Exploring the Links Between Ankylosing Spondylitis and Amyotrophic Lateral Sclerosis: A Bidirectional Mendelian Randomization Study
Abstract
1. Introduction
2. Methods
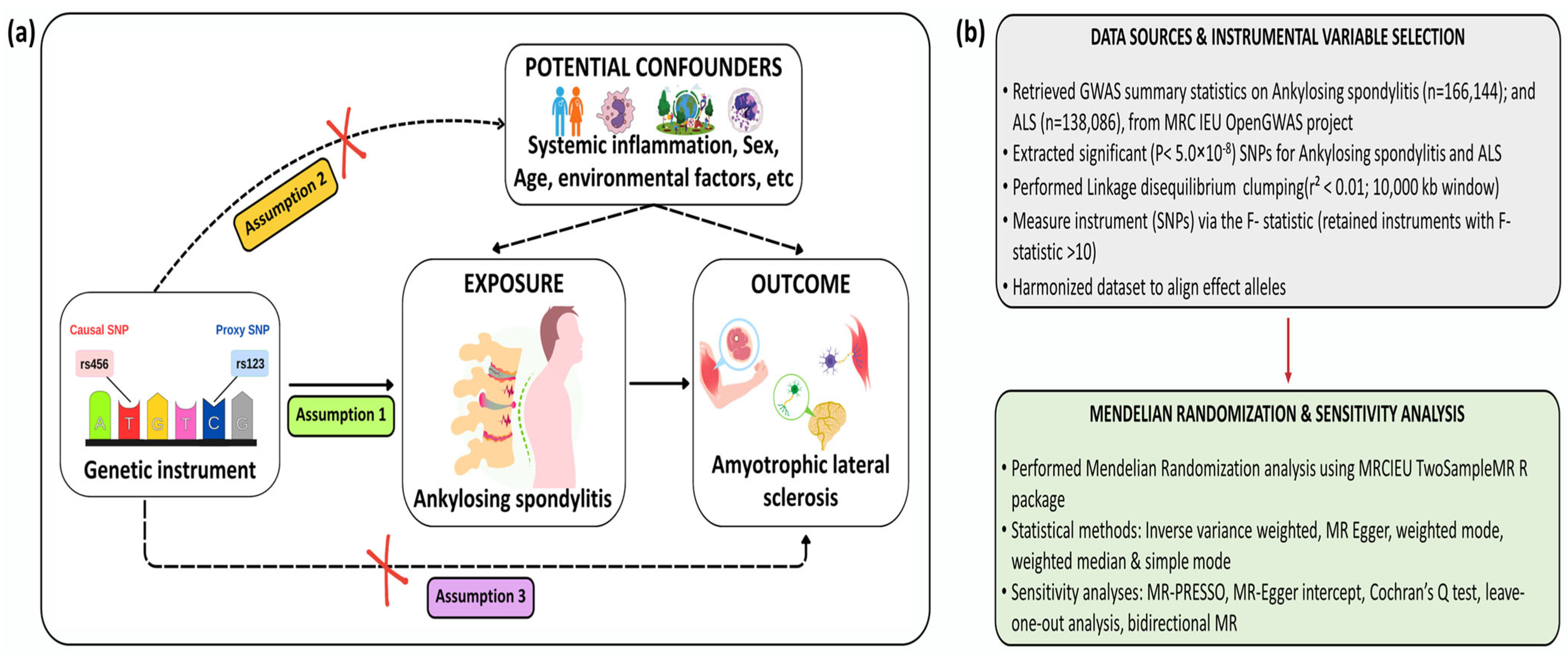
2.1. Study Design
2.2. Data Source
2.3. Genetic Variant Selection
2.4. Primary MR Analyses
2.5. Bidirectional MR Analyses
2.6. Sensitivity Analyses
3. Results
3.1. Genetic Instrument Strength
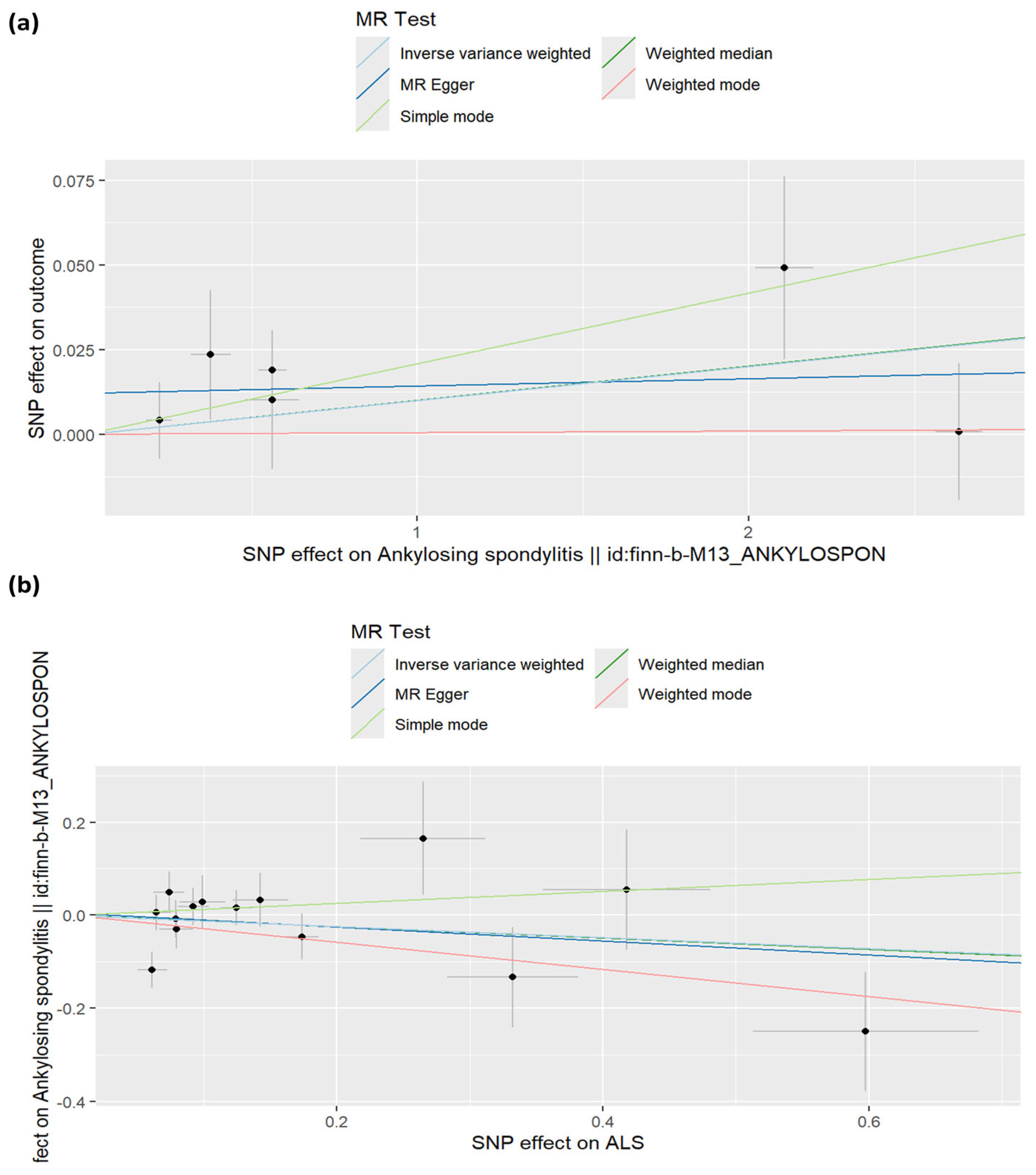
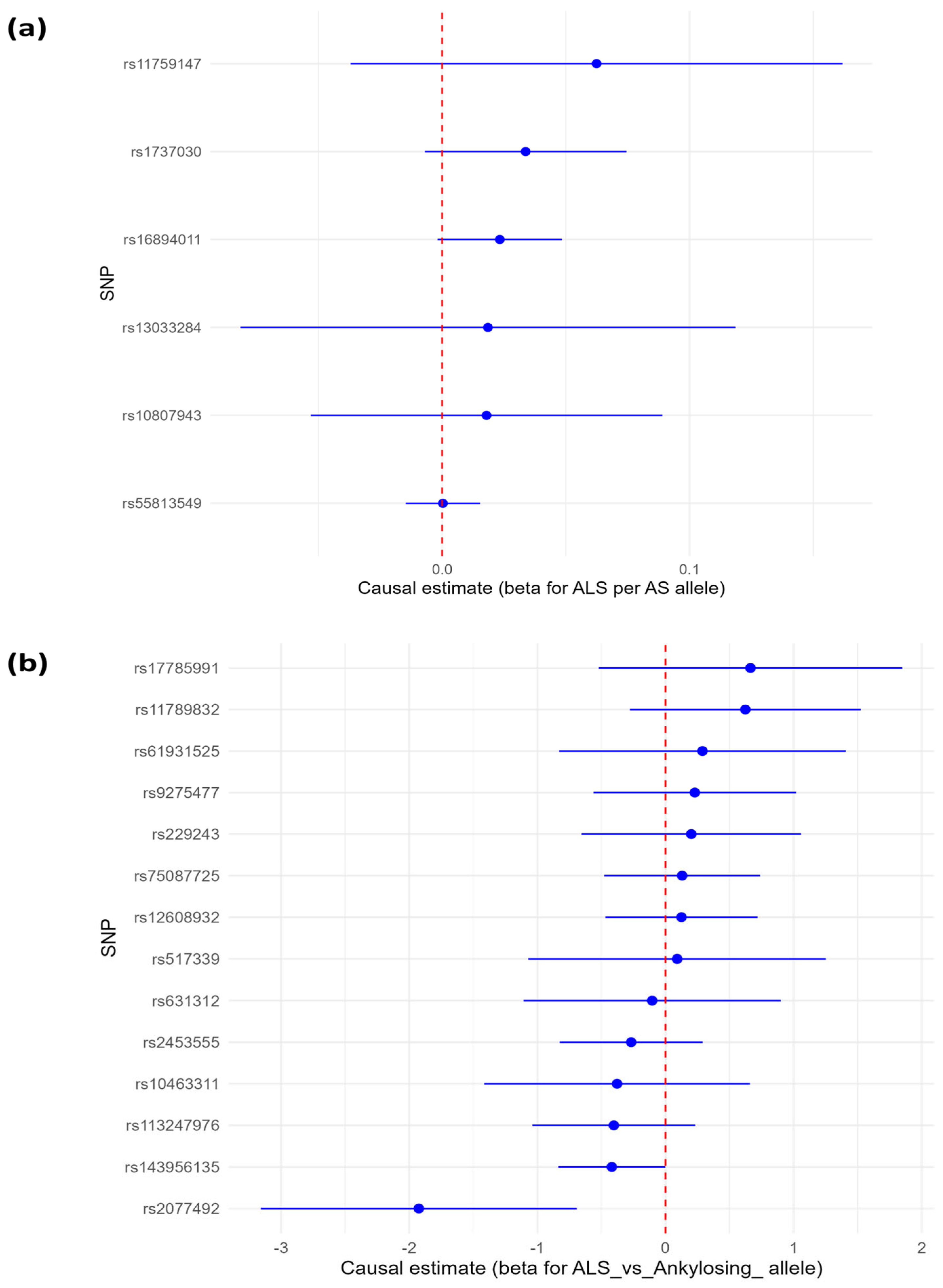
3.1.1. Causal Estimates of Genetic Liability to Ankylosing Spondylitis (AS) to Amyotrophic Lateral Sclerosis (ALS)
3.1.2. Causal Estimates of Effect of Genetic Susceptibility to Amyotrophic Lateral Sclerosis on Ankylosing Spondylitis Risk
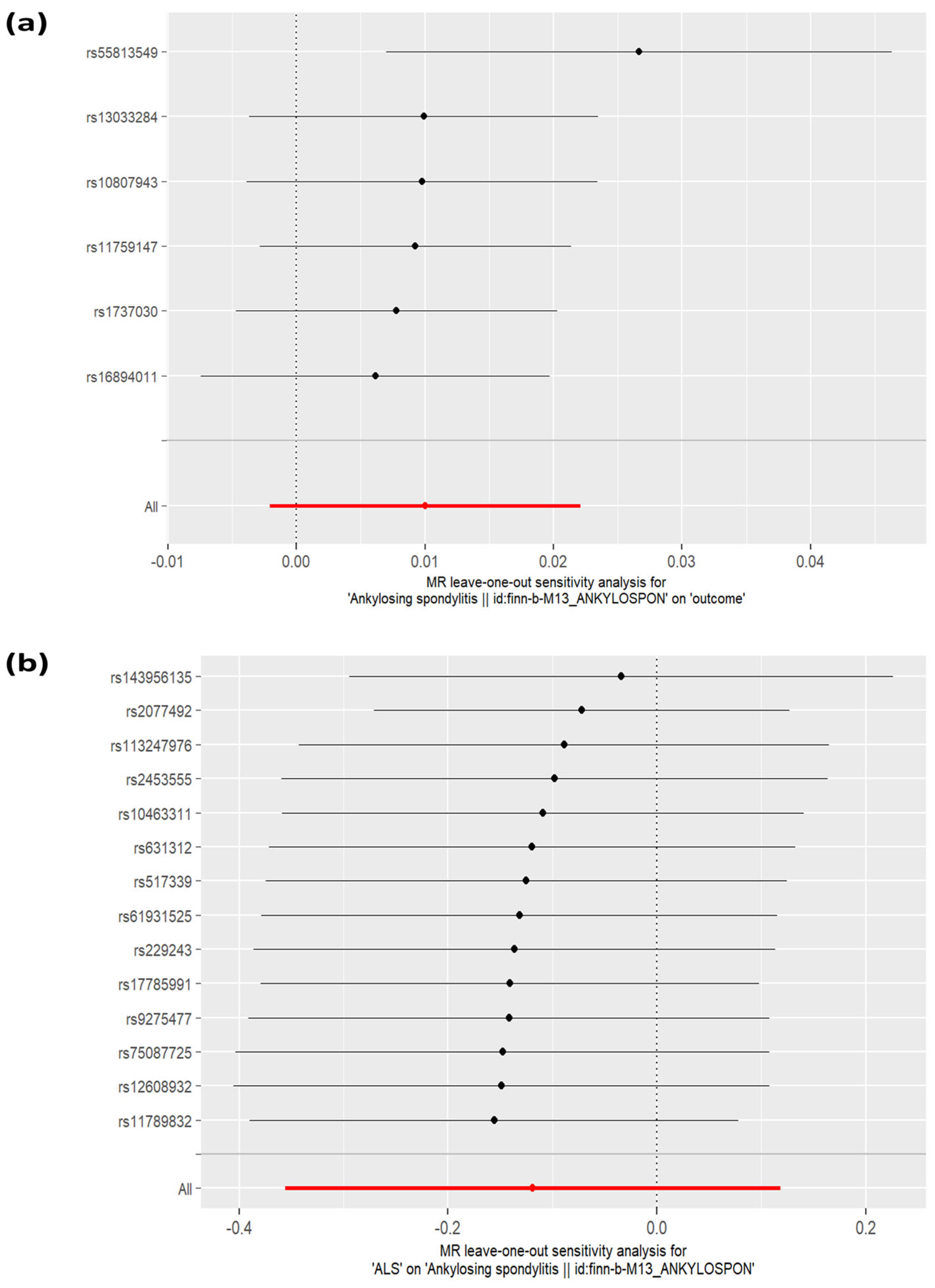
3.2. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef]
- Talbott, E.O.; Malek, A.M.; Lacomis, D. The epidemiology of amyotrophic lateral sclerosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 225–238. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From genes to mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Arthur, K.C.; Calvo, A.; Price, T.R.; Geiger, J.T.; Chio, A.; Traynor, B.J. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat. Commun. 2016, 7, 12408. [Google Scholar] [CrossRef] [PubMed]
- Bekele, B.K.; Kwizera, L.; Razzak, R.A.; Alfadul, E.S.; Anand, A.; Wojtara, M.; Nazir, A.; Uwishema, O. ALS in Africa: Current knowledge and exciting opportunities for future study—Short communication. Ann. Med. Surg. 2023, 85, 5827–5830. [Google Scholar] [CrossRef]
