina leptocarpa—Liang and Zhou, 1993 [101]: 231.
- Paracaridina leptocarpa—Liang, 2004 [102]: 318, fig. 155.
- Non Caridina serratirostris—Chace, 1997 [20]: 19, fig. 11.
- Caridina typus H. Milne Edwards, 1837 (in H. Milne Edwards, 1834–1840) [104]: 363, pl. 25bis, figs. 4, 5 (Type locality: Unknown, likely Mauritius).
- Caridina exilirostris Stimpson, 1860 [45]: 29 (Type locality (neotype): Okuma River, Okinawa Island, Ryukyu Islands, Japan).
- Caridina siamensis Giebel, 1863 [106]: 329 (Type locality: Siam).
- Caridina typus forme caledonica Bouvier, 1925 [72]: 253, figs. 296–297 (Type locality: New Caledonia).
- Caridina zhujiangensis Q.-H. Chen, W.-J. Chen and Guo, 2018 [108]: 319, figs. 4–6 (Type locality: Near the Resort Hotel, Dong’ao Island, Guangdong Province, China (E 113°42′03″, N 22°01′06″, al. 19 m, stn. 4)).
- Non Caridina typus—Bernardes et al., 2017 [105]: 1 (part, TAL clade).
- Caridina villadolidi Blanco, 1939 [79]: 389, pl. 1, figs. 1–9 (Type locality: Laoag River, Laoag, Ilocos Norte Province, Luzon, the Philippines).
- Caridina villadolidi—Hung et al., 1993 [112]: 485, figs. 1B, 3.—Chace, 1997 [20]: 21, fig. 12.—Shy and Yu, 1998 [113]: 62.—Cai and Ng, 2001 [114]: 668, fig. 4a–e.—Liang, 2004 [102]: 156, fig. 74.—Cai and Shokita, 2006 [12]: 248.—Cai et al., 2009 [13]: 66.—Bernardes et al., 2017 [105]: sup. fig. 2.—Vijayamma et al., 2021 [115]: 404 (key).
- Caridina typus var. longirostris De Man, 1892 [46]: 370: pl. 22, fig. 22f–I (junior homonym of Caridina longirostris H. Milne Edwards, 1837) (Type locality: River near Reo, Flores, Indonesia; River near Palopo, Sulawesi, Indonesia; Benteng, Saleyer, Indonesia).
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Hall, R. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: Computer-based reconstructions, model and animations. J. Asian Earth Sci. 2002, 20, 353–431. [Google Scholar] [CrossRef]
- Zahirovic, S.; Seton, M.; Müller, R.D. The Cretaceous and Cenozoic tectonic evolution of Southeast Asia. Solid Earth 2014, 5, 227–273. [Google Scholar] [CrossRef]
- Shih, H.-T.; Yeo, D.C.J.; Ng, P.K.L. The collision of the Indian plate with Asia: Molecular evidence for its impact on the phylogeny of freshwater crabs (Brachyura: Potamidae). J. Biogeogr. 2009, 36, 703–719. [Google Scholar] [CrossRef]
- Esselstyn, J.A.; Timm, R.M.; Brown, R.M. Do geological or climatic processes drive speciation in dynamic archipelagos? The tempo and mode of diversification in Southeast Asian shrews. Evolution 2009, 63, 2595–2610. [Google Scholar] [CrossRef]
- Ong, P.S.; Afuang, L.E.; Rosell-Ambal, R.G. (Eds.) Philippine Biodiversity Conservation Priorities: A Second Iteration of the National Biodiversity Strategy and Action Plan; Department of Environment and Natural Resources—Protected Areas and Wildlife Bureau, Conservation International Philippines, Biodiversity Conservation Program University of the Philippines Center for Integrative and Development Studies, and Foundation for the Philippine Environment: Quezon City, Philippines, 2002; 113p. [Google Scholar]
- Freitag, H.; Jäch, M.A.; Wewalka, G. Diversity of Aquatic Coleoptera of the Philippines—Checklist, State of Knowledge, Priorities for Conservation & Future Research. Aquat. Insects 2016, 37, 177–213. [Google Scholar] [CrossRef]
- Pelingen, A.L.; Zettel, H.; Pangantihon, C.; Aldaba, K.M.D.; Fatallo, E.K.; De Leon, J.M.; Freitag, H. Contributions to the knowledge of water bugs in Mindoro Island, Philippines, with a species checklist of Nepomorpha and Gerromorpha (Insecta, Hemiptera, Heteroptera). Biodivers. Data J. 2020, 8, e56883. [Google Scholar] [CrossRef]
- Ng PK, L.; Takeda, M. The Freshwater Crab Fauna (Crustacea: Brachyura) of the Philippines. I. The Family Potamidae Ortmann, 1896. Bull. Natl. Sci. Mus. Tokyo Ser. A 1992, 18, 149–166. [Google Scholar]
- Vidal, A.R.; Go, K.C.T.S.; Freitag, H. Hydraenidae (Insecta: Coleoptera) of Mindoro, Philippines.I: Hydraena Kugelann, 1794 of the Baroc River Basin, Roxas, Oriental Mindoro with Description of three New Species. Aquat. Insects 2017, 38, 1–20. [Google Scholar] [CrossRef]
- Philippine Statistics Authority. 2022. Available online: https://psada.psa.gov.ph/catalog/231 (accessed on 21 July 2023).
