From Gene to Clinic: The Role of APOL1 in Focal Segmental Glomerulosclerosis
Abstract
1. Introduction
2. Genetic Insights
2.1. Structure and Function of APOL1
2.2. APOL1 Variants (G1 and G2)
3. Pathogenic Mechanisms
3.1. Podocyte Injury
| Mechanism | Description | Cellular Effects | References |
|---|---|---|---|
| Podocyte Dysfunction | Cytotoxic ion channel activity | Proteinuria; podocyte detachment | [26,32] |
| Mitochondrial Dysfunction | Disruption of mitochondrial homeostasis | Energy deficits; oxidative stress | [35,36] |
| ER Stress | Induction of unfolded protein response | Cellular apoptosis | [34,37] |
| Lipid Dysregulation | Altered lipid metabolism in podocytes | Impaired membrane dynamics | [35] |
| Inflammation | Activation of innate immune pathways (e.g., STING, JAK-STAT) | Chronic inflammatory signaling | [27,38,39] |
3.2. Non-Podocyte Injury
3.3. Cellular Pathways
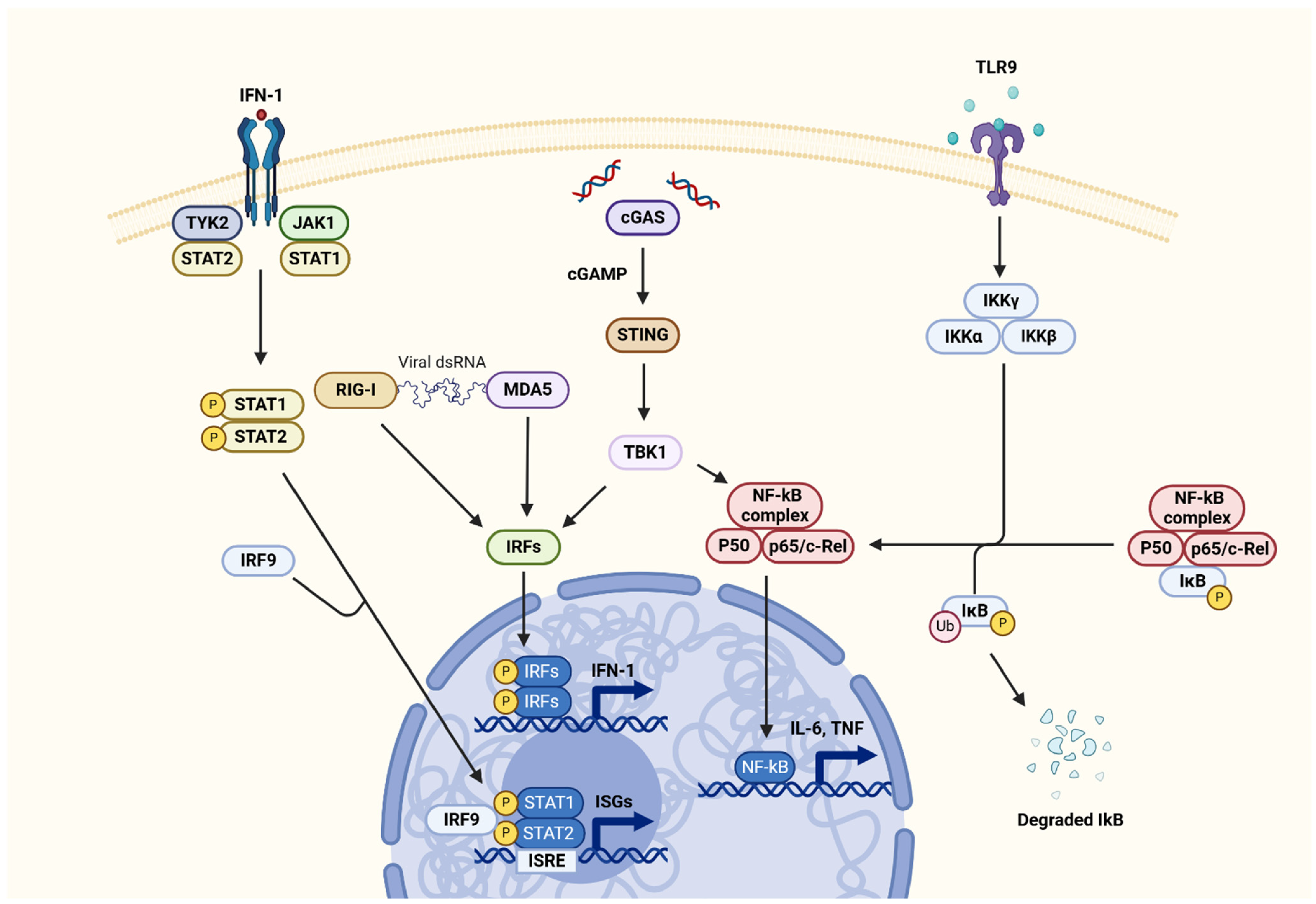
3.3.1. Interferon Pathway
3.3.2. STING Pathway
3.3.3. JAK-STAT Pathway
4. Clinical Implications
4.1. ARIC Study
4.2. NEPTUNE Study
4.3. CKiD Study
4.4. CureGN Study
4.5. APOLLO Study
5. Treatment
5.1. Baricitinib
5.2. Sparsentan
5.3. Diacylglycerol O-Acyltransferase 2 Inhibitors
5.4. Small-Molecule Inhibitors
5.5. Antisense Oligonucleotides
5.6. CRISPR-Cas9 Technology
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APOL1 | Apolipoprotein L1 |
| CKD | Chronic Kidney Disease |
| ESKD | End-Stage Kidney Disease |
| FSGS | Focal Segmental Glomerulosclerosis |
| HR | High Risk |
| IFN | Interferon |
| LR | Low Risk |
| eGFR | Estimated Glomerular Filtration Rate |
| SNP | Single-Nucleotide Polymorphism |
| SRNS | Steroid-Resistant Nephrotic Syndrome |
| FSGS-UC | FSGS of Undetermined Cause |
| HDL | High-Density Lipoprotein |
| SRA | Serum Resistance-Associated |
| TCA | Tricarboxylic Acid |
| OXPHOS | Oxidative Phosphorylation |
| UPS | Ubiquitin-Proteasome System |
| JAK-STAT | Janus Kinase-Signal Transducer and Activator of Transcription |
| STING | Stimulator of Interferon Genes |
| ASO | Antisense Oligonucleotide |
| LBW | Low Birth Weight |
| NEPTUNE | Nephrotic Syndrome Study Network |
| CKiD | Chronic Kidney Disease in Children |
| DUPLEX | Sparsentan FSGS Clinical Trial |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
References
