Evaluation of Early Initiation of Disease-Modifying Treatment for Patients with Multiple Sclerosis Within a Real-World Population for Long-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
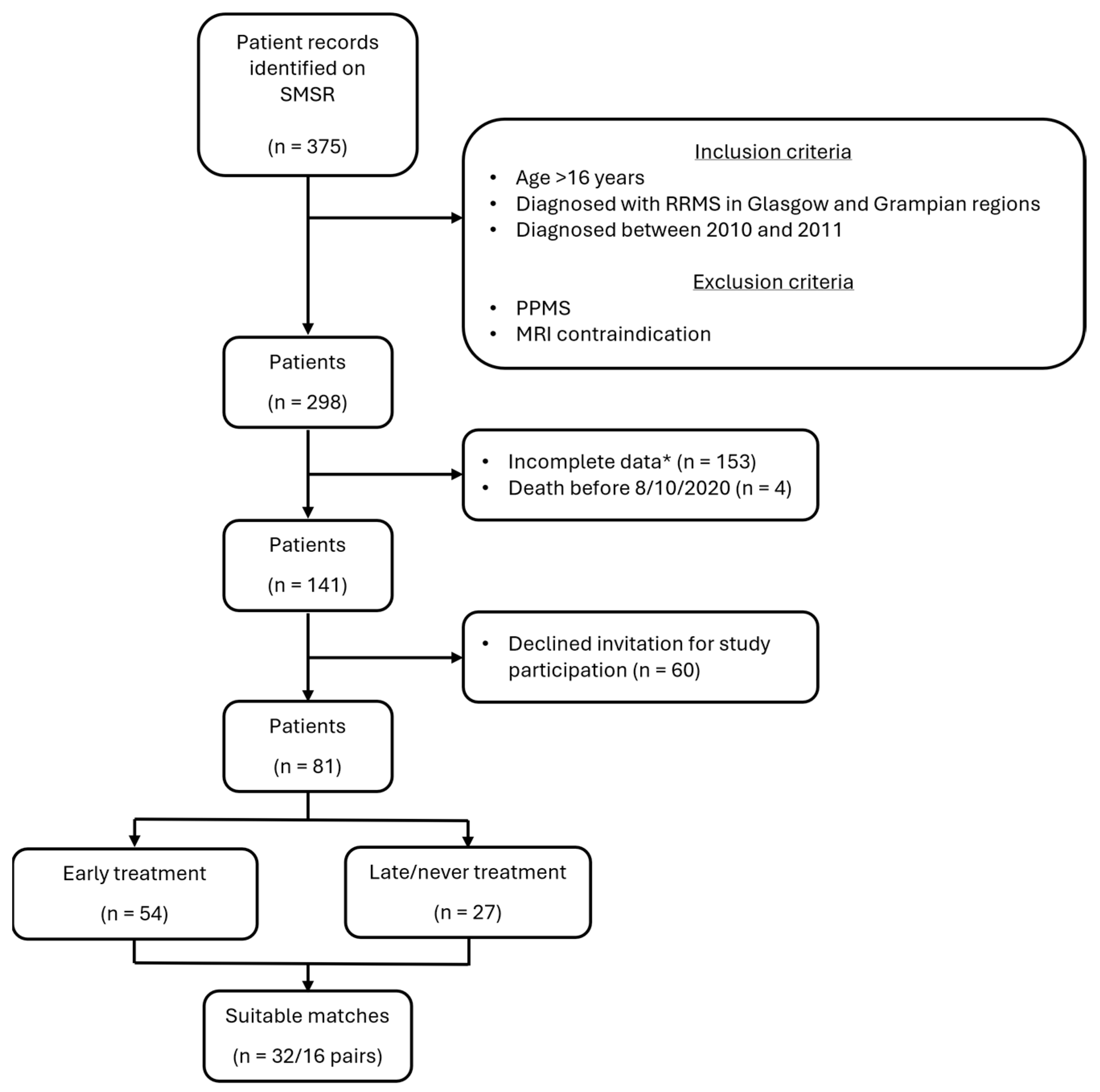
2.1. Study Population
2.2. Ethics
2.3. Study Design
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Measures of Physical Disability, Cognitive Dysfunction, Patient-Reported Function, and Quality of Life
3.2. Relapses
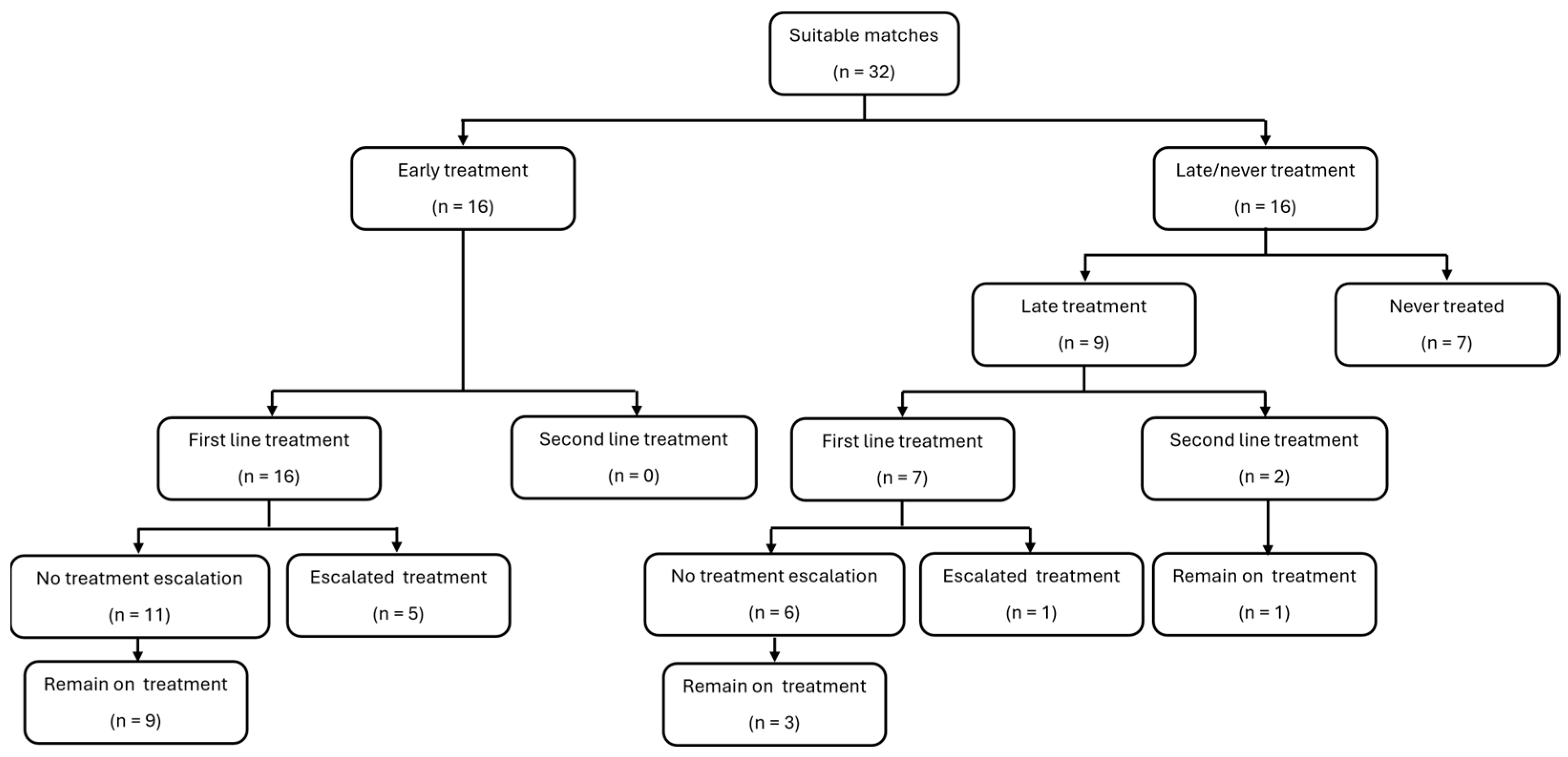
3.3. Treatments
3.4. Radiological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Multiple Sclerosis International Federation (MSIF). Atlast of MS, 3rd ed.; The Multiple Sclerosis International Federation (MSIF): London, UK, 2020. [Google Scholar]
- World Health Organisation. Atlas: Multiple Sclerosis Resources in the World; World Health Organisation: Geneva, Switzerland, 2023. [Google Scholar]
- Phadke, J.G.; Downie, A.W. Epidemiology of multiple sclerosis in the north-east (Grampian region) of Scotland—An update. J. Epidemiol. Community Health 1987, 41, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E.; Jarvis, L.; McLaughlin, R.; Fries, A.; Ebers, G.C.; Ramagopalan, S.V. The Epidemiology of Multiple Sclerosis in Scotland: Inferences from Hospital Admissions. PLoS ONE 2011, 6, e14606. [Google Scholar] [CrossRef]
- Cree, B.A.; Hartung, H.-P.; Barnett, M. New drugs for multiple sclerosis: New treatment algorithms. Curr. Opin. Neurol. 2022, 35, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Naismith, R.T. Multiple sclerosis therapeutic strategies: Start safe and effective, reassess early, and escalate if necessary. Neurol. Clin. Pract. 2011, 1, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, P. Concepts of induction and escalation therapy in multiple sclerosis. J. Neurol. Sci. 2009, 277, S42–S45. [Google Scholar] [CrossRef]
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993, 43, 655–661. [Google Scholar] [CrossRef]