- Cai, Y.; Shokita, S. Report on a collection of freshwater shrimps (Crustacea: Decapoda: Caridea) from the Philippines, with descriptions of four new species. Raffles Bull. Zool. 2006, 54, 245–270. [Google Scholar]
- Cai, Y.; Choy, S.; Ng, P.K.L. Epigean and hypogean freshwater shrimps of Bohol Island, central Philippines (Crustacea: Decapoda: Caridea). Raffles Bull. Zool. 2009, 57, 65–89. [Google Scholar]
- Han, C.C.; Klotz, W. Australatya obscura sp. nov., a new filter-feeding shrimp (Decapoda, Atyidae) from Taiwan and the Philippines. Crustaceana 2015, 88, 66–81. [Google Scholar] [CrossRef]
- Cai, Y.; Anker, A. On a collection of freshwater shrimps (Crustacea Decapoda Caridea) from the Philippines, with descriptions of five new species. Trop. Zool. 2004, 17, 233–266. [Google Scholar] [CrossRef]
- Cai, Y.; Husana, D.E.M. Cave shrimps of the genus Edoneus Holthuis, 1978, from Luzon, the Philippines, with descriptions of three new species (Crustacea: Decapoda: Atyidae). Raffles Bull. Zool. 2009, 57, 51–63. [Google Scholar]
- Woltereck, E. Systematisch–variationsanalytische Untersuchungen über die Rassen-und Artbildung bei Süßwassergarneelen aus der Gattung Caridina (Decapoda, Atyidae). Int. Rev. Gesamten Hydrobiol. Hydrogr. 1937, 34, 208–262. [Google Scholar] [CrossRef]
- Estampador, E.P. A Check List of Philippine Crustacean Decapods. Philipp. J. Sci. 1937, 62, 465–559. [Google Scholar]
- Estampador, E.P. Revised Check List of Philippine Crustacean Decapods. Nat. Appl. Sci. Bull. 1959, 17, 3–127. [Google Scholar]
- Chace, F.A. The Caridean shrimps (Crustacea: Decapoda) of the Albatross Philippine Expedition, 1907–1910, Part 7: Families Atyidae, Eugonatonotidae, Rhynchocinetidae, Bathypalaemonidae, Processidae, and Hippolytidae. Smithson. Contrib. Zool. 1997, 587, 1–106. [Google Scholar] [CrossRef]
- Freitag, H. Ancyronyx Erichson, 1847 (Coleoptera, Elmidae) from Mindoro, Philippines, with description of the larvae and two new species using DNA sequences for the assignment of the developmental stages. Zookeys 2013, 321, 35–64. [Google Scholar] [CrossRef]
- Garces, J.M.; Bauernfeind, E.; Freitag, H. Sparsorythus sescarorum, new species from Mindoro, Philippines (Ephemeroptera, Tricorythidae). ZooKeys 2018, 795, 13–30. [Google Scholar] [CrossRef]
- Garces, J.M.; Sartori, M.; Freitag, H. Integrative taxonomy of the genus Dudgeodes Sartori, 2008 (Insecta, Ephemeroptera, Teloganodidae) from the Philippines with description of new species and complementary description of Southeast Asia species. ZooKeys 2020, 910, 93–129. [Google Scholar] [CrossRef] [PubMed]
- Komarek, A.; Freitag, H. Revision of Anacaena Thomson, 1859 XI. Republic of the Philippines (Coleoptera: Hydrophilidae). Koleopterol. Rundsch. 2014, 84, 235–276. Available online: https://www.zobodat.at/pdf/KOR_84_2014_0235-0276.pdf (accessed on 21 July 2023).
- Komarek, A.; Freitag, H. Taxonomic revision of Agraphydrus Régimbart, 1903 IV. Philippine species and their first DNA barco3des (Coleoptera: Hydrophilidae: Acidocerinae). Koleopterol. Rundsch. 2020, 90, 201–242. Available online: https://www.zobodat.at/pdf/KOR_90_2020_0201-0242.pdf (accessed on 21 July 2023).
- Mey, W.; Freitag, H. Trichoptera of Mindoro, the Philippines I. New species and records from the Baroc River Catchment, Roxas, Oriental Mindoro (Insecta, Trichoptera). Esperiana 2013, 18, 259–269. Available online: https://archium.ateneo.edu/cgi/viewcontent.cgi?article=1017&context=biology-faculty-pubs (accessed on 21 July 2023).
- Pelingen, A.L.; Freitag, H. Description of Neoperla mindoroensis sp. nov., the first record of stonefly from Mindoro, Philippines (Plecoptera, Perlidae), and association of its life stages using COI barcodes. Zookeys 2020, 954, 47–63. [Google Scholar] [CrossRef]
- Zettel, H.; Pangantihon, C.V. Aphelocheirus (s.str.) freitagi nov. sp. from Mindoro Island and additional notes on Philippine Aphelocheiridae (Heteroptera). Linz. Biol. Beiträge 2010, 42, 1353–1362. [Google Scholar]
- BirdLife International. World Database of Key Biodiversity Areas. Developed by the KBA Partnership: BirdLife International, International Union for the Conservation of Nature, American Bird Conservancy, Amphibian Survival Alliance, Conservation International, Critical Ecosystem Partnership Fund, Global Environment Facility, Re:wild, NatureServe, Rainforest Trust, Royal Society for the Protection of Birds, Wildlife Conservation Society and World Wildlife Fund. September 2022 Version. 2022. Available online: http://keybiodiversityareas.org/kba-data/request (accessed on 21 July 2023).