- Tato, A.M.; Carrera, N.; García-Murias, M.; Shabaka, A.; Ávila, A.; Mora Mora, M.T.; Rabasco, C.; Soto, K.; De La Prada Alvarez, F.J.; Fernández-Lorente, L.; et al. Genetic Testing in Focal Segmental Glomerulosclerosis: In Whom and When? Clin. Kidney J. 2023, 16, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Winkler, C.A.; Vince, N.; Douillard, V.; Geffard, E.; Binns-Roemer, E.; Ng, D.K.; Gourraud, P.-A.; Reidy, K.; Warady, B.; et al. Identification of Novel Genetic Risk Factors for Focal Segmental Glomerulosclerosis in Children: Results from the Chronic Kidney Disease in Children (CKiD) Cohort. Am. J. Kidney Dis. 2023, 81, 635–646.e1. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, S.; Pillay, T.S.; Matsha, T.E. Gene of the Month: APOL1. J. Clin. Pathol. 2020, 73, 441–443. [Google Scholar] [CrossRef]
- Shukha, K.; Mueller, J.L.; Chung, R.T.; Curry, M.P.; Friedman, D.J.; Pollak, M.R.; Berg, A.H. Most ApoL1 Is Secreted by the Liver. J. Am. Soc. Nephrol. 2017, 28, 1079–1083. [Google Scholar] [CrossRef]
- Pays, E. Apolipoprotein-L1 (APOL1): From Sleeping Sickness to Kidney Disease. Cells 2024, 13, 1738. [Google Scholar] [CrossRef]
- Schaub, C.; Lee, P.; Racho-Jansen, A.; Giovinazzo, J.; Terra, N.; Raper, J.; Thomson, R. Coiled-Coil Binding of the Leucine Zipper Domains of APOL1 Is Necessary for the Open Cation Channel Conformation. J. Biol. Chem. 2021, 297, 101009. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Ge, M.; Merscher, S.; Rosenberg, A.Z.; Desante, M.; Roshanravan, H.; Okamoto, K.; Shin, M.K.; Hoek, M.; Fornoni, A.; et al. APOL1 Renal Risk Variants Promote Cholesterol Accumulation in Tissues and Cultured Macrophages from APOL1 Transgenic Mice. PLoS ONE 2019, 14, e0211559. [Google Scholar] [CrossRef]
- Blessing, N.A.; Wu, Z.; Madhavan, S.M.; Choy, J.W.; Chen, M.; Shin, M.K.; Hoek, M.; Sedor, J.R.; O’Toole, J.F.; Bruggeman, L.A. Lack of APOL1 in Proximal Tubules of Normal Human Kidneys and Proteinuric APOL1 Transgenic Mouse Kidneys. PLoS ONE 2021, 16, e0253197. [Google Scholar] [CrossRef]
- Mayanja, R.; Kintu, C.; Diabate, O.; Soremekun, O.; Oluwagbemi, O.O.; Wele, M.; Kalyesubula, R.; Jjingo, D.; Chikowore, T.; Fatumo, S. Molecular Dynamic Simulation Reveals Structure Differences in APOL1 Variants and Implication in Pathogenesis of Chronic Kidney Disease. Genes 2022, 13, 1460. [Google Scholar] [CrossRef]
- Abdu, A.; Duarte, R.; Dickens, C.; Dix-Peek, T.; Bala, S.M.; Ademola, B.; Naicker, S. High Risk APOL1 Genotypes and Kidney Disease among Treatment Naïve HIV Patients at Kano, Nigeria. PLoS ONE 2022, 17, e0275949. [Google Scholar] [CrossRef]