- Mehta, P.; Raymond, J.; Nair, T.; Han, M.; Berry, J.; Punjani, R.; Larson, T.; Mohidul, S.; Horton, D.K. Amyotrophic lateral sclerosis estimated prevalence cases from 2022 to 2030, data from the national ALS Registry. Amyotroph. Lateral Scler. Frontotemporal Degener. 2025, 26, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, C.; Gauvin, D.E.; Ishola, F.; Oskoui, M. Global Prevalence and Incidence of Amyotrophic Lateral Sclerosis. Neurology 2023, 101, e613–e623. [Google Scholar] [CrossRef]
- Mehta, P.; Kaye, W.; Bryan, L.; Larson, T.; Copeland, T.; Wu, J.; Muravov, O.; Horton, K. Prevalence of Amyotrophic Lateral Sclerosis—United States, 2012–2013. MMWR Surveill. Summ. 2016, 65, 1–12. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; van den Berg, L.H.; Veldink, J. Gene discovery in amyotrophic lateral sclerosis: Implications for clinical management. Nat. Rev. Neurol. 2017, 13, 96–104. [Google Scholar] [CrossRef]
- Ryan, M.; Heverin, M.; McLaughlin, R.L.; Hardiman, O. Lifetime Risk and Heritability of Amyotrophic Lateral Sclerosis. JAMA Neurol. 2019, 76, 1367. [Google Scholar] [CrossRef]
- Trabjerg, B.B.; Garton, F.C.; van Rheenen, W.; Fang, F.; Henderson, R.D.; Mortensen, P.B.; Agerbo, E.; Wray, N.R. ALS in Danish Registries. Neurol. Genet. 2020, 6, e398. [Google Scholar] [CrossRef] [PubMed]