- von Rintelen, K.; von Rintelen, T.; Meixner, M.; Lüter, C.; Cai, Y.; Glaubrecht, M. Freshwater shrimp-sponge association from an ancient lake. Biol. Lett. 2007, 3, 262–264. [Google Scholar] [CrossRef][Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F.; Nielsen, R.; Bollback, J.P. Bayesian inference of phylogeny and its impact on evolutionary biology. Science 2001, 294, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Uebeler, J.L.; Rumpf, C.M.A.; de Mazancourt, V.; von Rintelen, K. Workflow Protocol for the Digitization of Ethanol-Preserved Isopods to Accelerate the Process of Species Description; Museum für Naturkunde: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Coleman, C.O. “Digital inking”: How to make perfect line drawings on computers. Org. Divers. Evol. 2003, 3 (Suppl. 14), 1–14. [Google Scholar] [CrossRef]
- Coleman, C.O. Substituting time-consuming pencil drawings in arthropod taxonomy using stacks of digital photographs. Zootaxa 2006, 1360, 61–68. [Google Scholar] [CrossRef]
- Newport, G. Note on the genus Atya of Leach, with descriptions of four apparently new species, in the cabinets of the British Museum. Ann. Mag. Nat. Hist. 1847, 19, 158–160. [Google Scholar] [CrossRef]
- Chace, F.A. The Atya-like shrimps of the Indo-Pacific Region (Decapoda: Atyidae). Smithson. Contrib. Zool. 1983, 384, 1–54. [Google Scholar] [CrossRef]
- Stimpson, W. Prodromus descriptionis animalium evertebratorum, quae in Expeditione ad Oceanum Pacificum Septentrionalem, a Republica Federata missa, Cadwaladaro Ringgold et Johanne Rodgers Ducibus, observavit et descripsit. Pars VIII, Crustacea Macrura. Proc. Acad. Nat. Sci. Phila. 1860, 1860, 22–47. [Google Scholar]
- De Man, J.G. Decapoden des Indischen Archipels. In Zoologische Ergebnisse einer Reise in Niederländisch Ost-Indien; Weber, M., Ed.; Brill Publishers: Leiden, The Netherlands, 1892; Volume 2, pp. 265–527. [Google Scholar]
- Roux, J. Notes carcinologiques de l’Archipel Indo-Australien, I. Décapodes Macroures d’eau douce de l’Archipel Indo-Australien. Treubia 1928, 10, 197–224. [Google Scholar]
- Blanco, G.J. The Atyidae of the Philippine Islands. Philipp. J. Sci. 1935, 56, 29–39. [Google Scholar]
- De Grave, S.; Klotz, W.; Cai, Y. Atyoida pilipes (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T197920A147791194. [Google Scholar] [CrossRef]
- Thallwitz, J. Über einige neue indo-pacifische Crustaceen. Zool. Anz. 1891, 14, 96–103. [Google Scholar]
- Nobili, G. Decapodi e stomatopodi Indo-Malesi. Ann. Mus. Civ. Stor. Nat. Genova 1900, 40, 473–523. [Google Scholar]
- De Grave, S.; Shy, J.; Klotz, W. Atyopsis spinipes. IUCN Red List Threat. Species 2013, e.T198075A2510800. [Google Scholar] [CrossRef]
- De Man, J.G. On Caridina nilotica (Roux) and its varieties. Rec. Indian Mus. 1908, 2, 255–283. [Google Scholar]
- de Mazancourt, V.; Klotz, W.; Marquet, G.; Keith, P. Integrative taxonomy helps separate four species of freshwater shrimps commonly overlooked as Caridina longirostris (Crustacea: Decapoda: Atyidae) on Indo-West Pacific islands. Invertebr. Syst. 2018, 32, 1422. [Google Scholar] [CrossRef]
- Cai, Y. Species of Caridina nilotica group in China, with description of one new species (Crustacea, Decapoda, Atyidae). Crustaceana 2020, 93, 1405–1422. [Google Scholar] [CrossRef]
- Dang, N.T. The identities of north Vietnamese freshwater shrimp and crabs. Tap. San. Sinh. Vat-Dia Hoc. J. Biol. Geol. 1975, 13, 65–78. [Google Scholar]
- Cai, Y.; De Grave, S.; Klotz, W. Caridina gracilipes (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T198028A147793058. [Google Scholar] [CrossRef]
- Liang, X.-Q.; Yan, S.-L. New species and subspecies of Caridina (Decapoda, Caridea) from Fukien, China. Acta Hydrobiol. Sin. 1977, 6, 219–225. [Google Scholar]
