Adjuvant Pluronic F68 Is Compatible with a Plant Root-Colonizing Probiotic, Pseudomonas chlororaphis O6
Abstract
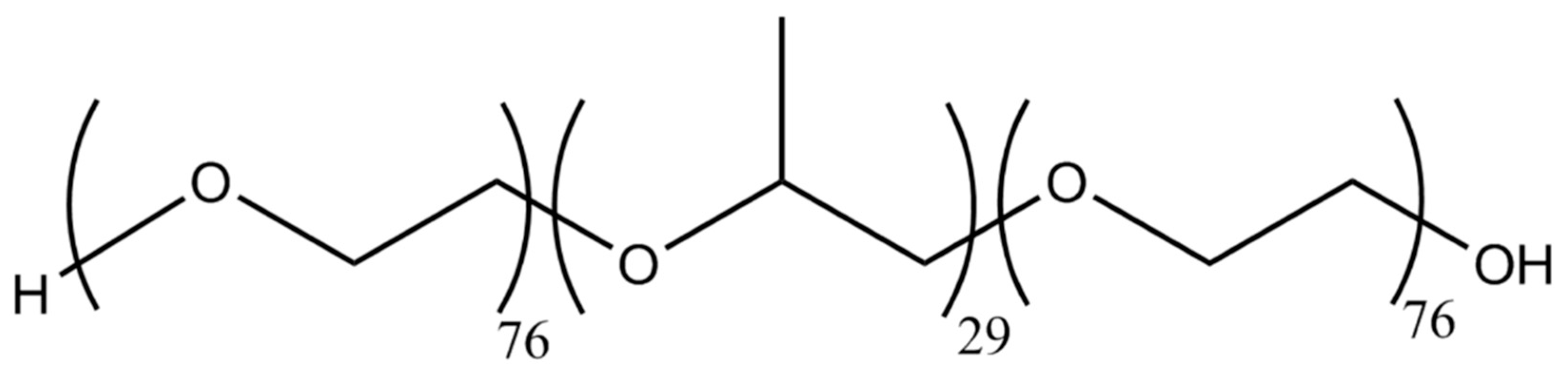
:1. Introduction
2. Materials and Methods
2.1. Effects of F68 on Growth of PcO6 in Liquid Culture
2.2. F68 Effects on PcO6 Swarming Mobility
2.3. Labeling of PcO6 Cells with fF68
2.4. Cryoprotection by F68 for PcO6 Cell Suspensions
3. Results
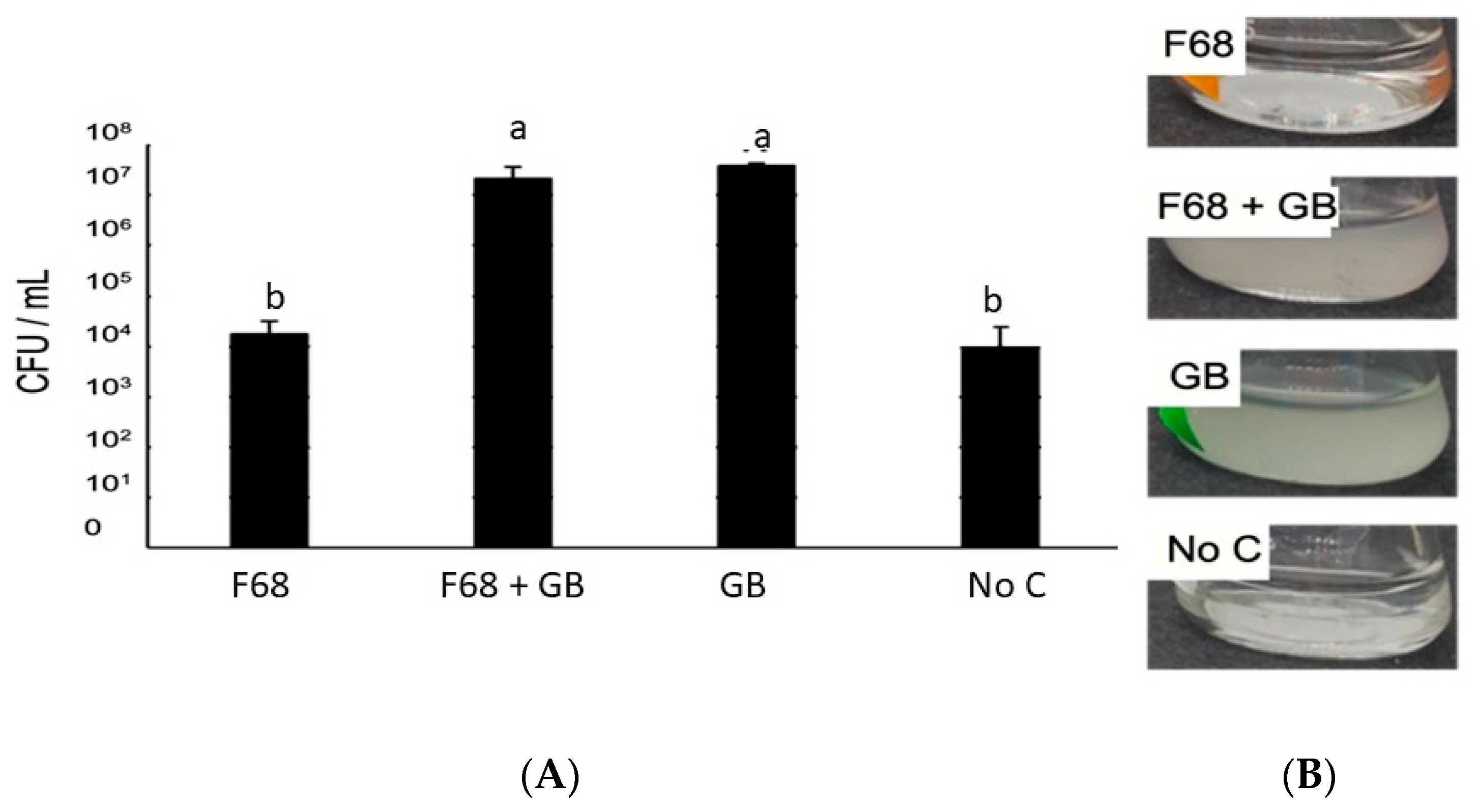
3.1. Growth of PcO6 in Liquid Cultures and Its Phenazine Production Are Not Altered by the Presence of F68
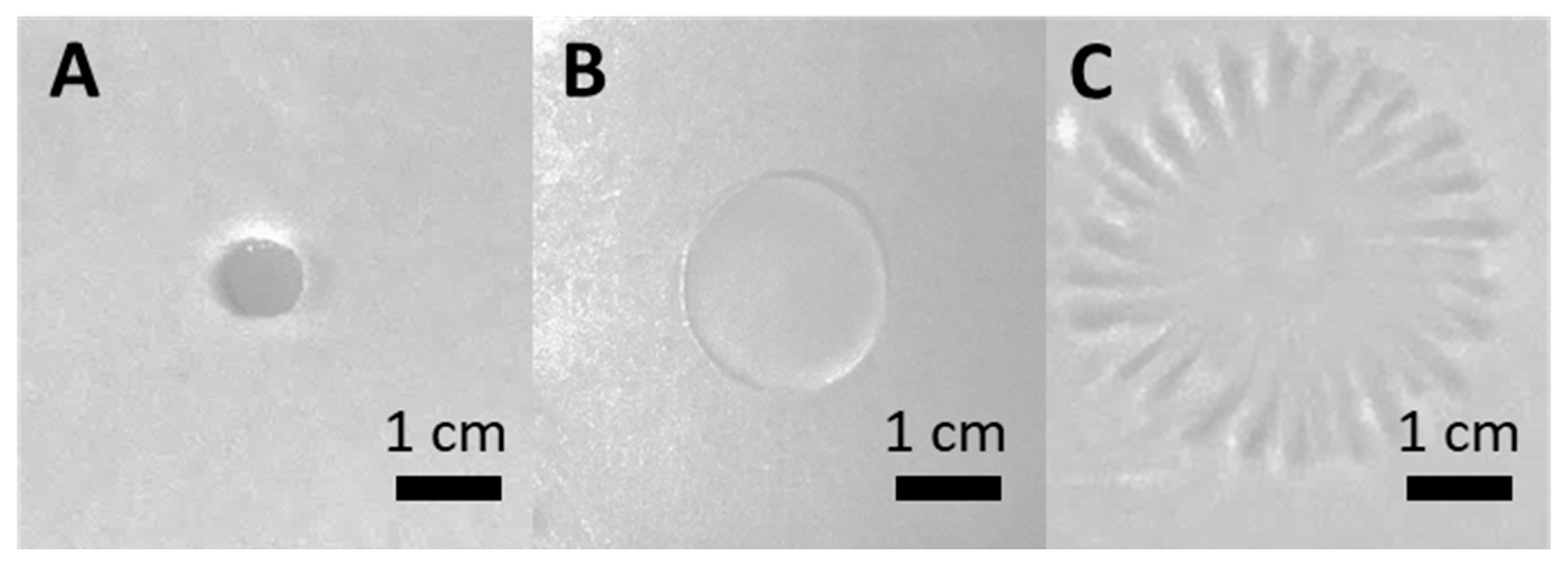
3.2. F68 Enhances the Swarming Behavior of PcO6
3.3. PcO6 Cells Become Fluorescent When Exposed to fF68
3.4. Suspension in F68 Does Not Provide Cryoprotection to PcO6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hazen, J.L. Adjuvants—Terminology, Classification, and Chemistry. Weed Technol. 2000, 14, 773–784. [Google Scholar] [CrossRef]
- Bertrand, A.; Castonguay, Y. Plant Adaptations to Overwintering Stresses and Implications of Climate Change. Can. J. Bot. 2003, 81, 1145–1152. [Google Scholar] [CrossRef]
- Willick, I.R.; Tanino, K.K.; Gusta, L.V. The Impact of Global Climate Change on the Freezing Tolerance of Winter Cereals in Western Canada. J. Agron. Crop Sci. 2021, 207, 88–99. [Google Scholar] [CrossRef]
- Murhammer, D.W.; Goochee, C.F. Sparged Animal Cell Bioreactors: Mechanism of Cell Damage and Pluronic F-68 Protection. Biotechnol. Prog. 1990, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Erickson, L.E.; Yiin, T.-Y.; Glasgow, L.A. A Review of the Effects of Shear and Interfacial Phenomena on Cell Viability. Crit. Rev. Biotechnol. 1993, 13, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Palomares, L.A.; González, M.; Ramírez, O.T. Evidence of Pluronic F-68 Direct Interaction with Insect Cells: Impact on Shear Protection, Recombinant Protein, and Baculovirus Production. Enzym. Microb. Technol. 2000, 26, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; River, L.P.; Pan, F.S.; Ji, L.; Wollmann, R.L. Surfactant-Induced Sealing of Electropermeabilized Skeletal Muscle Membranes in Vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 4524–4528. [Google Scholar] [CrossRef] [PubMed]