- Alipour, P.; Senkevich, K.; Ross, J.P.; Spiegelman, D.; Manousaki, D.; Dion, P.A.; Rouleau, G.A. Investigation of the causal relationship between ALS and autoimmune disorders: A Mendelian randomization study. BMC Med. 2022, 20, 382. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Appel, S.H. Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zeng, Y.; Zhang, F.; Xu, Y.; Duan, Q.; Long, S.; Lin, Y.; Wang, K.; Jiang, L. Genetic effects of circulating hormone and proteome on amyotrophic lateral sclerosis identified by Mendelian randomization. Sci. Rep. 2025, 15, 10782. [Google Scholar] [CrossRef]
- Khan, M.A.; Yong, S.; Wei, J.C. Ankylosing spondylitis: History, epidemiology, and HLA-B27. Int. J. Rheum. Dis. 2023, 26, 413–414. [Google Scholar] [CrossRef]
- Banciu, C.; Chiriac, S.; Pojoga, C.; Marian, L.; Fabian, A.; Gogulescu, A.; Simu, M.; Parvanescu, R.; Mioc, A.; Racoviceanu, R.; et al. An Uncommon Overlap Syndrome Between Ankylosing Spondylitis and Amyotrophic Lateral Sclerosis—Case Report. Medicina 2024, 60, 1703. [Google Scholar] [CrossRef]
- Roy, B.; Sengupta, S.; Ghosh, K.; Mukhopadhyay, S.; Ghosh, B. A curious case of ankylosing spondylosis and motor neuron disease: A mere coincidence or correlation? Int. J. Appl. Basic. Med. Res. 2018, 8, 266. [Google Scholar]
- Cui, C.; Longinetti, E.; Larsson, H.; Andersson, J.; Pawitan, Y.; Piehl, F.; Fang, F. Associations between autoimmune diseases and amyotrophic lateral sclerosis: A register-based study. Amyotroph. Lateral Scler. Frontotemporal Degener. 2021, 22, 211–219. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Burgess, S.; Smith, G.D.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2023, 4, 186. [Google Scholar] [CrossRef]