- Johnson, K.P.; Brooks, B.R.; Cohen, J.A.; Ford, C.C.; Goldstein, J.; Lisak, R.P.; Myers, L.W.; Panitch, H.S.; Rose, J.W.; Schiffer, R.B.; et al. Copolymer 1 reduces relapse rate and improves disability in relapsing remitting multiple sclerosis: Results of a phase III multicenter, double-blind placebo-controlled trial. Neurology 1995, 45, 1268–1276. [Google Scholar] [CrossRef]
- Jacobs, L.D.; Cookfair, D.L.; Rudick, R.A.; Herndon, R.M.; Richert, J.R.; Salazar, A.M.; Fischer, J.S.; Goodkin, D.E.; Granger, C.V.; Simon, J.H.; et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann. Neurol. 1996, 39, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Molazadeh, N.; Akaishi, T.; Bose, G.; Nishiyama, S.; Chitnis, T.; Levy, M. Progression independent of relapses in aquaporin4-IgG-seropositive neuromyelitis optica spectrum disorder, myelin oligodendrocyte glycoprotein antibody-associated disease, and multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 80, 105093. [Google Scholar] [CrossRef]
- Simon, J.; Jacobs, L.; Campion, M.; Rudick, R.; Cookfair, D.; Herndon, R.; Richert, J.; Salazar, A.; Fischer, J.; Goodkin, D.; et al. A longitudinal study of brain atrophy in relapsing multiple sclerosis. Neurology 1999, 53, 139. [Google Scholar] [CrossRef]
- Zipoli, V.; Goretti, B.; Hakiki, B.; Siracusa, G.; Sorbi, S.; Portaccio, E.; Amato, M.P. Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Mult. Scler. J. 2009, 16, 62–67. [Google Scholar] [CrossRef]
- Popescu, V.; Agosta, F.; Hulst, H.E.; Sluimer, I.C.; Knol, D.L.; Sormani, M.P.; Enzinger, C.; Ropele, S.; Alonso, J.; Sastre-Garriga, J.; et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1082–1091. [Google Scholar] [CrossRef]
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Rae-Grant, A.; Day, G.S.; Marrie, R.A.; Rabinstein, A.; Cree, B.A.; Gronseth, G.S.; Haboubi, M.; Halper, J.; Hosey, J.P.; Jones, D.E.; et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 777–788. [Google Scholar] [CrossRef]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. 2018, 24, 96–120, Erratum in: Mult. Scler. 2020, 26, 517. https://doi.org/10.1177/1352458520906383. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Lucisano, G.; Butzkueven, H.; Hillert, J.; Hyde, R.; Koch-Henriksen, N.; Magyari, M.; Pellegrini, F.; Spelman, T.; Sørensen, P.S.; et al. Early treatment delays long-term disability accrual in RRMS: Results from the BMSD network. Mult. Scler. J. 2021, 27, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Edan, G.; Kappos, L.; Montalban, X.; Polman, C.H.; Freedman, M.S.; Hartung, H.-P.; Miller, D.; Barkhof, F.; Herrmann, J.; Lanius, V.; et al. Long-term impact of interferon beta-1b in patients with CIS: 8-year follow-up of BENEFIT. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Martinelli, V.; Rodegher, M.; Moiola, L.; Bajenaru, O.; Carra, A.; Elovaara, I.; Fazekas, F.; Hartung, H.; Hillert, J.; et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): A randomised, double-blind, placebo-controlled trial. Lancet 2009, 374, 1503–1511. [Google Scholar] [CrossRef]
- Kinkel, R.P.; Dontchev, M.; Kollman, C.; Skaramagas, T.T.; O’connor, P.W.; Simon, J.H. Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance Investigators. Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: A 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch. Neurol. 2012, 69, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Chalmer, T.A.; Baggesen, L.M.; Nørgaard, M.; Koch-Henriksen, N.; Magyari, M.; Sorensen, P.S.; the Danish Multiple Sclerosis Group. Early versus later treatment start in multiple sclerosis: A register-based cohort study. Eur. J. Neurol. 2018, 25, 1262-E110. [Google Scholar] [CrossRef]
- Harding, K.; Williams, O.; Willis, M.; Hrastelj, J.; Rimmer, A.; Joseph, F.; Tomassini, V.; Wardle, M.; Pickersgill, T.; Robertson, N.; et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol. 2019, 76, 536–541. [Google Scholar] [CrossRef]
- Norborg, H.; Aarseth, J.H.; Mannseth, J.; Henriksen, H.N.; Grytten, N.; Myhr, K.-M.; Wergeland, S. Effect of early highly effective treatment compared to an escalating treatment strategy in multiple sclerosis. Mult. Scler. Relat. Disord. 2025, 103, 106702. [Google Scholar] [CrossRef]