- Moloughney, J.G.; Weisleder, N. Poloxamer 188 (P188) as a Membrane Resealing Reagent in Biomedical Applications. Recent Pat. Biotechnol. 2012, 6, 200–211. [Google Scholar] [CrossRef]
- Houang, E.M.; Bates, F.S.; Sham, Y.Y.; Metzger, J.M. All-Atom Molecular Dynamics-Based Analysis of Membrane-Stabilizing Copolymer Interactions with Lipid Bilayers Probed under Constant Surface Tensions. J. Phys. Chem. B 2017, 121, 10657–10664. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef]
- Gill, M.I.S.; Cancino, G.O.; Anthony, P.; Davey, M.R.; Power, J.B.; Lowe, K.C. Pluronic F-68 Enhanced Shoot Regeneration in Micropropagated Citrus Rootstock and Passiflora Species. Acta Biotechnol. 2003, 23, 349–358. [Google Scholar] [CrossRef]
- Kumar, V.; Laouar, L.; Davey, M.R.; Mulligan, B.J.; Lowe, K.C. Pluronic F-68 Stimulates Growth of Solanum dulcamara in Culture. J. Exp. Bot. 1992, 43, 487–493. [Google Scholar] [CrossRef]
- Kok, A.D.-X.; Wan Abdullah, W.M.A.N.; Tan, N.-P.; Ong-Abdullah, J.; Sekeli, R.; Wee, C.-Y.; Lai, K.-S. Growth Promoting Effects of Pluronic F-68 on Callus Proliferation of Recalcitrant Rice Cultivar. 3 Biotechnol. 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.D.-X.; Mohd Yusoff, N.F.; Sekeli, R.; Wee, C.-Y.; Lamasudin, D.U.; Ong-Abdullah, J.; Lai, K.-S. Pluronic F-68 Improves Callus Proliferation of Recalcitrant Rice Cultivar via Enhanced Carbon and Nitrogen Metabolism and Nutrients Uptake. Front. Plant Sci. 2021, 12, 667434. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.; Jelodar, N.B.; Lowe, K.C.; Power, J.B.; Davey, M.R. Pluronic F-68 Increases the Post-Thaw Growth of Cryopreserved Plant Cells. Cryobiology 1996, 33, 508–514. [Google Scholar] [CrossRef]
- Lowe, K.C.; Anthony, P.; Davey, M.R.; Power, J.B. Beneficial Effects of Pluronic F-68 and Artificial Oxygen Carriers on the Post-Thaw Recovery of Cryopreserved Plant Cells. Artif. Cells Blood Substit. Biotechnol. 2001, 29, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, A.; Anderson, A.J.; Britt, D.W. Pluronic F68-Capped SiO2 Nanoparticles Are Compatible as Delivery Vehicles to Roots and Shoots. MRS Adv. 2022, 7, 327–332. