Corneal Neovascularisation and Anti-VEGF Therapy
Abstract
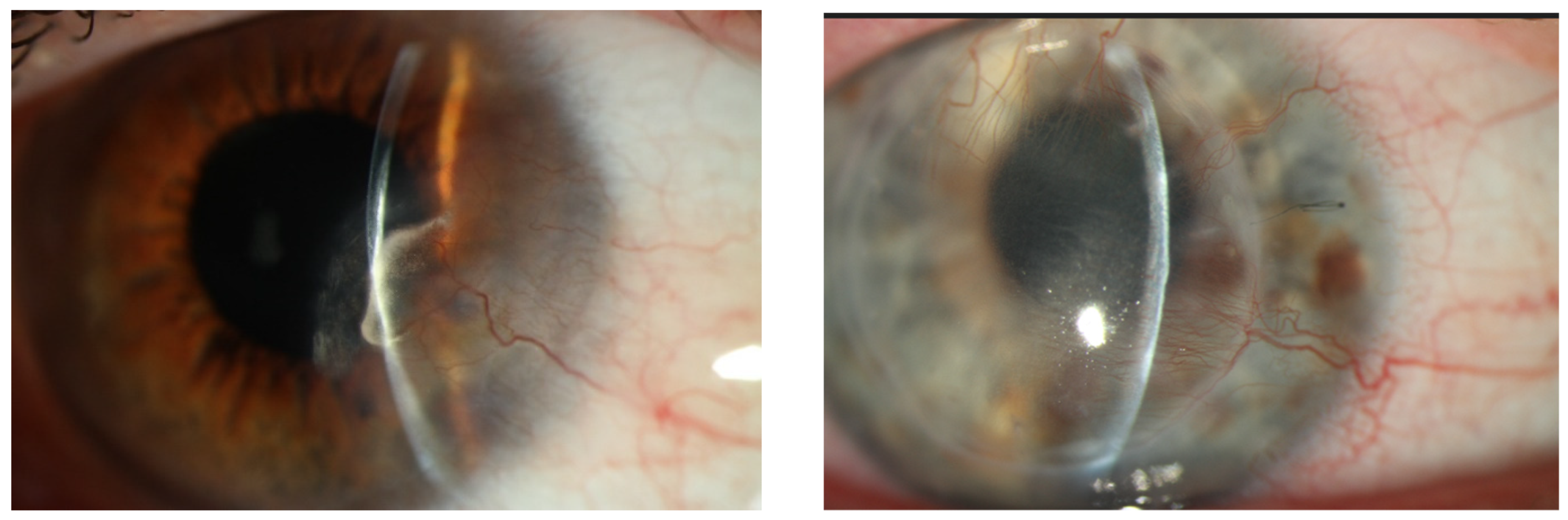
1. Introduction
2. Efficacy of Anti-VEGF Agents in the Treatment of Experimental Corneal Neovascularisation
3. Efficacy of Anti-VEGF Agents in Clinical Studies
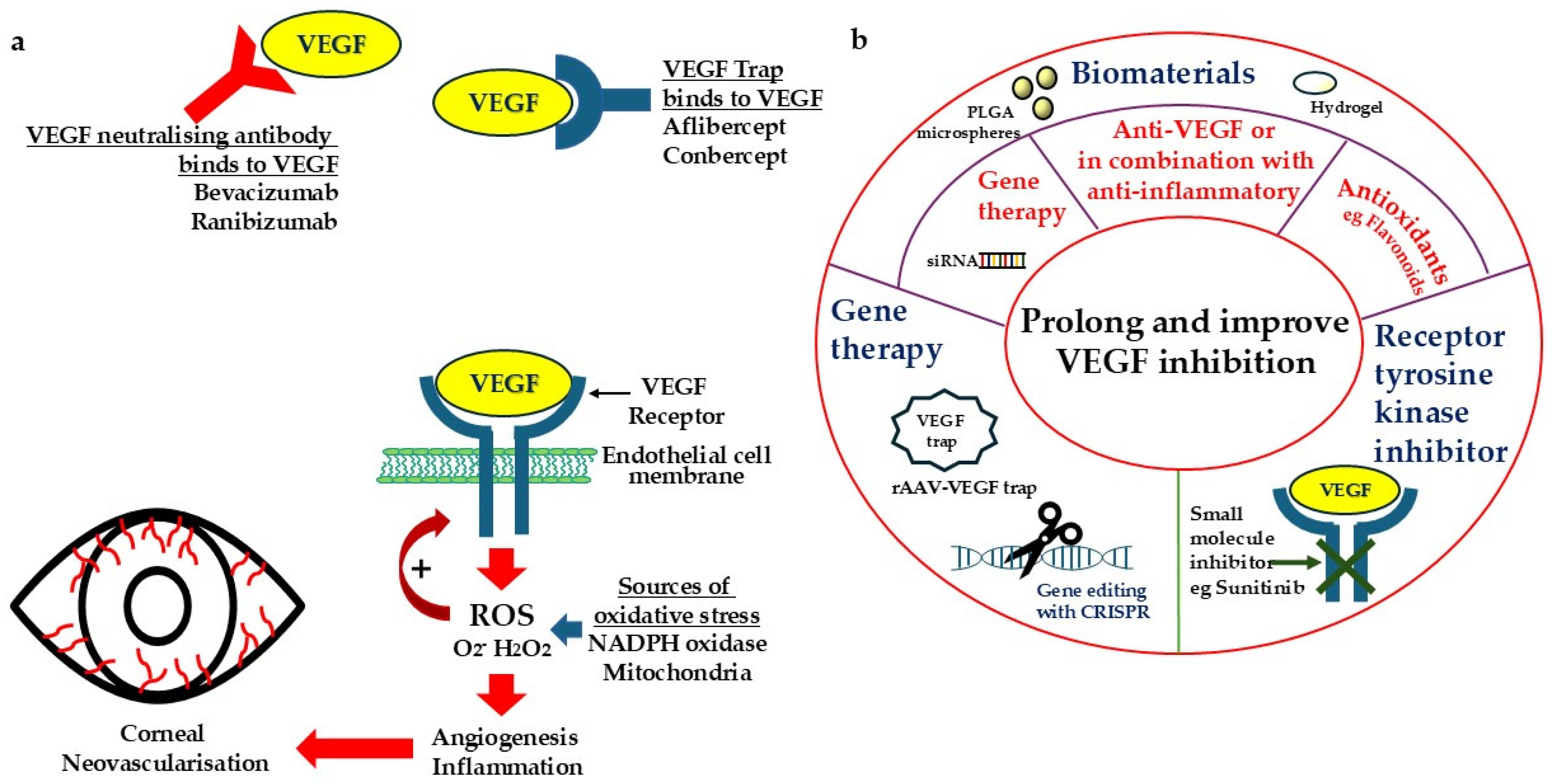
4. Developing New Strategies to Overcome the Challenges and Limitations of Anti-VEGF Treatment
4.1. Gene-Based Approach to Long-Term Inhibition of VEGF
4.2. Sustained-Release Formulation for Progressive Inhibition of VEGF
4.3. Combined Therapy or Multiple Targeting to Improve and Prolong Efficacy
4.4. Simultaneous Targeting of Oxidative Stress and VEGF
5. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| AAV | Adeno-associated virus |
| bFGF | basic fibroblast growth factor |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| ECCG | Epigallocatechin gallate |
| NOX | NADPH oxidase |
| PDGF | Platelet-derived growth factor |
| PLGA | poly(D,L-lactic-co-glycolic acid |
| r | Recombinant |
| TGF | Transforming growth factor |
| TNF | Tumour necrosis factor |
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factors |
References
- Lasagni Vitar, R.M.; Triolo, G.; Fonteyne, P.; Acuti Martellucci, C.; Manzoli, L.; Rama, P.; Ferrari, G. Epidemiology of Corneal Neovascularization and Its Impact on Visual Acuity and Sensitivity: A 14-Year Retrospective Study. Front. Med. 2021, 8, 733538. [Google Scholar] [CrossRef]
- Sharif, Z.; Sharif, W. Corneal neovascularization: Updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [PubMed]
- Poon, S.H.L.; Wong, W.H.L.; Bu, Y.; Lo, A.C.Y.; Jhanji, V.; Chan, Y.K.; Shih, K.C. A Systematic Review of Emerging Therapeutic Strategies in the Management of Chemical Injuries of the Ocular Surface. Eye Contact Lens 2020, 46, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chodosh, J. Angiography of the Limbus and Cornea. Int. Ophthalmol. Clin. 2019, 59, 19–29. [Google Scholar] [CrossRef]
- Drzyzga, L.; Spiewak, D.; Dorecka, M.; Wygledowska-Promienska, D. Available Therapeutic Options for Corneal Neovascularization: A Review. Int. J. Mol. Sci. 2024, 25, 5479. [Google Scholar] [CrossRef]
- Yang, J.F.; Walia, A.; Huang, Y.H.; Han, K.Y.; Rosenblatt, M.I.; Azar, D.T.; Chang, J.H. Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv. Ophthalmol. 2016, 61, 272–296. [Google Scholar] [CrossRef]