- Wowor, D.; Cai, Y.; Ng PK, L. Crustacea: Decapoda, Caridea. In Freshwater Invertebrates of the Malaysian Region; Yule, C.M., Sen, Y.H., Eds.; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2004; pp. 337–357. [Google Scholar]
- Cai, Y.; Ng, P.K.L.; Choy, S. Freshwater shrimps of the family Atyidae (Crustacea: Decapoda: Caridea) from Peninsular Malaysia and Singapore. Raffles Bull. Zool. 2007, 55, 277–309. [Google Scholar]
- Cai, Y. Atyid shrimps of Hainan Island, southern China, with the description of a new species of Caridina (Crustacea, Decapoda, Atyidae). In Advances in Freshwater Decapod Systematics and Biology; Yeo, D.C.J., Cumberlidge, N., Klaus, S., Eds.; BRILL: Leiden, The Netherlands, 2014; Volume 19, pp. 207–231. [Google Scholar] [CrossRef]
- Yeo, D.C.J.; Cai, Y.; Ng, P.K.L. The freshwater and terrestrial decapod Crustacea of Pulau Tioman, Peninsular Malaysia. Raffles Bull. Zool. 1999, 47 (Suppl. 6), 197–244. [Google Scholar]
- De Grave, S. Caridina elongopoda. IUCN Red List Threat. Species 2013, e.T197578A2491525. [Google Scholar] [CrossRef]
- Choy, S.C. Caridina bruneiana, a new species of freshwater shrimp (Decapoda, Caridea, Atyidae) from Negara Brunei Darussalam, Borneo. Zool. Scr. 1992, 21, 49–55. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Boseto, D.; Marquet, G.; Keith, P. Solomon’s Gold Mine: Description or redescription of 24 species of Caridina (Crustacea: Decapoda: Atyidae) freshwater shrimps from the Solomon Islands, including 11 new species. Eur. J. Taxon. 2020, 696, 1–86. [Google Scholar] [CrossRef]
- De Grave, S.; Cai, Y. Caridina bruneiana (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T197857A147790079. [Google Scholar] [CrossRef]
- Ortmann, A.E. Crustaceen. In Zoologische Forschungsreisen in Australien und dem Malayischen Archipel. Mit Unterstutzung des Herrn Dr. Paul von Ritter ausgefuhrt in den Jahren 1891–1893. V. Denkschriften Der Medizinisch–Naturwissenschaftlichen Gesellschaft Zu Jena; Semon, R., Ed.; Gustav Fischer: Iena, Germany, 1894; Volume 8, pp. 3–80. [Google Scholar]
- Ortmann, A.E. A Study of the Systematic and Geographical Distribution of the Decapod Family Atyidæ Kingsley. Proc. Acad. Nat. Sci. Phila. 1894, 46, 397–416. [Google Scholar]
- Roux, J. Décapodes d’eau douce de Célèbes (Genres Caridina et Potamon). Rev. Suisse Zool. 1904, 12, 539–572. [Google Scholar] [CrossRef]
- Bouvier, E.L. Sur la classification du genre Caridina et les variations extraordinaires d’une espèce de ce genre, la Caridina brevirostris Stimpson. Comptes Rendus Hebd. Séances L’Académie Sci. 1912, 154, 915–922. [Google Scholar]
- Bouvier, E.-L. Les Caridines des Seychelles. Trans. Linn. Soc. Lond. 1913, 15, 447–472. [Google Scholar] [CrossRef][Green Version]
- Bouvier, E.L. Recherches sur la morphologie, les variations, la distribution géographique des Crevettes de la famille des Atyidés. In Encyclopédie Entomologique; de Roret: Paris, France, 1925. [Google Scholar]
- Page, T.J.; von Rintelen, K.; Hughes, J.M. An island in the stream: Australia’s place in the cosmopolitan world of Indo-West Pacific freshwater shrimp (Decapoda: Atyidae: Caridina). Mol. Phylogenetics Evol. 2007, 43, 645–659. [Google Scholar] [CrossRef] [PubMed]
- de Mazancourt, V.; Marquet, G.; Klotz, W.; Keith, P.; Castelin, M. When molecules and morphology work together: Lines of evidence for the validity of Caridina buehleri Roux (Crustacea: Decapoda: Atyidae) and for C. gueryi Marquet, Keith & Kalfatak as its junior synonym. Invertebr. Syst. 2017, 31, 220–230. [Google Scholar] [CrossRef]
- De Grave, S.; Cai, Y.; Wowor, D. Caridina brevicarpalis (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T197757A147787449. [Google Scholar] [CrossRef]
