Contrast-Enhanced Mammography as a Functional Biomarker in Breast Cancer: Correlation of Enhancement Patterns with Ki-67 and Histological Grade
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Approval
2.3. Imaging Protocol
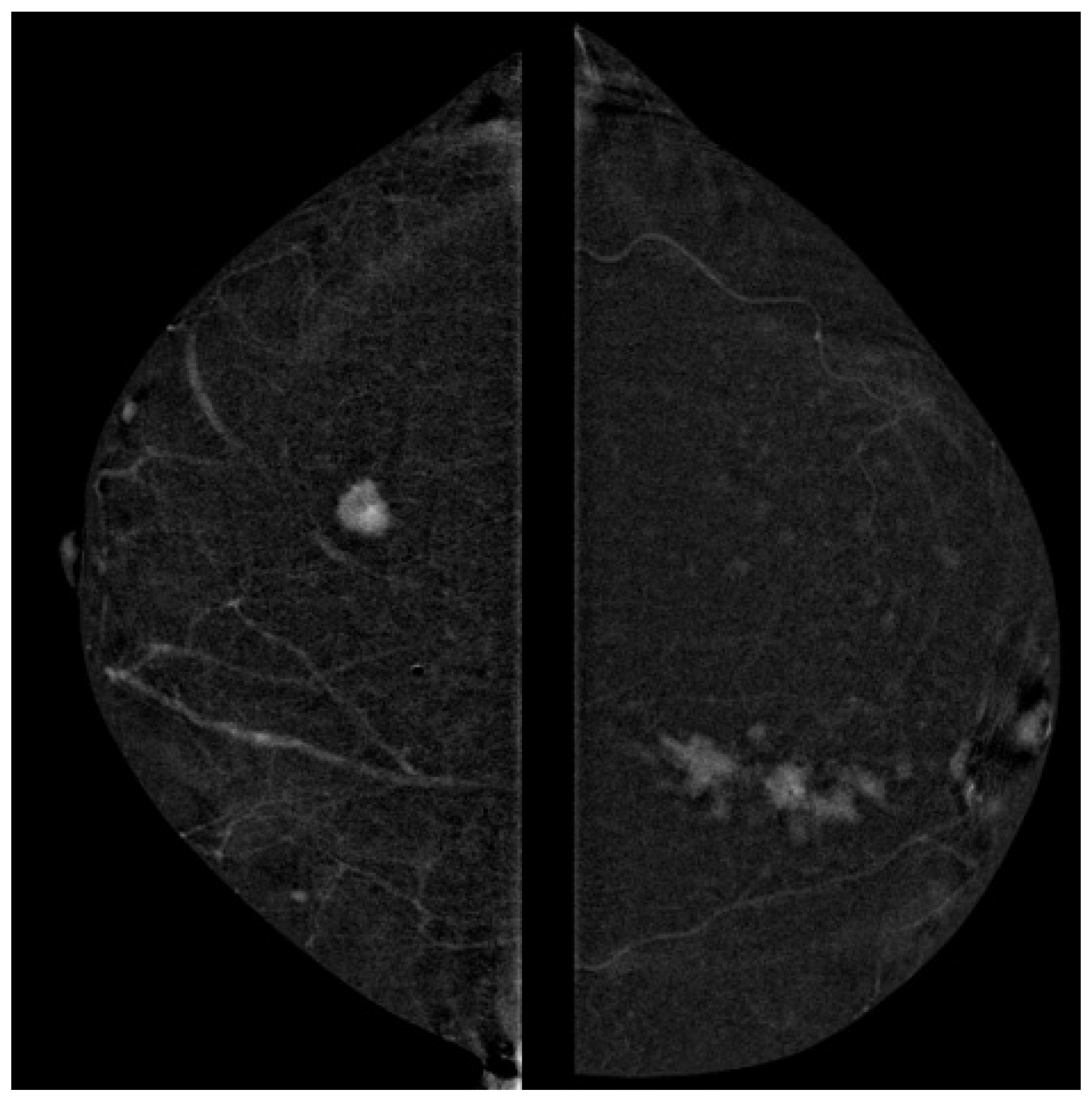
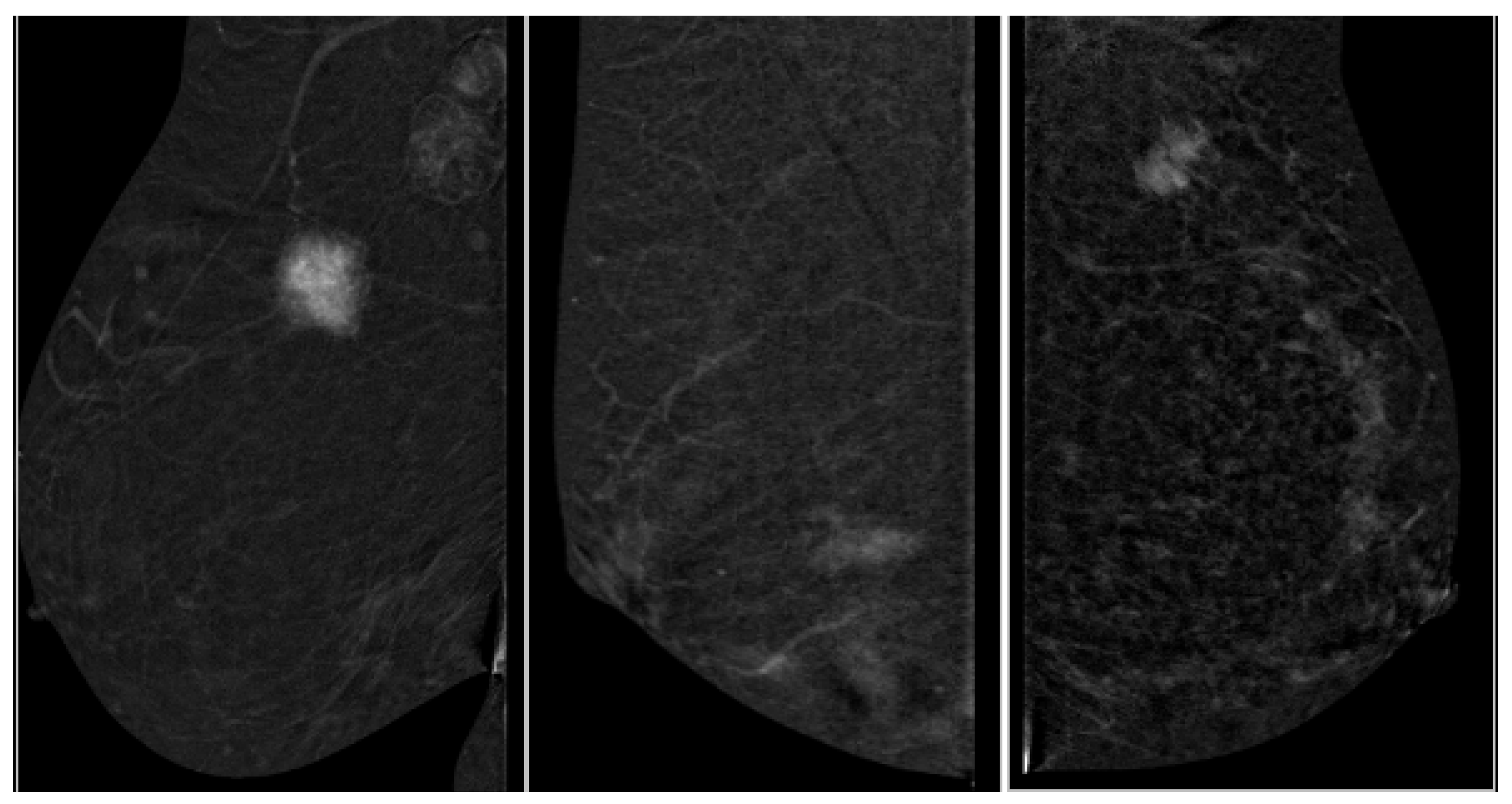
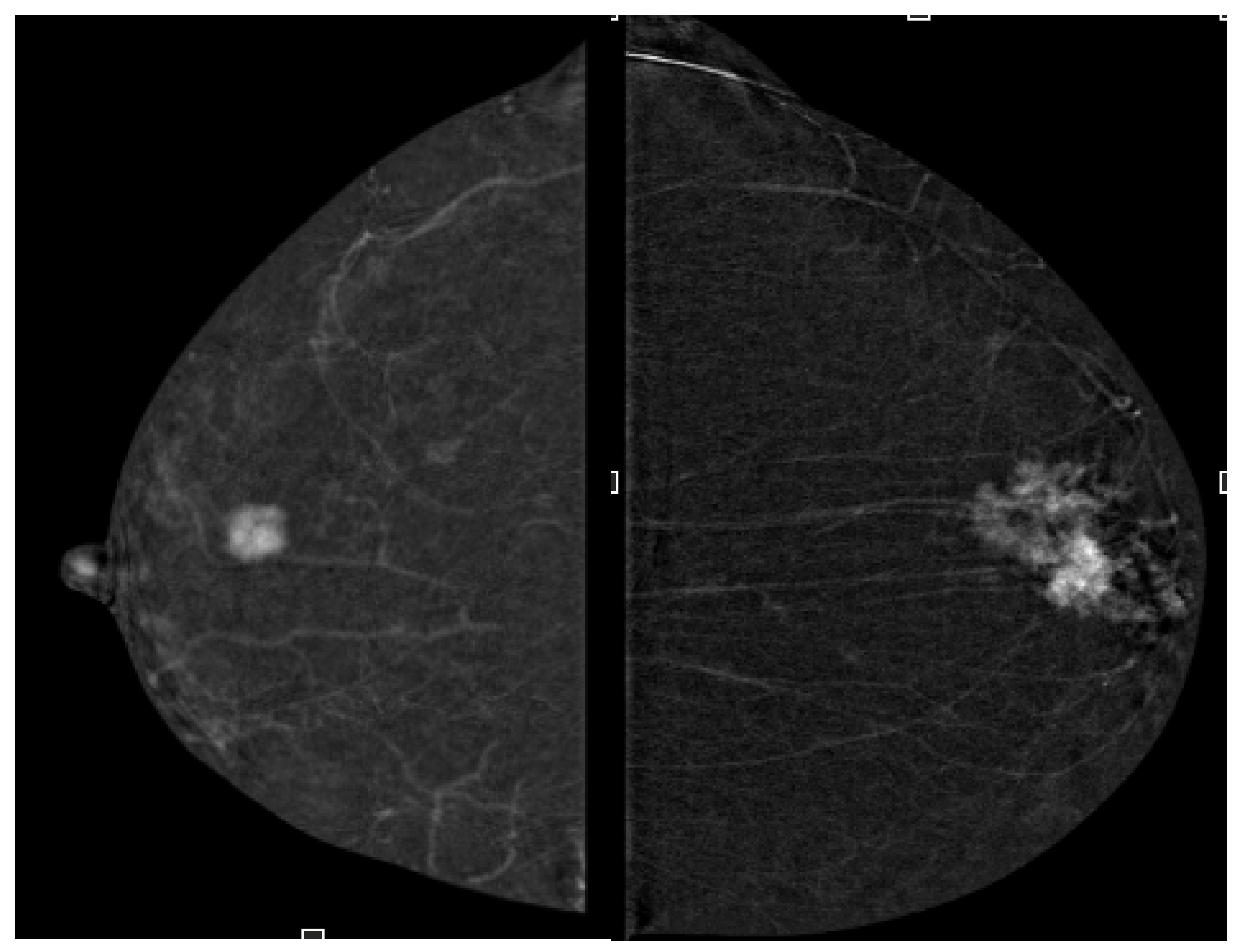
2.4. Image Analysis
- Enhancement intensity: high, moderate, low (visual signal relative to parenchyma),
- Enhancement homogeneity: homogeneous vs. heterogeneous (uniformity of contrast distribution).