- Zhao, Y.; Singh, R.P. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context 2018, 7, 212532. [Google Scholar] [CrossRef]
- Philipp, W.; Speicher, L.; Humpel, C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2514–2522. [Google Scholar]
- Zakaria, N.; Van Grasdorff, S.; Wouters, K.; Rozema, J.; Koppen, C.; Lion, E.; Cools, N.; Berneman, Z.; Tassignon, M.J. Human tears reveal insights into corneal neovascularization. PLoS ONE 2012, 7, e36451. [Google Scholar] [CrossRef]
- Khanna, S.; Komati, R.; Eichenbaum, D.A.; Hariprasad, I.; Ciulla, T.A.; Hariprasad, S.M. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: A comparative review. BMJ Open Ophthalmol. 2019, 4, e000398. [Google Scholar] [CrossRef] [PubMed]
- Kuckelkorn, R.; Schrage, N.; Keller, G.; Redbrake, C. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol. Scand. 2002, 80, 4–10. [Google Scholar] [CrossRef]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bovone, C.; Spena, R.; Senni, C.; Scorcia, V.; Busin, M. Anti-VEGF Treatment in Corneal Diseases. Curr. Drug Targets 2020, 21, 1159–1180. [Google Scholar] [CrossRef] [PubMed]
- Akar, E.E.; Oner, V.; Kucukerdonmez, C.; Aydin Akova, Y. Comparison of subconjunctivally injected bevacizumab, ranibizumab, and pegaptanib for inhibition of corneal neovascularization in a rat model. Int. J. Ophthalmol. 2013, 6, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- Vinores, S.A. Pegaptanib in the treatment of wet, age-related macular degeneration. Int. J. Nanomed. 2006, 1, 263–268. [Google Scholar]
- Stewart, M.W. Aflibercept (VEGF Trap-eye): The newest anti-VEGF drug. Br. J. Ophthalmol. 2012, 96, 1157–1158. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Semeraro, F.; Morescalchi, F.; Duse, S.; Parmeggiani, F.; Gambicorti, E.; Costagliola, C. Aflibercept in wet AMD: Specific role and optimal use. Drug Des. Dev. Ther. 2013, 7, 711–722. [Google Scholar] [CrossRef]
- Lu, X.; Sun, X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des. Dev. Ther. 2015, 9, 2311–2320. [Google Scholar] [CrossRef]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar] [PubMed]
- Sener, E.; Yuksel, N.; Yildiz, D.K.; Yilmaz, B.; Ozdemir, O.; Caglar, Y.; Degirmenci, E. The impact of subconjuctivally injected EGF and VEGF inhibitors on experimental corneal neovascularization in rat model. Curr. Eye Res. 2011, 36, 1005–1013. [Google Scholar] [CrossRef]