- Karge, A.; Klotz, W. Süßwassergarnelen aus aller Welt; Dähne: Ettlingen, Baden-Wuerttemberg, Germany, 2007. [Google Scholar]
- De Grave, S.; Wowor, D.; Cai, Y. Caridina endehensis (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T197868A147790217. [Google Scholar] [CrossRef]
- Cai, Y.; Ng, P.K.L. A revision of the Caridina gracilirostris de Man, 1892, species group, with descriptions of two new taxa (Decapoda; Caridea; Atyidae). J. Nat. Hist. 2007, 41, 1585–1602. [Google Scholar] [CrossRef]
- Blanco, G.J. Four new philippine species of fresh-water shrimps of the genus Caridina. Philipp. J. Sci. 1939, 70, 389–403. [Google Scholar]
- Cai, Y.; Shokita, S. Atyid shrimps (Crustacea: Decapoda: Caridea) of the Ryukyu Islands, southern Japan, with descriptions of two new species. J. Nat. Hist. 2006, 40, 2123–2172. [Google Scholar] [CrossRef]
- Inui, N.; Maruyama, T.; Okamoto, K. First record of Australatya obscura Han & Klotz, 2015 (Decapoda, Atyidae) from the Ryukyu Islands, Japan. Biodivers. Data J. 2019, 7, e30507. [Google Scholar] [CrossRef]
- Dwiyanto, D.; Annawaty, A.; Farajallah, A.; Wowor, D. A preliminary survey of the freshwater shrimp genus Caridina from Eastern Sulawesi, Indonesia. Trop. Nat. Hist. 2021, 21, 337–342. [Google Scholar]
- De Grave, S.; Cai, Y. Caridina laoagensis (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T197704A147786522. [Google Scholar] [CrossRef]
- Nobili, G. Decapodi e isopodi della Nuova Guinea Tedesca raccolti dal Sign. L. Biró. Ann. Musei Natl. Hung. 1905, 3, 480–507. [Google Scholar]
- Roux, J. Crustacés. (Expédition de 1903). Nova Guinea. Résultats de l’Expédition Scientifique Néerlandaise à La Nouvelle-Guinée En 1903 Sous Les Auspices de Arthur Wichmann 5 (Zoologie); Brill: Leiden, The Netherlands, 1917; pp. 589–621. [Google Scholar]
- Roux, J. Notes de Carcinologie mélanésienne I. Décapodes d’eau douce de l’Archipel Bismarck et des iles de l’Amirauté. Rev. Suisse Zool. 1934, 41, 217–234. [Google Scholar] [CrossRef]
- de Mazancourt, V.; Klotz, W.; Marquet, G.; Mos, B.; Rogers, D.C.; Keith, P. The complex study of complexes: The first well-supported phylogeny of two species complexes within genus Caridina (Decapoda: Caridea: Atyidae) sheds light on evolution, biogeography, and habitat. Mol. Phylogenetics Evol. 2019, 131, 164–180. [Google Scholar] [CrossRef] [PubMed]
- De Grave, S.; Wowor, D.; Cai, Y.; Klotz, W. Caridina papuana (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T198195A147796299. [Google Scholar] [CrossRef]
- Bouvier, E.-L. Observations nouvelles sur les crevettes de la famille des Atyidés. Bull. Sci. La Fr. La Belg. 1905, 39, 57–134. [Google Scholar]
- De Grave, S.; Cai, Y.; Wowor, D. Caridina parvirostris (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T197886A147790562. [Google Scholar] [CrossRef]
- Cai, Y. Atydina, a new genus for Caridina atyoides Nobili, 1900, from Indonesia (Crustacea: Decapoda: Atyidae). Zootaxa 2010, 2372, 75–79. [Google Scholar] [CrossRef]
- De Grave, S.; Wowor, D.; Cai, Y. Atydina atyoides (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T198170A147795833. [Google Scholar] [CrossRef]
- Kamita, T. Notes on the freshwater shrimps from the Iwami and Izumo districts of San-in Province, Japan. Bull. Shimane Univ. (Nat. Sci.) 1951, 1, 71–82. [Google Scholar]
- Kamita, T. Studies of the Freshwater Shrimps, Prawns and Crawfishs from Japan; Sonoyama-shoten: Matsue, Japan, 1961. [Google Scholar]
- Fujino, T. Taxonomy of Freshwater shrimps from Japan, with a key to all species. Nat. Study 1972, 18, 5–10. [Google Scholar]
- Shokita, S. The distribution and speciation of the inland water shrimps and prawns from the Ryukyu Islands—I. Bull. Sci. Eng. Div. Univ. Ryukyus Math. Nat. Sci. 1975, 18, 115–136. [Google Scholar]