2.5. Histopathological Assessment
2.6. Statistical Analysis
3. Results
3.1. Lesion Size Concordance
3.2. Lesion Morphology and Histology
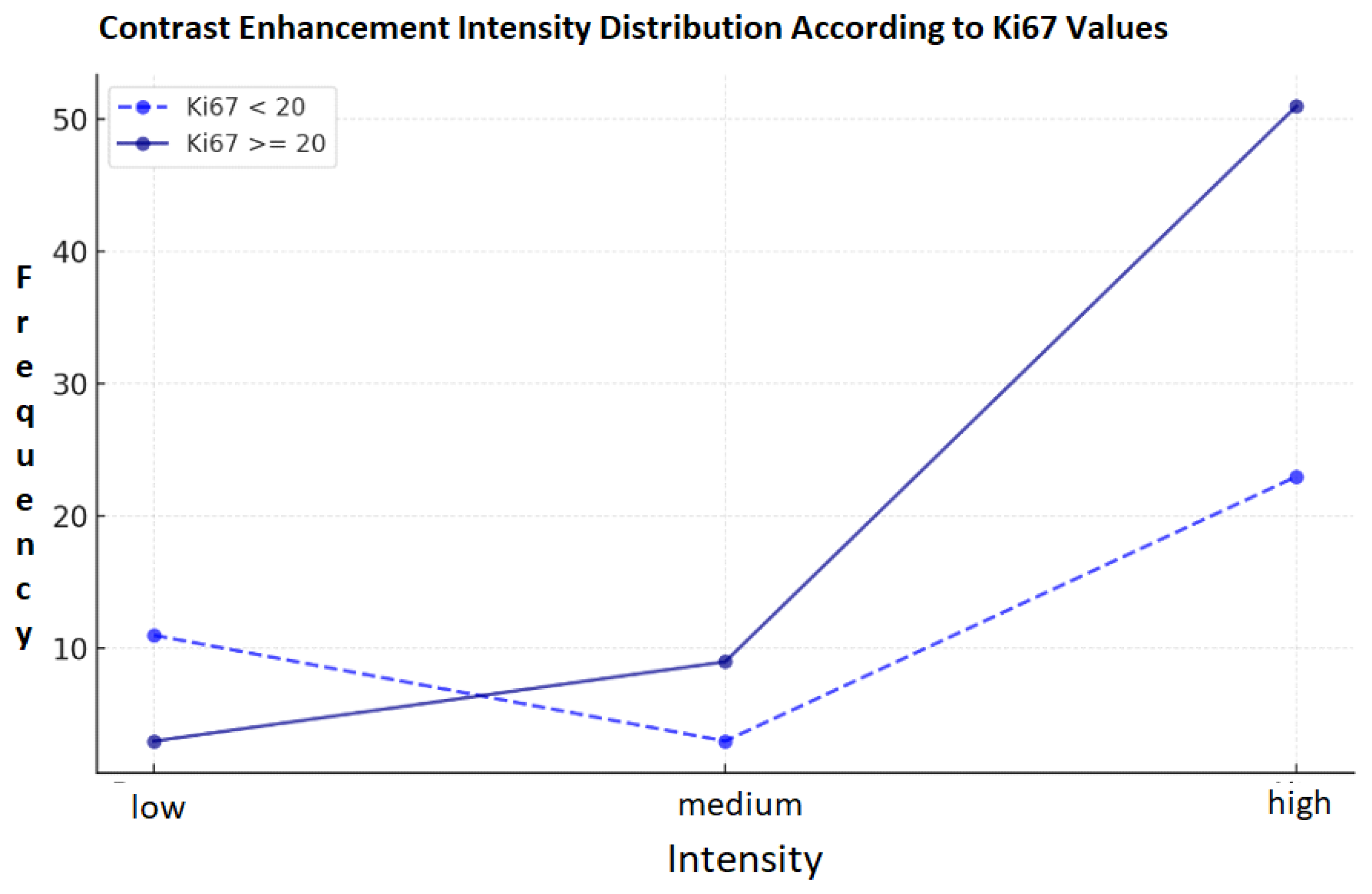
3.3. Correlation of Ki-67 and Enhancement Intensity
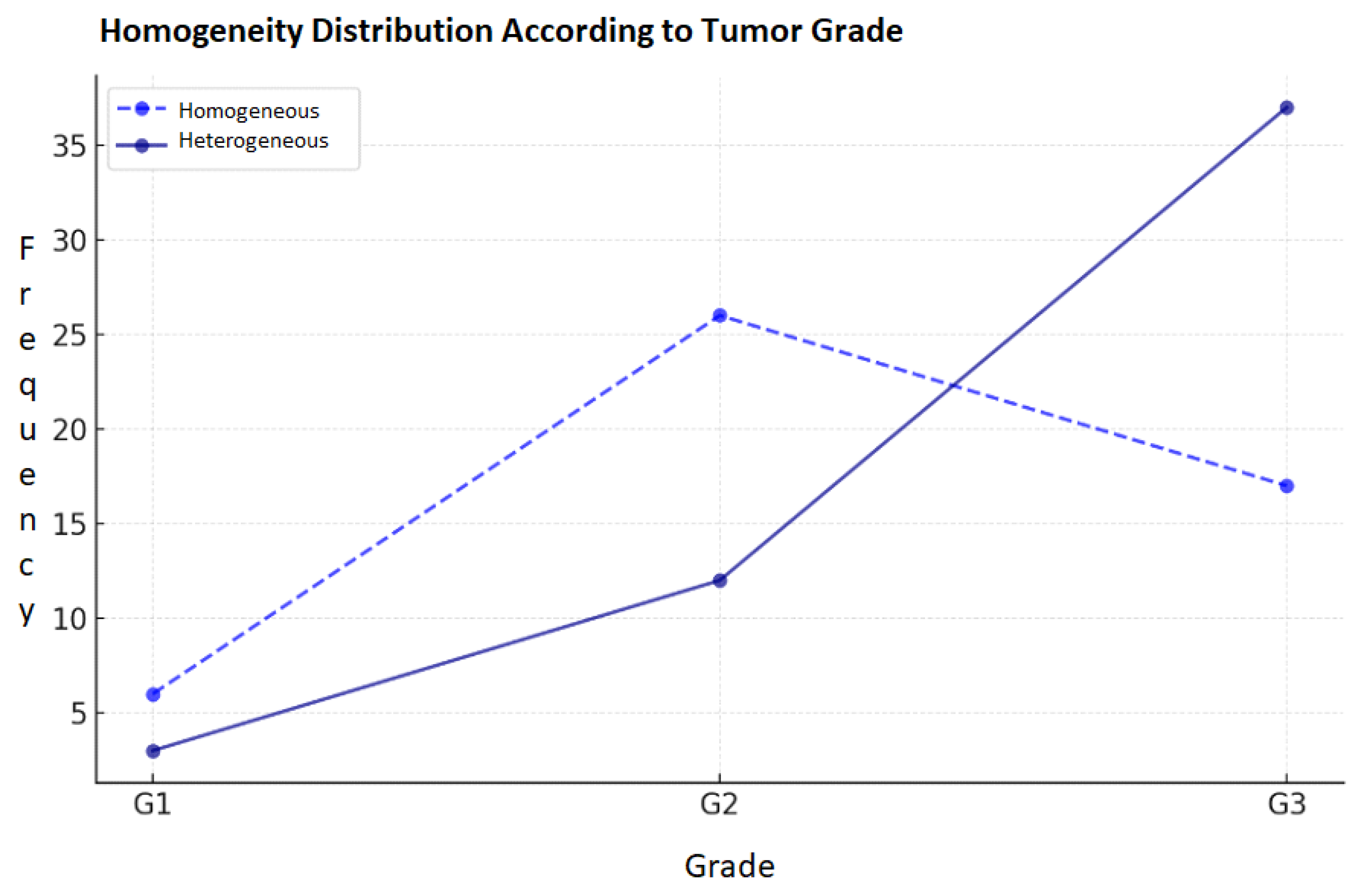
3.4. Correlation of Contrast Homogeneity and Tumor Grade
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jochelson, M.S.; Lobbes, M.B.I. Contrast-enhanced Mammography: State of the Art. Radiology 2021, 299, 36–48. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Bignotti, B.; Rossi, F.; Signori, A.; Sormani, M.P.; Valdora, F.; Calabrese, M.; Houssami, N. Diagnostic performance of contrast-enhanced spectral mammography: Systematic review and meta-analysis. Breast 2016, 28, 13–19. [Google Scholar] [CrossRef]
- Fallenberg, E.M.; Schmitzberger, F.F.; Amer, H.; Ingold-Heppner, B.; Balleyguier, C.; Diekmann, F.; Engelken, F.; Mann, R.M.; Renz, D.M.; Bick, U.; et al. Contrast-enhanced spectral mammography vs. mammography and MRI—clinical performance in a multi-reader evaluation. Eur. Radiol. 2017, 27, 2752–2764. [Google Scholar] [CrossRef]
- Wu, W.Y.-Y.; Tabar, L.; Tot, T.; Fann, C.-Y.; Yen, A.M.-F.; Chen, S.L.-S.; Chiu, S.Y.-H.; Ku, M.M.-S.; Hsu, C.-Y.; Beckmann, K.R.; et al. Imaging biomarkers as predictors for breast cancer death. J. Oncol. 2019, 2019, 2087983. [Google Scholar] [CrossRef]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn, E.A.; DeSouza, N.M. Functional magnetic resonance: Biomarkers of response in breast cancer. Breast Cancer Res. 2011, 13, 204, Erratum in Breast Cancer Res. 2011, 13, 405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buadu, L.D.; Murakami, J.; Murayama, S.; Hashiguchi, N.; Sakai, S.; Masuda, K.; Toyoshima, S.; Kuroki, S.; Ohno, S. Breast lesions: Correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology 1996, 200, 639–649. [Google Scholar] [CrossRef]