- Kwon, J.W.; Choi, J.A.; Shin, E.Y.; La, T.Y.; Jee, D.H.; Chung, Y.W.; Cho, Y.K. Effect of trapping vascular endothelial growth factor-A in a murine model of dry eye with inflammatory neovascularization. Int. J. Ophthalmol. 2016, 9, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.R.; Xu, H.C.; Ma, Y.; Huang, L.Y.; Zhai, L.Y.; Zhao, Y. Effects of VEGF Inhibitor Conbercept on Corneal Neovascularization Following Penetrating Keratoplasty in Rabbit Model. Clin. Ophthalmol. 2020, 14, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, C.; Lu, Y.; Huang, Z. The inhibitory effect of different concentrations of KH902 eye drops on corneal neovascularization induced by alkali burn. Indian J. Ophthalmol. 2017, 65, 1127–1132. [Google Scholar] [CrossRef]
- Yoeruek, E.; Ziemssen, F.; Henke-Fahle, S.; Tatar, O.; Tura, A.; Grisanti, S.; Bartz-Schmidt, K.U.; Szurman, P.; Tubingen Bevacizumab Study, G. Safety, penetration and efficacy of topically applied bevacizumab: Evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008, 86, 322–328. [Google Scholar] [CrossRef]
- Hashemian, M.N.; Moghimi, S.; Kiumehr, S.; Riazi, M.; Amoli, F.A. Prevention and treatment of corneal neovascularization: Comparison of different doses of subconjunctival bevacizumab with corticosteroid in experimental rats. Ophthalmic Res. 2009, 42, 90–95. [Google Scholar] [CrossRef]
- Gore, A.; Horwitz, V.; Cohen, M.; Gutman, H.; Cohen, L.; Gez, R.; Kadar, T.; Dachir, S. Successful single treatment with ziv-aflibercept for existing corneal neovascularization following ocular chemical insult in the rabbit model. Exp. Eye Res. 2018, 171, 183–191. [Google Scholar] [CrossRef]
- Papathanassiou, M.; Theodoropoulou, S.; Analitis, A.; Tzonou, A.; Theodossiadis, P.G. Vascular endothelial growth factor inhibitors for treatment of corneal neovascularization: A meta-analysis. Cornea 2013, 32, 435–444. [Google Scholar] [CrossRef]
- DeStafeno, J.J.; Kim, T. Topical bevacizumab therapy for corneal neovascularization. Arch. Ophthalmol. 2007, 125, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Ha, B.J.; Kim, E.K.; Tchah, H.; Kim, T.I. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology 2008, 115, e33–e38. [Google Scholar] [CrossRef]
- Lai, S.C.; Loh, E.W.; Chiou, D.I.; Hong, C.T. Efficacy and safety of anti-vascular endothelial growth factor agents on corneal neovascularization: A meta-analysis. World J. Clin. Cases 2023, 11, 7337–7349. [Google Scholar] [CrossRef] [PubMed]