- Hayashi, K.-I. Prawns, shrimps and lobsters from Japan (49), Family Atyidae—Genus Caridina (3) and Antecaridina. Aquabiololgy 1989, 64, 376–379. [Google Scholar]
- Shokita, S. Atyidae. In The Flora and Fauna of Inland Waters in the Ryukyu Islands; Nishida, M., Shikatani, N., Shokita, S., Eds.; Tokai University Press: Yoyogi, Tokyo, 2003; pp. 249–254. [Google Scholar]
- von Rintelen, K.; Karge, A.; Klotz, W. News from a small island—First record of a freshwater shrimp (Decapoda, Atyidae, Caridina) from Peleng, Banggai Islands, Indonesia. J. Nat. Hist. 2008, 42, 2243–2256. [Google Scholar] [CrossRef]
- Liang, X.Q.; Zheng, M.-Q. Notes on Caridina from Fujian, China (Decapoda: Caridea). Acta Zootaxonomica Sin. 1988, 13, 15–19. [Google Scholar]
- Liang, X.Q.; Zhou, J. Study on new atyid shrimps (Decapoda, Caridea) from Guangxi, China. Acta Hydrobiol. Sin. 1993, 17, 231–239. [Google Scholar]
- Liang, X.-Q. Fauna Sinica. Invertebrata Vol. 36. Crustacea Decapoda Atyidae; Science Press: Beijing, China, 2004. [Google Scholar]
- De Grave, S.; Shy, J.; Cai, Y. Caridina celebensis (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T198287A147797943. [Google Scholar] [CrossRef]
- Milne Edwards, H. Histoire Naturelle des Crustaces, Comprenant L’anatomie, la Physiologie et la Classification de ces Animaux; Librairie encyclopédique de Roret: Paris, France, 1837. [Google Scholar]
- Bernardes, S.C.; Pepato, A.R.; von Rintelen, T.; von Rintelen, K.; Page, T.J.; Freitag, H.; de Bruyn, M. The complex evolutionary history and phylogeography of Caridina typus (Crustacea: Decapoda): Long-distance dispersal and cryptic allopatric species. Sci. Rep. 2017, 7, 9044. [Google Scholar] [CrossRef]
- Giebel, C.G. Caridina siamensis n. sp. Z. Für Die Gesammten Nat. 1863, 21, 329–330. [Google Scholar]
- De Grave, S. Caridina typus. IUCN Red List Threat. Species 2013, e.T198327A2520928. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Chen, W.-J.; Guo, Z.-L. Caridean prawn (Crustacea, Decapoda) from Dong’ao Island, Guangdong, China. Zootaxa 2018, 4399, 315–328. [Google Scholar] [CrossRef]
- Xu, D.-J.; Li, D.-X.; Zheng, X.-Z.; Guo, Z.-L. Caridina sinanensis, a new species of stygobiotic atyid shrimp (Decapoda, Caridea, Atyidae) from a karst cave in the Guizhou Province, southwestern China. ZooKeys 2020, 1008, 17–35. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Chen, W.-J.; Zheng, X.-Z.; Guo, Z.-L. Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 2020, 923, 15–32. [Google Scholar] [CrossRef]
- Feng, S.; Chen, Q.-H.; Guo, Z.-L. Utilizing integrative taxonomy uncovers a new stygobiotic Caridina species (Decapoda: Caridea: Atyidae) from Guizhou Province, China. ZooKeys 2021, 1028, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-S.; Chan, T.-Y.; Yu, H.-P. Atyid Shrimps (Decapoda: Caridea) of Taiwan, With Descriptions of Three New Species. J. Crustacean Biol. 1993, 13, 481–503. [Google Scholar] [CrossRef]
- Shy, J.Y.; Yu, H.P. Freshwater Shrimps of Taiwan; National Museum of Marine Biology and Aquarium: Pingtung County, Taiwan, 1998. [Google Scholar]
- Cai, Y.; Ng, P.K.L. The freshwater decapod crustaceans of Halmahera, Indonesia. J. Crustac. Biol. 2001, 21, 665–695. [Google Scholar] [CrossRef][Green Version]
- Vijayamma, J.K.; Dhamorikar, A.; Manchi, S. A new species of Caridina H. Milne Edwards, 1837 (Family: Atyidae) from a limestone cave on Interview Island, Andaman and Nicobar Islands, India. Zootaxa 2021, 5057, 402–414. [Google Scholar] [CrossRef]
- De Grave, S.; Shy, J.; Cai, Y.; Wowor, D. Caridina villadolidi (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T198063A147793726. [Google Scholar] [CrossRef]
- Hancock, M.A. The relationship between egg size and embryonic and larval development in the freshwater shrimp Paratya australiensis Kemp (Decapoda: Atyidae). Freshw. Biol. 1998, 39, 715–723. [Google Scholar] [CrossRef]