- Li, L.; Roth, R.; Germaine, P.; Ren, S.; Lee, M.; Hunter, K.; Tinney, E.; Liao, L. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn. Interv. Imaging 2017, 98, 113–123. [Google Scholar] [CrossRef]
- Kalia, M. Biomarkers for personalized oncology: Recent advances and future challenges. Metabolism 2015, 64, S16–S21. [Google Scholar] [CrossRef]
- Qi, Y.J.; Su, G.H.; You, C.; Zhang, X.; Xiao, Y.; Jiang, Y.Z.; Shao, Z.M. Radiomics in breast cancer: Current advances and future directions. Cell Rep. Med. 2024, 5, 101719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uematsu, T. Non-mass-like lesions on breast ultrasonography: A systematic review. Breast Cancer 2012, 19, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Sakorafas, G.H.; Tsiotou, A.G. Selection criteria for breast conservation in breast cancer. Eur. J. Surg. 2000, 166, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef]
- James, J. Contrast-enhanced spectral mammography (CESM)-guided breast biopsy as an alternative to MRI-guided biopsy. Br. J. Radiol. 2022, 95, 20211287. [Google Scholar] [CrossRef]
- Schell, A.M.; Rosenkranz, K.; Lewis, P.J. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. Am. J. Roentgenol. 2009, 192, 1438–1444. [Google Scholar] [CrossRef]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Lee, A.H.S.; Elston, C.W.; Grainge, M.J.; Hodi, Z.; Blamey, R.W.; Ellis, I.O. Prognostic Significance of Nottingham Histologic Grade in Invasive Breast Carcinoma. J. Clin. Oncol. 2008, 26, 3153–3158. [Google Scholar] [CrossRef]
- Cox, T.F. Medical Statistics for Cancer Studies; Chapman & Hall/CRC: Boca Raton, FL, USA, 2022; ISBN 9780367486150. [Google Scholar]
- Barra, F.R.; de Souza, F.F.; Camelo, R.E.F.A.; Ribeiro, A.C.O.; Farage, L. Accuracy of contrast-enhanced spectral mammography for estimating residual tumor size after neoadjuvant chemotherapy in patients with breast cancer: A feasibility study. Radiol. Bras. 2017, 50, 224–230. [Google Scholar] [CrossRef]