- Petsoglou, C.; Balaggan, K.S.; Dart, J.K.; Bunce, C.; Xing, W.; Ali, R.R.; Tuft, S.J. Subconjunctival bevacizumab induces regression of corneal neovascularisation: A pilot randomised placebo-controlled double-masked trial. Br. J. Ophthalmol. 2013, 97, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, T.H.; McSoley, M.; Amparo, F.; Carreno-Galeano, T.; Wang, M.; Dastjerdi, M.; Singh, R.B.; Coco, G.; Di Zazzo, A.; Shikari, H.; et al. Bevacizumab in High-Risk Corneal Transplantation: A Pilot Multicenter Prospective Randomized Control Trial. Ophthalmology 2022, 129, 865–879. [Google Scholar] [CrossRef]
- Dohlman, T.H.; Singh, R.B.; Amparo, F.; Carreno-Galeano, T.; Dastjerdi, M.; Coco, G.; Di Zazzo, A.; Shikari, H.; Saboo, U.; Sippel, K.; et al. Suppression of Neovascularization by Topical and Subconjunctival Bevacizumab After High-Risk Corneal Transplantation. Ophthalmol. Sci. 2024, 4, 100492. [Google Scholar] [CrossRef]
- Sun, C.; Ruan, F.; Li, S.; Zhang, J.Q.; Jie, Y. Subconjunctival conbercept for the treatment of corneal neovascularization. Int. J. Ophthalmol. 2023, 16, 871–875. [Google Scholar] [CrossRef]
- Quaggin, S.E. Turning a blind eye to anti-VEGF toxicities. J. Clin. Investig. 2012, 122, 3849–3851. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Kataoka, Y.; Nakao, K.; Nakamura, K.; Morikawa, S.; Tanaka, S.; Katsuki, M.; Maru, Y.; Shibuya, M. Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos. Mol. Cell Biol. 2005, 25, 355–363. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, S.; Ye, X.; Wang, Y.; Wang, Q.; Chen, Z.; Wang, Z.; Zhang, J.; Wang, Q.; Chen, L.; et al. Genome Editing VEGFA Prevents Corneal Neovascularization In Vivo. Adv. Sci. 2024, 11, e2401710. [Google Scholar] [CrossRef]
- Su, W.; Sun, S.; Tian, B.; Tai, P.W.L.; Luo, Y.; Ko, J.; Zhan, W.; Ke, X.; Zheng, Q.; Li, X.; et al. Efficacious, safe, and stable inhibition of corneal neovascularization by AAV-vectored anti-VEGF therapeutics. Mol. Ther. Methods Clin. Dev. 2021, 22, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.; Philipp, S.I.; Stylianos, M.; Martin, B.; Barbara, W.; Felix, R.; Alexander, O.G.; Eberhart, Z.; Marius, U.; Birgit, K.; et al. Safety and Toxicology of Ocular Gene Therapy with Recombinant AAV Vector rAAV.hCNGA3 in Nonhuman Primates. Hum. Gene Ther. Clin. Dev. 2019, 30, 50–56. [Google Scholar] [CrossRef]