- Han, C.C.; Chang, C.S.; Cheng, I.M.; Fang, L.S.; Tew, K.S. Population dynamics of a landlock and amphidromous freshwater shrimp, Caridina gracilipes (Decapoda: Caridea) in subtropical waters. J. Crustac. Biol. 2011, 31, 278–285. [Google Scholar] [CrossRef]
- De Grave, S.; Cai, Y.; Klotz, W.; Wowor, D. Edoneus atheatus (errata version published in 2019). IUCN Red List Threat. Species 2013, e.T198113A147794626. [Google Scholar] [CrossRef]
- Nagai, H.; Kitano, T.; Imai, H. Molecular Phylogenetic Analysis of Caridina weberi Species Group around Japan, with the First Record of Caridina tupaia de Mazancourt, Marquet & Keith, 2019 (Crustacea: Decapoda: Atyidae) from Japan. Eur. J. Aquat. Sci. 2022, 1, 1–8. [Google Scholar] [CrossRef]
- von Rintelen, K.; Page, T.J.; Cai, Y.; Roe, K.; Stelbrink, B.; Kuhajda, B.R.; Iliffe, T.M.; Hughes, J.; von Rintelen, T. Drawn to the dark side: A molecular phylogeny of freshwater shrimps (Crustacea: Decapoda: Caridea: Atyidae) reveals frequent cave invasions and challenges current taxonomic hypotheses. Mol. Phylogenetics Evol. 2012, 63, 82–96. [Google Scholar] [CrossRef]
- Klotz, W.; von Rintelen, T. To “bee” or not to be—On some ornamental shrimp from Guangdong Province, Southern China and Hong Kong SAR, with descriptions of three new species. Zootaxa 2014, 3889, 151–184. [Google Scholar] [CrossRef]



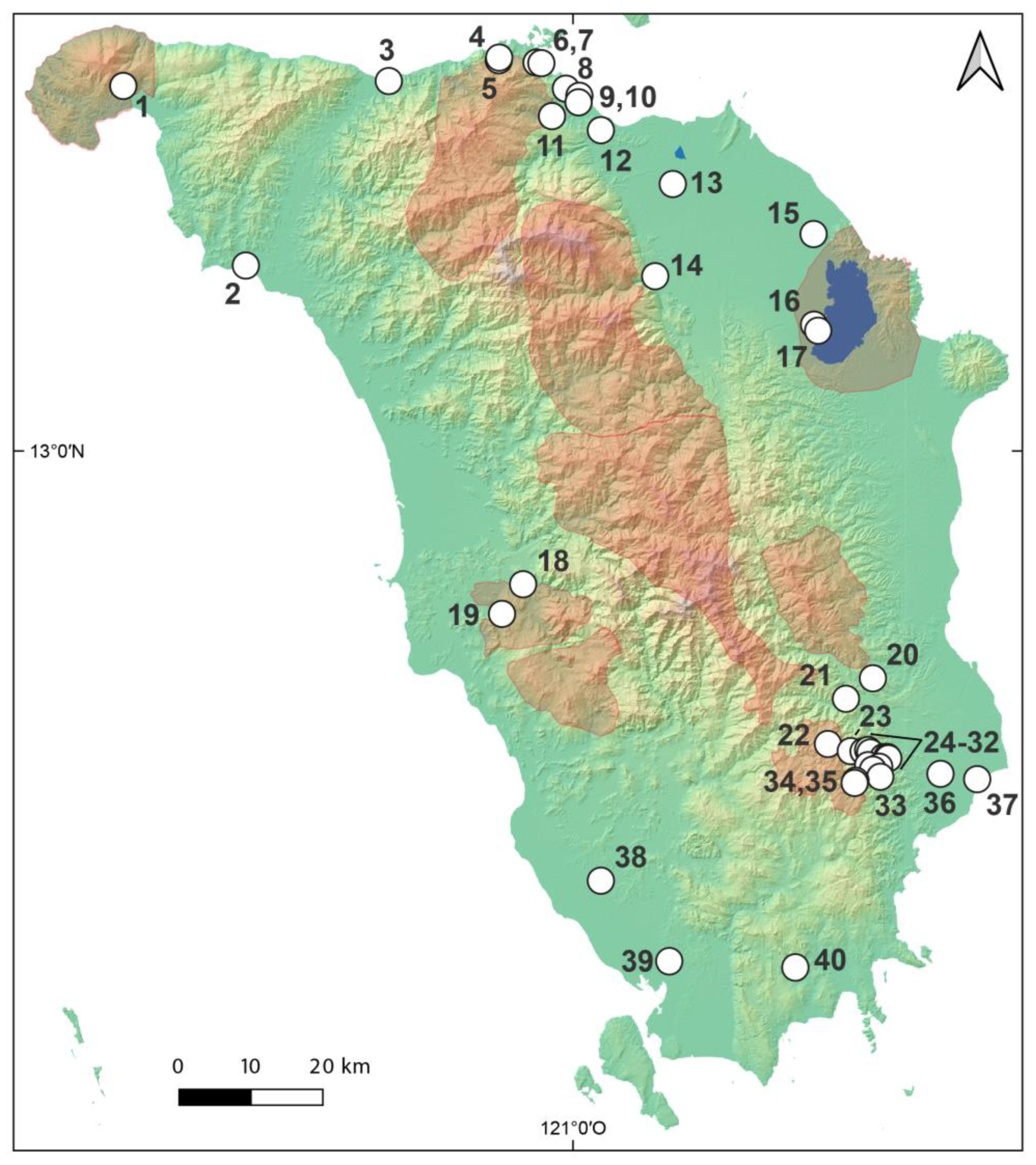
| Locality | Locality | Latitude | Longitude | Species Found |
|---|---|---|---|---|
| 1 | Paluan, Bgy. Harrison, small mountain river NE of summit Mt. Calavite | 13.4542 | 120.425 | Atydina atyoides Caridina parvirostris |
| 2 | Mamburao, Bgy. Tayainaan, Tugilan River/Sitio, 1 km upstream of estuary | 13.2308 | 120.5817 | Caridina gracilipes Caridina villadolidi |
| 3 | Abra de Ilog, lowland creek in a rural area | 13.4603 | 120.7644 | Caridina celebensis Caridina gracilipes |
| 4 | Puerto Galera, NR km 59, downstr. Aniuan Falls | 13.4862 | 120.905 | Caridina laoagensis Caridina papuana Caridina villadolidi |
| 5 | Puerto Galera, 8 km W of Muelle, Talipanan Riv. | 13.4894 | 120.9056 | Caridina elongapoda Caridina laoagensis Caridina papuana Caridina typus |
| 6 | Puerto Galera, NR km 49, Tagbinai Munti River | 13.4833 | 120.953 | Caridina papuana |
| 7 | Puerto Galera, NR km 48.2, Tagbinai Malaki River | 13.4825 | 120.9594 | Caridina endehensis |
| 8 | Puerto Galera, NR km 37.2, downstream of Tamaraw Falls | 13.4508 | 120.9908 | Caridina brevicarpalis Caridina celebensis Caridina cf. elongapoda Caridina endehensis Caridina laoagensis Caridina papuana Caridina parvirostris |
| 9 | Puerto Galera, small creek | 13.4622 | 120.9864 | Caridina brevicarpalis Caridina celebensis Caridina gracilipes Caridina sp. ‘Palawan’ |
| 10 | Puerto Galera, Bisayaan River | 13.4333 | 120.9731 | Caridina laoagensis Caridina papuana Caridina parvirostris |
| 11 | Puerto Galera, Calsapa, lower Bisaan River | 13.4153 | 120.9575 | Caridina serratirostris |
| 12 | Baco, small creek | 13.3992 | 121.0356 | Caridina typus |
| 13 | Baco, Pinagsabangan River | 13.3322 | 121.1275 | Caridina brevicarpalis Caridina endehensis Caridina gracilipes Caridina laoagensis Caridina serratirostris |
| 14 | Baco, Tagbungan, Lantuyan mountain river | 13.3044 | 121.0642 | Caridina laoagensis Caridina papuana Caridina typus |
| 15 | Naujan, Bancuro | 13.2700 | 121.3075 | Caridina celebensis |
| 16 | Victoria, Malayas, Malayas River, W Naujan tributary | 13.1572 | 121.3081 | Caridina endehensis |
| 17 | Victoria, Malayas, W coast Naujan Lake | 13.1497 | 121.3133 | Caridina leptopoda sp. nov. |
| 18 | Sablayan, large mountain river | 12.8339 | 120.9361 | Caridina endehensis |