- Adamson, W.E.; Noyes, H.; Johnson, P.; Cooper, A.; Monckton, D.G.; Ogunsola, J.; Sullivan, M.; Mark, P.; Parekh, R.S.; MacLeod, A. Phenome-Wide Analysis of APOL1 Risk Variants Reveals Associations between One Combination of Haplotypes and Multiple Disease Phenotypes in Addition to Chronic Kidney Disease. medRxiv 2023. [Google Scholar] [CrossRef]
- Brandenburg, J.-T.; Govender, M.A.; Winkler, C.A.; Boua, P.R.; Agongo, G.; Fabian, J.; Ramsay, M. Apolipoprotein L1 High-Risk Genotypes and Albuminuria in Sub-Saharan African Populations. Clin. J. Am. Soc. Nephrol. 2022, 17, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Dumas, S.J.; Ma, L.; Wang, G.; Witjas, F.; Berg, C.W.V.D.; Rocco, M.V.; Freedman, B.I.; Rabelink, T.J.; Spijker, S. APOL1 Risk Variants Induce Mitochondrial Dysfunction in Patient-Derived Kidney Organoids: SA-PO789. J. Am. Soc. Nephrol. 2023, 34, 947–948. [Google Scholar] [CrossRef]
- Pollak, M.R.; Friedman, D.J. APOL1 and APOL1-Associated Kidney Disease: A Common Disease, an Unusual Disease Gene—Proceedings of the Henry Shavelle Professorship. Glomerular Dis. 2023, 3, 75–87. [Google Scholar] [CrossRef]
- Gupta, Y.; Friedman, D.J.; McNulty, M.T.; Khan, A.; Lane, B.; Wang, C.; Ke, J.; Jin, G.; Wooden, B.; Knob, A.L.; et al. Strong Protective Effect of the APOL1 p.N264K Variant against G2-Associated Focal Segmental Glomerulosclerosis and Kidney Disease. Nat. Commun. 2023, 14, 7836. [Google Scholar] [CrossRef]
- Bruno, J.; Edwards, J.C. Kidney-Disease-Associated Variants of Apolipoprotein L1 Show Gain of Function in Cation Channel Activity. J. Biol. Chem. 2021, 296, 100238. [Google Scholar] [CrossRef]
- Datta, S.; Kataria, R.; Zhang, J.-Y.; Moore, S.; Petitpas, K.; Mohamed, A.; Zahler, N.; Pollak, M.R.; Olabisi, O.A. Kidney Disease-Associated APOL1 Variants Have Dose-Dependent, Dominant Toxic Gain-of-Function. J. Am. Soc. Nephrol. 2020, 31, 2083–2096. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Wang, M.; Tian, L.; Genovese, G.; Yan, P.; Wilson, J.G.; Thadhani, R.; Mottl, A.K.; Appel, G.B.; Bick, A.G.; et al. UBD Modifies APOL1 -Induced Kidney Disease Risk. Proc. Natl. Acad. Sci. USA 2018, 115, 3446–3451. [Google Scholar] [CrossRef]
- Zee, J.; McNulty, M.T.; Hodgin, J.B.; Zhdanova, O.; Hingorani, S.; Jefferson, J.A.; Gibson, K.L.; Trachtman, H.; Fornoni, A.; Dell, K.M.; et al. APOL1 Genotype-Associated Morphologic Changes among Patients with Focal Segmental Glomerulosclerosis. Pediatr. Nephrol. 2021, 36, 2747–2757. [Google Scholar] [CrossRef]
- Hogg, R.J. Childhood Nephrotic Syndrome Associated with Diffuse Mesangial Hypercellularity. A Report of the Southwest Pediatric Nephrology Study Group. Kidney Int. 1983, 24, 87–94. [Google Scholar] [CrossRef]