- Lawlor, D.A. Commentary: Two-sample Mendelian randomization: Opportunities and challenges. Int. J. Epidemiol. 2016, 45, 908–915. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, W.; van der Spek, R.A.; Bakker, M.K.; van Vugt, J.J.; Hop, P.J.; Zwamborn, R.A.; De Klein, N.; Westra, H.J.; Bakker, O.B.; Deelen, P.; et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat. Genet. 2021, 53, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.A.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013, 42, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Loustau, V.; Foltz, V.; Poulain, C.; Rozenberg, S.; Bruneteau, G. Diagnosis of amyotrophic lateral sclerosis in a patient treated with TNFα blockers for ankylosing spondylitis: Fortuitus association or new side effect of TNFα blockers? Joint Bone Spine 2009, 76, 213–214. [Google Scholar] [CrossRef]
- Dalmau-Carolà, J. Amyotrophic Lateral Sclerosis: A Causal or Casual Relationship with Anti-Tnf Therapy?—A Clinical Case. Arch. Med. 2015, 1, 8. [Google Scholar]
- Petitpain, N.; Devos, D.; Bagheri, H.; Rocher, F.; Gouraud, A.; Masmoudi, K.; Coquerel, A. Is TNF inhibitor exposure a risk factor for amyotrophic lateral sclerosis? Fundam. Clin. Pharmacol. 2019, 33, 689–694. [Google Scholar] [CrossRef]
- Callhoff, J.; Sieper, J.; Weiß, A.; Zink, A.; Listing, J. Efficacy of TNFα blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: A meta-analysis. Ann. Rheum. Dis. 2015, 74, 1241–1248. [Google Scholar] [CrossRef]