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Berendsen, R.L.; de Jonge, R.; Stringlis, I.A.; Van Dijken, A.J.H.; Van Pelt, J.A.; Van Wees, S.C.M.; Yu, K.; Zamioudis, C.; Bakker, P.A.H.M. Pseudomonas simiae WCS417: Star Track of a Model Beneficial Rhizobacterium. Plant Soil 2021, 461, 245–263. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Dar, D.; Thomashow, L.S.; Weller, D.M.; Newman, D.K. Global Landscape of Phenazine Biosynthesis and Biodegradation Reveals Species-specific Colonization Patterns in Agricultural Soils and Crop Microbiomes. Elife 2020, 9, e59726. [Google Scholar] [CrossRef]
- Radtke, C.; Cook, W.S.; Anderson, A. Factors Affecting Antagonism of the Growth of Phanerochaete chrysosporium by Bacteria Isolated from Soils. Appl. Microbiol. Biotechnol. 1994, 41, 274–280. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R,3R-Butanediol, a Bacterial Volatile Produced by Pseudomonas chlororaphis O6, Is Involved in Induction of Systemic Tolerance to Drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Pierson, L.S., III; Pierson, E.A. Phenazine Antibiotic Production in Pseudomonas aureofaciens: Role in Rhizosphere Ecology and Pathogen Suppression. FEMS Microbiol. Lett. 1996, 136, 101–108. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phenazines in Plant-Beneficial Pseudomonas Spp.: Biosynthesis, Regulation, Function and Genomics. Environ. Microbiol. 2018, 20, 3905–3917. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.R.; Han, S.H.; Zdor, R.E.; Anderson, A.J.; Spencer, M.; Yang, K.Y.; Kim, Y.H.; Lee, M.C.; Cho, B.H.; Kim, Y.C. Inhibition of Seed Germination and Induction of Systemic Disease Resistance by Pseudomonas chlororaphis O6 Requires Phenazine Production Regulated by the Global Regulator, GacS. J. Microbiol. Biotechnol. 2007, 17, 586–593. [Google Scholar] [PubMed]
- Housley, L.; Anderson, T.; Sontag, N.; Han, S.-H.; Britt, D.W.; Anderson, A.J. Pluronics’ Influence on Pseudomonad Biofilm and Phenazine Production. FEMS Microbiol. Lett. 2009, 293, 148–153. [Google Scholar] [CrossRef]