- Shen, H.H.; Chan, E.C.; Lee, J.H.; Bee, Y.S.; Lin, T.W.; Dusting, G.J.; Liu, G.S. Nanocarriers for treatment of ocular neovascularization in the back of the eye: New vehicles for ophthalmic drug delivery. Nanomedicine 2015, 10, 2093–2107. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, W.; Yu, J.; Yu, X.; Zheng, Q.; Peng, F.; He, Z.; Zhao, W.; Zhang, Z.; Li, X.; et al. Combination of dexamethasone and Avastin((R)) by supramolecular hydrogel attenuates the inflammatory corneal neovascularization in rat alkali burn model. Colloids Surf. B Biointerfaces 2017, 159, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, L.; Yang, R.; Hu, R.; Zheng, Q.; Zan, X. Combined delivery of small molecule and protein drugs as synergistic therapeutics for treating corneal neovascularization by a one-pot coassembly strategy. Mater. Today Bio 2022, 17, 100456. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, Y.; Xing, C.; Li, S.; Bao, Z.; Lei, L.; Lin, D.; Wang, Y.; Chen, H.; Xu, X. An injectable thermosensitive hydrogel for dual delivery of diclofenac and Avastin(R) to effectively suppress inflammatory corneal neovascularization. Int. J. Pharm. 2022, 625, 122081. [Google Scholar] [CrossRef]
- Zhou, C.; Lei, F.; Sharma, J.; Hui, P.C.; Wolkow, N.; Dohlman, C.H.; Vavvas, D.G.; Chodosh, J.; Paschalis, E.I. Sustained Inhibition of VEGF and TNF-alpha Achieves Multi-Ocular Protection and Prevents Formation of Blood Vessels after Severe Ocular Trauma. Pharmaceutics 2023, 15, 2059. [Google Scholar] [CrossRef]
- Voiculescu, O.B.; Voinea, L.M. Infliximab eye drops treatment in corneal neovascularization. J. Med. Life 2015, 8, 566–567. [Google Scholar]
- Gladkauskas, T.; Bruland, O.; Abu Safieh, L.; Edward, D.P.; Rodahl, E.; Bredrup, C. Corneal Vascularization Associated with a Novel PDGFRB Variant. Investig. Ophthalmol. Vis. Sci. 2023, 64, 9. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Sunitinib: A VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem. Biophys. Res. Commun. 2007, 356, 323–328. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Perez-Santonja, J.J.; Campos-Mollo, E.; Lledo-Riquelme, M.; Javaloy, J.; Alio, J.L. Inhibition of corneal neovascularization by topical bevacizumab (Anti-VEGF) and Sunitinib (Anti-VEGF and Anti-PDGF) in an animal model. Am. J. Ophthalmol. 2010, 150, 519–528.e511. [Google Scholar] [CrossRef]