- Morgan, A.; Tallantyre, E.; Ontaneda, D. The benefits and risks of escalation versus early highly effective treatment in patients with multiple sclerosis. Expert Rev. Neurother. 2023, 23, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.L.; Coles, A.; Horakova, D.; Havrdova, E.; Izquierdo, G.; Prat, A.; Girard, M.; Duquette, P.; Trojano, M.; Lugaresi, A.; et al. Association of Initial Disease-Modifying Therapy with Later Conversion to Secondary Progressive Multiple Sclerosis. JAMA 2019, 321, 175–187. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Merkel, B.; Brown, J.W.L.; Ryerson, L.Z.; Kister, I.; Malpas, C.B.; Sharmin, S.; Horakova, D.; Havrdova, E.K.; Spelman, T.; et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol. 2020, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
| No DMT in First Year Mean (SD) Median [IQR] | DMT in First Year Mean (SD) Median [IQR] | p † (Effect Size r) | |
|---|---|---|---|
| Patient numbers | 16 | 16 | |
| Age at diagnosis | 34.3 (7.07) 33 [11.5] | 34.3 (7.07) 33 [11.5] | 0.19 (0.23) |
| Duration of disease * | 2.01 (2.05) 1.44 [2.08] | 2.20 (2.42) 1.48 [2.13] | 0.84 (0.04) |
| Years between relapse * | 1.92 (1.87) 1.13 [3.15] | 1.75 (2.05) 1.07 [2.05] | 0.57 (0.10) |
| EDSS score | 2.22 (1.59) 1.75 [2.50] | 1.69 (1.48) 1.5 [1.0] | 0.38 (0.16) |
| Number of relapses | 2.0 (0.63) 2.0 [0.0] | 2.19 (0.75) 2.0 [0.75] | 0.45 (0.13) |
N (%) | N (%) | ||
| SEX (% female) | 9 (56%) | 11 (69%) | 0.69 ‡ (0.17) |
Initial relapse symptoms Sensory only Motor Sensorimotor Polysymptoms | 9 (56%) 5 (31%) 2 (13%)0 | 9 (56%) 3 (19%) 3 (19%) 1 (6%) | 0.62 (0.09) |
Number T2 lesions zero Less than 9 9 or more | 2 (13%) 4 (25%) 10 (63%) | 2 (13%) 4 (25%) 10 (63%) | 1.0 |
Recovery from 1st relapse Complete Partial None | 9 (57%) 1 (6%) 6 (38%) | 8 (50%) 3 (19%) 5 (31%) | 0.90 (0.02) |
| No DMT in First Year (n = 16) Mean (SD) Median [IQR] {Range} | DMT in First Year (n = 16) Mean (SD) Median [IQR] {Range} | p † (Effect Size r) | |
|---|---|---|---|
| EDSS at diagnosis | 2.22 (1.59) 1.75 [2.5] {0–6} | 1.69(1.48) 1.50 [1.00] {0–6} | 0.38 (0.16) |
| EDSS at visit | 3.93 (2.41) 3.25 [3.9] {0–7.5} | 4.53 (2.00) 4.50 [2.50] {1–9} | 0.57 (0.10) |
| Change in EDSS | 1.72 (1.58) 1.50 [2.25] {−0.5–5.0} | 2.84 (1.88) 3.0 [3.4] {0–7} | 0.09 (0.30) |
| % EDSS >= 3.0 | 10 (62.5%) | 13 (81.3%) | 0.45 ‡ (0.21) |