- Silverstein, D.M.; Craver, R.D. Mesangial Hypercellularity in Children: Presenting Features and Outcomes. Pediatr. Nephrol. 2008, 23, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, S.; Urushihara, Y. Favorable Outcome in Children with Idiopathic Steroid-Resistant Nephrotic Syndrome Due to Mesangial Hypercellularity: A Distinct Disease Entity? Pediatr. Nephrol. 2016, 31, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Wenderfer, S.E.; Swinford, R.D.; Braun, M.C. C1q Nephropathy in the Pediatric Population: Pathology and Pathogenesis. Pediatr. Nephrol. 2010, 25, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-F.; Wang, S.-X.; Zhang, Y.-K.; Zhao, M.-H.; Zou, W.-Z. Ultrastructural Features and Expression of Cytoskeleton Proteins of Podocyte from Patients with Minimal Change Disease and Focal Segmental Glomerulosclerosis. Ren. Fail. 2008, 30, 477–483. [Google Scholar] [CrossRef][Green Version]
- Mann, N.; Sun, H.; Majmundar, A.J. Mechanisms of Podocyte Injury in Genetic Kidney Disease. Pediatr. Nephrol. 2024. [Google Scholar] [CrossRef]
- Wakashin, H.; Heymann, J.; Roshanravan, H.; Daneshpajouhnejad, P.; Rosenberg, A.; Shin, M.K.; Hoek, M.; Kopp, J.B. APOL1 Renal Risk Variants Exacerbate Podocyte Injury by Increasing Inflammatory Stress. BMC Nephrol. 2020, 21, 371. [Google Scholar] [CrossRef]
- Wu, J.; Raman, A.; Coffey, N.J.; Sheng, X.; Wahba, J.; Seasock, M.J.; Ma, Z.; Beckerman, P.; Laczkó, D.; Palmer, M.B.; et al. The Key Role of NLRP3 and STING in APOL1-Associated Podocytopathy. J. Clin. Investig. 2021, 131, e136329. [Google Scholar] [CrossRef]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 Inflammasome Promotes Renal Inflammation and Contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef]
- Xiong, W.; Meng, X.-F.; Zhang, C. Inflammasome Activation in Podocytes: A New Mechanism of Glomerular Diseases. Inflamm. Res. 2020, 69, 731–743. [Google Scholar] [CrossRef]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal Proteins and Human Diseases: Molecular Mechanisms and Targeted Therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Yoshida, T.; Latt, K.Z.; Rosenberg, A.Z.; Shrivastav, S.; Heymann, J.; Halushka, M.K.; Winkler, C.A.; Kopp, J.B. Transcriptomic Analysis of Human Podocytes In Vitro: Effects of Differentiation and APOL1 Genotype. Kidney Int. Rep. 2023, 8, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Pays, E. APOL1 Variant–Associated Kidney Disease: From Trypanosomes to Podocyte Cytoskeleton. Kidney Int. 2020, 98, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Heintz, L.; Meyer-Schwesinger, C. The Intertwining of Autophagy and the Ubiquitin Proteasome System in Podocyte (Patho)Physiology. Cell Physiol. Biochem. 2021, 55, 68–95. [Google Scholar] [CrossRef] [PubMed]