| 19 | Sablayan, SPPF, small limestone river | 12.7969 | 120.9092 | Caridina laoagensis |
| 20 | Bongabong, Formon, Pastuhan, Tangisan Falls | 12.7167 | 121.3833 | Caridina laoagensis |
| 21 | Bongabong, Lisap, lower Siange River | 12.6911 | 121.3489 | Caridina laoagensis |
| 22 | Roxas, San Vicente, Sitio Tauga Diit, Baroc River tributary Tauga Daka | 12.6347 | 121.3258 | Caridina laoagensis |
| 23 | Roxas, San Vicente, Sitio Taugad Diit, Baroc River tributary Taugad Daka | 12.6325 | 121.3367 | Caridina laoagensis |
| 24 | Roxas, San Vicente, Sitio Taugad Diit, Baroc River tributary Taugad Diit River | 12.6256 | 121.3714 | Caridina laoagensis |
| 25 | Roxas, San Vicente, Taugad River unnamed tributary | 12.6272 | 121.3772 | Caridina laoagensis |
| 26 | Roxas, San Vicente, Baroc River tributary Hiyong Creek | 12.6242 | 121.3808 | Caridina laoagensis |
| 27 | Roxas, San Vicente, Taugad River | 12.6183 | 121.3970 | Caridina laoagensis |
| 28 | Roxas, San Vicente, Baroc River | 12.6169 | 121.4031 | Caridina zhujiangensis |
| 29 | Roxas, Baroc River | 12.6169 | 121.4031 | Caridina bruneiana Caridina laoagensis |
| 30 | Roxas, San Vicente, Sitio Quirao, Hinundungan River tributary Tagugoy Creek | 12.6083 | 121.3772 | Caridina laoagensis |
| 31 | Roxas, San Vicente, Sitio Quirao, Baroc River tributary Hinundungan River | 12.6064 | 121.3914 | Caridina laoagensis |
| 32 | Roxas, San Vicente, Sitio Quirao, Hinundungan River tributary Quirao Buhay Creek | 12.6028 | 121.3833 | Caridina laoagensis |
| 33 | Roxas, San Vicente, Sitio Quirao, Hinundungan River tributary Quirao na Balete Creek | 12.5939 | 121.3928 | Caridina laoagensis |
| 34 | Roxas, San Vicente, Sitio Tagaskan, Hinundungan River tributary Quianao Creek | 12.5889 | 121.3611 | Caridina laoagensis |
| 35 | Roxas, San Vicente, Upper Hinundungan River | 12.5861 | 121.3600 | Caridina laoagensis Caridina sp. |
| 36 | Roxas, Baroc River | 12.5975 | 121.4697 | Caridina laoagensis Caridina villadolidi |
| 37 | Roxas, Bagumbayan, Magugo River | 12.5928 | 121.5175 | Caridina serratirostris Caridina elongapoda |
| 38 | San Jose, “Central”, Purok Tunnel, Busuanga River | 12.4642 | 121.0356 | Atyopsis spinipes Caridina endehensis Caridina laoagensis Caridina villadolidi |
| 39 | San Jose, Palangeran River | 12.3638 | 121.1230 | Caridina gracilipes Caridina serratirostris |
| 40 | Bulalacao, Bagonsikat, Paluguan River | 12.3561 | 121.2842 | Caridina laoagensis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mazancourt, V.; Freitag, H.; von Rintelen, K.; Manuel-Santos, M.; von Rintelen, T. Updated Checklist of the Freshwater Shrimps (Decapoda: Caridea: Atyidae) of Mindoro Island, the Philippines, with a Description of a New Species of Caridina. Arthropoda 2023, 1, 374-397. https://doi.org/10.3390/arthropoda1040015
de Mazancourt V, Freitag H, von Rintelen K, Manuel-Santos M, von Rintelen T. Updated Checklist of the Freshwater Shrimps (Decapoda: Caridea: Atyidae) of Mindoro Island, the Philippines, with a Description of a New Species of Caridina. Arthropoda. 2023; 1(4):374-397. https://doi.org/10.3390/arthropoda1040015
Chicago/Turabian Stylede Mazancourt, Valentin, Hendrik Freitag, Kristina von Rintelen, Marivene Manuel-Santos, and Thomas von Rintelen. 2023. "Updated Checklist of the Freshwater Shrimps (Decapoda: Caridea: Atyidae) of Mindoro Island, the Philippines, with a Description of a New Species of Caridina" Arthropoda 1, no. 4: 374-397. https://doi.org/10.3390/arthropoda1040015
APA Stylede Mazancourt, V., Freitag, H., von Rintelen, K., Manuel-Santos, M., & von Rintelen, T. (2023). Updated Checklist of the Freshwater Shrimps (Decapoda: Caridea: Atyidae) of Mindoro Island, the Philippines, with a Description of a New Species of Caridina. Arthropoda, 1(4), 374-397. https://doi.org/10.3390/arthropoda1040015