- Perneel, M.; D’hondt, L.; De Maeyer, K.; Adiobo, A.; Rabaey, K.; Höfte, M. Phenazines and Biosurfactants Interact in the Biological Control of Soil-Borne Diseases Caused by Pythium Spp. Environ. Microbiol. 2008, 10, 778–788. [Google Scholar] [CrossRef]
- Stanghellini, M.E. Inhibitory and Lytic Effects of a Nonionic Surfactant on Various Asexual Stages in the Life Cycle of Pythium and Phytophthora Species. Phytopathology 1987, 77, 112. [Google Scholar] [CrossRef]
- Goodman, J.; Mclean, J.E.; Britt, D.W.; Anderson, A.J. Sublethal Doses of ZnO Nanoparticles Remodel Production of Cell Signaling Metabolites in the Root Colonizer Pseudomonas chlororaphis O6. Environ. Sci. Nanotechnol. 2016, 3, 1103–1113. [Google Scholar] [CrossRef]
- Pierson, L.S.; Pierson, E.A. Metabolism and Function of Phenazines in Bacteria: Impacts on the Behavior of Bacteria in the Environment and Biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef]
- Kearns, D.B. A Field Guide to Bacterial Swarming Motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.T.; Pocard, J.A.; Bernard, T.; Le Rudulier, D. Osmotic Control of Glycine Betaine Biosynthesis and Degradation in Rhizobium meliloti. J. Bacteriol. 1988, 170, 3142–3149. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants Under Abiotic Stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- de Weger, L.A.; van der Bij, A.J.; Dekkers, L.C.; Simons, M.; Wijffelman, C.A.; Lugtenberg, B.J.J. Colonization of the Rhizosphere of Crop Plants by Plant-Beneficial Pseudomonads. FEMS Microbiol. Ecol. 1995, 17, 221–227. [Google Scholar] [CrossRef]
- Cane, J. Global Warming, Advancing Bloom and Evidence for Pollinator Plasticity from Long-Term Bee Emergence Monitoring. Insects 2021, 12, 457. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Loper, J.E. The Sigma Factor RpoS Is Required for Stress Tolerance and Environmental Fitness of Pseudomonas fluorescens Pf-5. Microbiology 2005, 151, 3001–3009. [Google Scholar] [CrossRef]
- Oh, S.A.; Kim, J.S.; Han, S.H.; Park, J.Y.; Dimkpa, C.; Edlund, C.; Anderson, A.J.; Kim, Y.C. The GacS-Regulated Sigma Factor RpoS Governs Production of Several Factors Involved in Biocontrol Activity of the Rhizobacterium Pseudomonas chlororaphis O6. Can. J. Microbiol. 2013, 59, 556–562. [Google Scholar] [CrossRef]