- Montemurro, F.; Martincich, L.; Sarotto, I.; Bertotto, I.; Ponzone, R.; Cellini, L.; Redana, S.; Sismondi, P.; Aglietta, M. Relationship between DCE-MRI morphological and functional features and histopathological characteristics of breast cancer. Eur. Radiol. 2007, 17, 1490–1497. [Google Scholar] [CrossRef]
- Kim, S.H.; Cha, E.S.; Park, C.S.; Kang, B.J.; Whang, I.Y.; Lee, A.W.; Song, B.J.; Park, J. Imaging features of invasive lobular carcinoma: Comparison with invasive ductal carcinoma. Jpn. J. Radiol. 2011, 29, 475–482. [Google Scholar] [CrossRef]
- Kontzoglou, K.; Palla, V.; Karaolanis, G.; Karaiskos, I.; Alexiou, I.; Pateras, I.; Konstantoudakis, K.; Stamatakos, M. Correlation between Ki67 and breast cancer prognosis. Oncology 2013, 84, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, W.; Heinze, S.; Niemiec, J.; Kojs, Z.; Sas-Korczynska, B.; Hendrick, E.; Luczynska, E. Correlation between quantitative assessment of contrast enhancement in contrast-enhanced spectral mammography (CESM) and histopathology—Preliminary results. Eur. Radiol. 2019, 29, 6220–6226. [Google Scholar] [CrossRef] [PubMed]
- Lobbes, M.B.; Lalji, U.C.; Nelemans, P.J.; Houben, I.; Smidt, M.L.; Heuts, E.; de Vries, B.; Wildberger, J.E.; Beets-Tan, R.G. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J. Cancer 2015, 6, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Jensen, H.M.; Cooke, G.; Han, H.L. Relationship between mammographic and histological risk factors for breast cancer. J. Natl. Cancer Inst. 1992, 84, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Iotti, V.; Ravaioli, S.; Vacondio, R.; Coriani, C.; Caffarri, S.; Sghedoni, R.; Nitrosi, A.; Ragazzi, M.; Gasparini, E.; Masini, C.; et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: A comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017, 19, 106. [Google Scholar] [CrossRef]