- Ko, B.Y.; Kim, Y.S.; Baek, S.G.; Lee, G.W.; Kim, J.M.; Jean, W.S.; Lee, N.S.; Kang, J. Inhibition of corneal neovascularization by subconjunctival and topical bevacizumab and sunitinib in a rabbit model. Cornea 2013, 32, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, L.; Oh, Y.; Meng, T.; Chai, G.; Xia, S.; Emmert, D.; Wang, B.; Eberhart, C.G.; Lee, S.; et al. Sunitinib malate-loaded biodegradable microspheres for the prevention of corneal neovascularization in rats. J. Control. Release 2020, 327, 456–466. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Ferrari, G.; Giacomini, C.; Bignami, F.; Moi, D.; Ranghetti, A.; Doglioni, C.; Naldini, L.; Rama, P.; Mazzieri, R. Angiopoietin 2 expression in the cornea and its control of corneal neovascularisation. Br. J. Ophthalmol. 2016, 100, 1005–1010. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Bohm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; van Wijngaarden, P.; Chan, E.; Ngo, D.; Wang, J.H.; Peshavariya, H.M.; Dusting, G.J.; Liu, G.S. NADPH oxidase 2 plays a role in experimental corneal neovascularization. Clin. Sci. 2016, 130, 683–696. [Google Scholar] [CrossRef]
- Hakami, N.Y.; Dusting, G.J.; Chan, E.C.; Shah, M.H.; Peshavariya, H.M. Wound Healing After Alkali Burn Injury of the Cornea Involves Nox4-Type NADPH Oxidase. Invest. Ophthalmol. Vis. Sci. 2020, 61, 20. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, M.Y.; Li, Q.G.; Wang, X.H.; Wan, Y.Y.; Yang, Z.J.; He, M.; Yi, Y.M.; Jiang, L.P.; Qu, X.H.; et al. Drp1-dependent mitochondrial fission mediates corneal injury induced by alkali burn. Free Radic. Biol. Med. 2021, 176, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hua, X.; Li, L.; Zhou, X.; Tian, Y.; Deng, Y.; Zhang, M.; Yuan, X.; Chi, W. AIP1 suppresses neovascularization by inhibiting the NOX4-induced NLRP3/NLRP6 imbalance in a murine corneal alkali burn model. Cell Commun. Signal 2022, 20, 59. [Google Scholar] [CrossRef]
- Chan, E.C.; van Wijngaarden, P.; Liu, G.S.; Jiang, F.; Peshavariya, H.; Dusting, G.J. Involvement of Nox2 NADPH oxidase in retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7061–7067. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ye, J.; Wu, Y.; Cheng, Y.; Su, M.; Dai, Q.; Han, Y.; Pan, J.; Wu, Z.; Chen, C.; et al. A Synergistic Therapy With Antioxidant and Anti-VEGF: Toward its Safe and Effective Elimination for Corneal Neovascularization. Adv. Healthc. Mater. 2024, 13, e2302192. [Google Scholar] [CrossRef]