| Average 9-HPT * | 38.35 (28.14) 23.75 [25.75] {18.75–123} | 36.09 (27.19) 28.25 [7.56] {20.75–132.75} | 1.0 (0) |
| Information Processing (SDMT) * | 47.00 (18.02) 53.0 [8.0] {0–67} | 42.10 (11.52) 42.0 [15.0] {20–61} | 0.14 (0.27) |
| Visual Memory (BVMT-R) * | 21.93 (10.07) 26.0 [14.0] {0–31} | 24.13 (5.57) 25.0 [8.0] {14–32} | 0.86 (0.03) |
| Verbal Memory (CVLT-II) | 14.69 (3.40) 16.0 [0] {6–16} | 15.00 (2.07) 16.0 [1] {9–16} | 0.89 (0.02) |
| Lower limb function (NeuroQol) | 32.13 (9.99) 37.0 [13.0] {9–40} | 29.44 (8.79) 31.5 [12.0] {8–40} | 0.41 (0.15) |
| Upper limb function (NeuroQol) | 36.06 (5.36) 39.0 [7.75] {22–40} | 33.81 (8.83) 35.0 [6.75] {8–40} | 0.55 (0.11) |
| Cognition (PROMIS) | 124.0 (27.56) 132.5 [35.25] {70–164} | 93.19 (31.74) 94.0 [40.25] {47–165} | 0.02 (0.40) |
| Quality of life (MSIS-29) | 68.0 (26.25) 68.5 [44.5] {31–122} | 87.25 (26.33) 89.5 [39.0] {33–139} | 0.11 (0.28) |
| Number of relapses | 1.88 (1.50) 1.50 [1.75] {0–5} | 2.0 (1.10) 2.50 [1.50] {0–3} | 0.75 (0.06) |
| Annualised relapse rate | 0.15 (0.12) 0.12 [0.13] {0–0.41} | 0.17 (0.09) 0.20 [0.17] {0–0.27} | 0.57 (0.10) |
| N (%) | N (%) | ||
| Any severe relapses | 5 (41%) | 7 (44%) | 0.73 ‡ (0.13) |
| Any disabling relapses | 8 (50%) | 10 (62%) | 0.69 ‡ (0.17) |
| Any incomplete recovery | 6 (38%) | 10 (62%) | 0.29 ‡ (0.25) |
| Treatment | No DMT in First yr (n = 16) Mean (SD) Median [IQR] {Range} | DMT in First Year (n = 16) Mean (SD) Median [IQR] {Range} | p † (Effect Size r) |
|---|---|---|---|
| No. of DMTs | 0.75 (0.78) 1.0 [1.0] {0–2} | 2.81 (0.83) 3.0 [0] {1–4} | 0.001 (0.61) |
| Time on DMT (years) | 4.2 (4.4) 4.0 [9.1] {0–11.5} | 10.4 (2.9) 10.8 [1.5] {0.4–12.5} | 0.001 (0.59) |
| No. of 1st-line DMTs | 0.56 (0.73) 0 [1.0] {0–2} | 2.38 (0.96) 3.0 [1.75] {1–4} | 0.001 (0.61) |
| Time on 1st-line DMT (years) | 3.1 (3.9) 0 [8.5] {0–10.2} | 8.5 (4.0) 10.2 [6.2] {0.4–12.5} | 0.002 (0.55) |
| No. of 2nd-line DMTs | 0.19 (0.40) 0 [0] {0–1} | 0.44 (0.73) 0 [1] {0–2} | 0.27 (0.19) |
| Time on 2nd-line DMT (days) | 402 (966) 0 [0] {0–3428} | 684 (1179) 0 [1572] {0–3596} | 0.48 (0.12) |