- Ashwood-Smith, M.J.; Voss, W.A.G.; Warby, C. Cryoprotection of Mammalian Cells in Tissue Culture with Pluronic polyols. Cryobiology 1973, 10, 502–504. [Google Scholar] [CrossRef]
- Doğan, A.; Yalvaç, M.E.; Yılmaz, A.; Rizvanov, A.; Şahin, F. Effect of F68 on Cryopreservation of Mesenchymal Stem cells derived from Human Tooth Germ. Appl. Biochem. Biotech. 2013, 171, 1819–1831. [Google Scholar] [CrossRef]
- Glauser, S.C.; Talbot, T.R. Some studies on freezing and thawing human erythrocytes. Am. J. Med. Sci. 1956, 231, 75–81. [Google Scholar] [CrossRef]
- González Hernández, Y.; Fischer, R.W. Serum-free Culturing of Mammalian Cells—Adaptation to and Cryopreservation in Fully Defined Media. ALTEX 2007, 24, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lei, J.; Zhang, M.; Wu, F.; Ren, M.; Yang, J.; Wu, Q.; Shi, X. Optimization of Preservation Methods Provides Insights into Photosynthetic Picoeukaryotes in Lakes. Microbiol. Spectr. 2022, 10, e02557-21. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streeter, A.R.; Cartwright, A.; Zargaran, M.; Wankhade, A.; Anderson, A.J.; Britt, D.W. Adjuvant Pluronic F68 Is Compatible with a Plant Root-Colonizing Probiotic, Pseudomonas chlororaphis O6. Agrochemicals 2024, 3, 1-11. https://doi.org/10.3390/agrochemicals3010001
Streeter AR, Cartwright A, Zargaran M, Wankhade A, Anderson AJ, Britt DW. Adjuvant Pluronic F68 Is Compatible with a Plant Root-Colonizing Probiotic, Pseudomonas chlororaphis O6. Agrochemicals. 2024; 3(1):1-11. https://doi.org/10.3390/agrochemicals3010001
Chicago/Turabian StyleStreeter, Amanda R., Anthony Cartwright, Mohammad Zargaran, Anagha Wankhade, Anne J. Anderson, and David W. Britt. 2024. "Adjuvant Pluronic F68 Is Compatible with a Plant Root-Colonizing Probiotic, Pseudomonas chlororaphis O6" Agrochemicals 3, no. 1: 1-11. https://doi.org/10.3390/agrochemicals3010001
APA StyleStreeter, A. R., Cartwright, A., Zargaran, M., Wankhade, A., Anderson, A. J., & Britt, D. W. (2024). Adjuvant Pluronic F68 Is Compatible with a Plant Root-Colonizing Probiotic, Pseudomonas chlororaphis O6. Agrochemicals, 3(1), 1-11. https://doi.org/10.3390/agrochemicals3010001