- Börjesson, A.; Grundmark, B.; Olaisson, H.; Waldenlind, L. Is there a link between amyotrophic lateral sclerosis and treatment with TNF-alpha inhibitors? Ups. J. Med. Sci. 2013, 118, 199–200. [Google Scholar] [CrossRef]
- Ou, J.; Xiao, M.; Huang, Y.; Tu, L.; Chen, Z.; Cao, S.; Wei, Q.; Gu, J. Serum Metabolomics Signatures Associated with Ankylosing Spondylitis and TNF Inhibitor Therapy. Front. Immunol. 2021, 12, 630791. [Google Scholar] [CrossRef]
- Du, Y.; Wen, Y.; Guo, X.; Hao, J.; Wang, W.; He, A.; Fan, Q.; Li, P.; Liu, L.; Liang, X.; et al. A Genome-wide Expression Association Analysis Identifies Genes and Pathways Associated with Amyotrophic Lateral Sclerosis. Cell. Mol. Neurobiol. 2018, 38, 635–639. [Google Scholar] [CrossRef]

| Phenotype/Trait | Data Source | OpenGWAS ID | Sample/Cohort | Ancestry | Sample Sizes |
|---|---|---|---|---|---|
| Ankylosing Spondylitis | FinnGen Consortium | finn-b-M13_ANKYLOSPON | FinnGen R9 | (Finnish) (100%) | 1462 (cases) 164,682 (controls) |
| Amyotrophic lateral sclerosis | van Rheenen et al., 2021 [24] | ebi-a-GCST90027164 | SLAP, MND Bank, SLALOM, BePS, SLAGEN, PARALS, QSkin | Pan-European (100%) | 27,205 (cases) 110,881 (controls) |
| Exposure | Outcome | Sensitivity Test | |||||
|---|---|---|---|---|---|---|---|
| Cochran’s Q | |||||||
| MR-Egger | IVW | ||||||
| Q | df | p value | Q | df | p value | ||
| AS | ALS | 3.28 | 4 | 0.51 | 5.13 | 5 | 0.40 |
| ALS | AS | 18.9 | 12 | 0.09 | 19 | 13 | 0.12 |