- Gao, F.J.; Zhang, S.H.; Xu, P.; Yang, B.Q.; Zhang, R.; Cheng, Y.; Zhou, X.J.; Huang, W.J.; Wang, M.; Chen, J.Y.; et al. Quercetin Declines Apoptosis, Ameliorates Mitochondrial Function and Improves Retinal Ganglion Cell Survival and Function in In Vivo Model of Glaucoma in Rat and Retinal Ganglion Cell Culture In Vitro. Front. Mol. Neurosci. 2017, 10, 285. [Google Scholar] [CrossRef]
- Kim, M.H. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J. Cell Biochem. 2003, 89, 529–538. [Google Scholar] [CrossRef]
- Fan Gaskin, J.C.; Kong, R.C.K.; Shah, M.H.; Edgley, A.J.; Peshavariya, H.M.; Chan, E.C. Inhibitory Effects of 3′,4′-Dihydroxyflavonol in a Mouse Model of Glaucoma Filtration Surgery and TGFbeta1-Induced Responses in Human Tenon’s Fibroblasts. Transl. Vis. Sci. Technol. 2022, 11, 18. [Google Scholar] [CrossRef]
- Pasvanis, Z.; Kong, R.C.K.; Shah, M.H.; Chan, E.C.; Fan Gaskin, J.C. 3′,4′-Dihydroxyflavonol Inhibits Fibrotic Response in a Rabbit Model of Glaucoma Filtration Surgery. Int. J. Mol. Sci. 2024, 25, 10767. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Hu, J.; Wu, T.; Xu, W.; Meng, Q.; Cao, K.; Luo, X. Effects of Flavonoid Supplementation on Nanomaterial-Induced Toxicity: A Meta-Analysis of Preclinical Animal Studies. Front. Nutr. 2022, 9, 929343. [Google Scholar] [CrossRef]
- Clements, J.L.; Dana, R. Inflammatory corneal neovascularization: Etiopathogenesis. Semin. Ophthalmol. 2011, 26, 235–245. [Google Scholar] [CrossRef]
- Chu, X.; Wang, X.; Zhang, C.; Liu, H.; Li, F.; Li, G.; Zhao, S. A deep learning-based model for automatic segmentation and evaluation of corneal neovascularization using slit-lamp anterior segment images. Quant. Imaging Med. Surg. 2023, 13, 6778–6788. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, E.; Fan Gaskin, J.; Chan, E.C. Corneal Neovascularisation and Anti-VEGF Therapy. Targets 2025, 3, 9. https://doi.org/10.3390/targets3010009
Chan E, Fan Gaskin J, Chan EC. Corneal Neovascularisation and Anti-VEGF Therapy. Targets. 2025; 3(1):9. https://doi.org/10.3390/targets3010009
Chicago/Turabian StyleChan, Elsie, Jennifer Fan Gaskin, and Elsa C. Chan. 2025. "Corneal Neovascularisation and Anti-VEGF Therapy" Targets 3, no. 1: 9. https://doi.org/10.3390/targets3010009
APA StyleChan, E., Fan Gaskin, J., & Chan, E. C. (2025). Corneal Neovascularisation and Anti-VEGF Therapy. Targets, 3(1), 9. https://doi.org/10.3390/targets3010009