| Switching 1st- to 2nd-line [n(%)] | 3 (18.8%) | 5 (31.3%) | 0.73 ‡ (0.13) |
| Happy with treatment [n(%)] | 8 (50%) | 9 (100%) | 0.008 ‡ (0.50) |
| No DMT in First Year (n = 12) Mean (SD) Median [IQR] {Range} | DMT in First Year (n = 12) Mean (SD) Median [IQR] {Range} | p † (Effect Size r) | |
|---|---|---|---|
| FLAIR lesion volume (mL) | 7.3 (6.3) 5.55 [11.35] {0.7–18.5} | 9.8 (9.5) 6.60 [15.1] {1.7–30.8} | 0.53 (0.13) |
| T1 lesion volume (mL) | 5.43 (4.77) 4.0 [9.66] {0.1 –13.1} | 7.5 (7.6) 4.7 [11.6] {1.3–24.9} | 0.58 (0.11) |
| Whole-brain volume (mL) | 1515 (86.6) 1512 [140] {1346–1647} | 1463 (50.0) 1453 [88] {1390–1542} | 0.14 (0.30) |
| Whole-brain volume population percentile | 30.2 (26.6) 26.3 [57.7] {1–68} | 13.6 (21.3) 2.45 [17.0] {1–68} | 0.12 (0.32) |
| Grey matter volume (mL) | 913 (60) 910 [61] {802–1041} | 819 (233) 864 [73] {90–959} | 0.15 (0.30) |
| Grey matter volume population percentile | 48.7 (28.6) 49.8 [47.3] {1–98.8} | 32.4 (35.7) 20.6 [70.7] {1–89.1} | 0.39 (0.18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonald, M.; Macleod, A.D.; Gallagher, P. Evaluation of Early Initiation of Disease-Modifying Treatment for Patients with Multiple Sclerosis Within a Real-World Population for Long-Term Outcomes. Sclerosis 2025, 3, 35. https://doi.org/10.3390/sclerosis3040035
McDonald M, Macleod AD, Gallagher P. Evaluation of Early Initiation of Disease-Modifying Treatment for Patients with Multiple Sclerosis Within a Real-World Population for Long-Term Outcomes. Sclerosis. 2025; 3(4):35. https://doi.org/10.3390/sclerosis3040035
Chicago/Turabian StyleMcDonald, Menai, Angus D. Macleod, and Paul Gallagher. 2025. "Evaluation of Early Initiation of Disease-Modifying Treatment for Patients with Multiple Sclerosis Within a Real-World Population for Long-Term Outcomes" Sclerosis 3, no. 4: 35. https://doi.org/10.3390/sclerosis3040035
APA StyleMcDonald, M., Macleod, A. D., & Gallagher, P. (2025). Evaluation of Early Initiation of Disease-Modifying Treatment for Patients with Multiple Sclerosis Within a Real-World Population for Long-Term Outcomes. Sclerosis, 3(4), 35. https://doi.org/10.3390/sclerosis3040035