- Łuczyńska, E.; Heinze-Paluchowska, S.; Hendrick, E.; Dyczek, S.; Ryś, J.; Herman, K.; Blecharz, P.; Jakubowicz, J. Comparison between breast MRI and contrast-enhanced spectral mammography. Med. Sci. Monit. 2015, 21, 1358–1367. [Google Scholar] [CrossRef]
- Wong, C.Y.Y.; Lee, S.Y.S.; Mahmood, R.D. Contrast-enhanced spectral mammography. Singap. Med. J. 2024, 65, 195–201. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Juan, M.W.; Yu, J.; Peng, G.X.; Jun, L.J.; Feng, S.P.; Fang, L.P. Correlation between DCE-MRI radiomics features and Ki-67 expression in invasive breast cancer. Oncol. Lett. 2018, 16, 5084–5090. [Google Scholar] [CrossRef]
- Khan, Q.J.; Kimler, B.F.; O’Dea, A.P.; Zalles, C.M.; Sharma, P.; Fabian, C.J. Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer. Breast Cancer Res. 2007, 9, R35. [Google Scholar] [CrossRef] [PubMed]
- James, J.R.; Pavlicek, W.; Hanson, J.A.; Boltz, T.F.; Patel, B.K. Breast Radiation Dose With CESM Compared With 2D FFDM and 3D Tomosynthesis Mammography. AJR Am. J. Roentgenol. 2017, 208, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Xiang, C.; Yang, Q. The diagnostic performance of CESM and CE-MRI in evaluating the pathological response to neoadjuvant therapy in breast cancer: A systematic review and meta-analysis. Br. J. Radiol. 2020, 93, 20200301. [Google Scholar] [CrossRef] [PubMed]
- Macura, K.J.; Ouwerkerk, R.; Jacobs, M.A.; Bluemke, D.A. Patterns of enhancement on breast MR images: Interpretation and imaging pitfalls. Radiographics 2006, 26, 1719–1734, quiz 1719. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Shi, S. Ultrasound elastography: Advances and challenges in early detection of breast cancer. Front. Oncol. 2025, 15, 1589142. [Google Scholar] [CrossRef]