| MR-Egger intercept | |||||||
| Egger intercept | p-value | ||||||
| AS | ALS | 0.012 | 0.25 | ||||
| ALS | AS | 0.005 | 0.86 | ||||
| MR-PRESSO (Global test) | |||||||
| RSS obs | p-value | ||||||
| AS | ALS | 16.35 | 0.39 | ||||
| ALS | AS | 21.87 | 0.15 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinade, A.T.; Jacobs, E.D.; Obere, C.M.; Ogaji, V.O.; Alakunle, E.; Awe, O.I. Exploring the Links Between Ankylosing Spondylitis and Amyotrophic Lateral Sclerosis: A Bidirectional Mendelian Randomization Study. Sclerosis 2025, 3, 37. https://doi.org/10.3390/sclerosis3040037
Akinade AT, Jacobs ED, Obere CM, Ogaji VO, Alakunle E, Awe OI. Exploring the Links Between Ankylosing Spondylitis and Amyotrophic Lateral Sclerosis: A Bidirectional Mendelian Randomization Study. Sclerosis. 2025; 3(4):37. https://doi.org/10.3390/sclerosis3040037
Chicago/Turabian StyleAkinade, Adeyemi Timothy, Ezekiel Damilare Jacobs, Chucks Marvellous Obere, Victor Omeiza Ogaji, Emmanuel Alakunle, and Olaitan I. Awe. 2025. "Exploring the Links Between Ankylosing Spondylitis and Amyotrophic Lateral Sclerosis: A Bidirectional Mendelian Randomization Study" Sclerosis 3, no. 4: 37. https://doi.org/10.3390/sclerosis3040037
APA StyleAkinade, A. T., Jacobs, E. D., Obere, C. M., Ogaji, V. O., Alakunle, E., & Awe, O. I. (2025). Exploring the Links Between Ankylosing Spondylitis and Amyotrophic Lateral Sclerosis: A Bidirectional Mendelian Randomization Study. Sclerosis, 3(4), 37. https://doi.org/10.3390/sclerosis3040037