- Marino, M.A.; Leithner, D.; Sung, J.; Avendano, D.; Morris, E.A.; Pinker, K.; Jochelson, M.S. Radiomics for Tumor Characterization in Breast Cancer Patients: A Feasibility Study Comparing Contrast-Enhanced Mammography and Magnetic Resonance Imaging. Diagnostics 2020, 10, 492. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, X.; Shi, K.; Rominger, A.; Caobelli, F. AI in Breast Cancer Imaging: An Update and Future Trends. Semin. Nucl. Med. 2025, 55, 358–370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balbino, M.; Montatore, M.; Masino, F.; Ancona, A.; Carpagnano, F.A.; Capuano, G.; Guglielmi, R.; Guglielmi, G. Contrast-Enhanced Mammography as a Functional Biomarker in Breast Cancer: Correlation of Enhancement Patterns with Ki-67 and Histological Grade. Targets 2025, 3, 29. https://doi.org/10.3390/targets3030029
Balbino M, Montatore M, Masino F, Ancona A, Carpagnano FA, Capuano G, Guglielmi R, Guglielmi G. Contrast-Enhanced Mammography as a Functional Biomarker in Breast Cancer: Correlation of Enhancement Patterns with Ki-67 and Histological Grade. Targets. 2025; 3(3):29. https://doi.org/10.3390/targets3030029
Chicago/Turabian StyleBalbino, Marina, Manuela Montatore, Federica Masino, Antonietta Ancona, Francesca Anna Carpagnano, Giulia Capuano, Riccardo Guglielmi, and Giuseppe Guglielmi. 2025. "Contrast-Enhanced Mammography as a Functional Biomarker in Breast Cancer: Correlation of Enhancement Patterns with Ki-67 and Histological Grade" Targets 3, no. 3: 29. https://doi.org/10.3390/targets3030029
APA StyleBalbino, M., Montatore, M., Masino, F., Ancona, A., Carpagnano, F. A., Capuano, G., Guglielmi, R., & Guglielmi, G. (2025). Contrast-Enhanced Mammography as a Functional Biomarker in Breast Cancer: Correlation of Enhancement Patterns with Ki-67 and Histological Grade. Targets, 3(3), 29. https://doi.org/10.3390/targets3030029