- Heymann, J.; Winkler, C.A.; Hoek, M.; Susztak, K.; Kopp, J.B. Therapeutics for APOL1 Nephropathies: Putting out the Fire in the Podocyte. Nephrol. Dial. Transplant. 2017, 32, i65–i70. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Molina, J.; Ducasa, G.M.; Mallela, S.K.; Varona Santos, J.; Mitrofanova, A.; Kim, J.-J.; Liu, X.; Sloan, A.; Mendez, A.J.; et al. APOL1 Risk Variants Affect Podocyte Lipid Homeostasis and Energy Production in Focal Segmental Glomerulosclerosis. Hum. Mol. Genet. 2021, 30, 182–197. [Google Scholar] [CrossRef]
- Ma, L.; Palmer, N.D.; Choi, Y.A.; Murea, M.; Snipes, J.A.; Parks, J.S.; Langefeld, C.D.; Freedman, B.I. APOL1 Risk Variants Impair Multiple Mitochondrial Pathways in a Metabolomics Analysis. Kidney360 2020, 1, 1353–1362. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, J.-G.; Fu, Y.; Van De Leemput, J.; Ray, P.E.; Han, Z. APOL1-G2 Accelerates Nephrocyte Cell Death by Inhibiting the Autophagy Pathway. Dis. Models Mech. 2023, 16, dmm050223. [Google Scholar] [CrossRef]
- Vasquez-Rios, G.; De Cos, M.; Campbell, K.N. Novel Therapies in APOL1-Mediated Kidney Disease: From Molecular Pathways to Therapeutic Options. Kidney Int. Rep. 2023, 8, 2226–2234. [Google Scholar] [CrossRef]
- Nystrom, S.E.; Li, G.; Datta, S.; Soldano, K.L.; Silas, D.; Weins, A.; Hall, G.; Thomas, D.B.; Olabisi, O.A. JAK Inhibitor Blocks COVID-19 Cytokine–Induced JAK/STAT/APOL1 Signaling in Glomerular Cells and Podocytopathy in Human Kidney Organoids. JCI Insight 2022, 7, e157432. [Google Scholar] [CrossRef]
- Hartleben, B.; Gödel, M.; Meyer-Schwesinger, C.; Liu, S.; Ulrich, T.; Köbler, S.; Wiech, T.; Grahammer, F.; Arnold, S.J.; Lindenmeyer, M.T.; et al. Autophagy Influences Glomerular Disease Susceptibility and Maintains Podocyte Homeostasis in Aging Mice. J. Clin. Investig. 2010, 120, 1084–1096. [Google Scholar] [CrossRef]
- Pell, J.; Nagata, S.; Menon, M.C. Nonpodocyte Roles of APOL1 Variants: An Evolving Paradigm. Kidney360 2023, 4, e1325–e1331. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.F. APOL1 Risk Variants in Kidney Transplantation: A Modulation of Immune Cell Function. J. Clin. Investig. 2021, 131, e154676. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Latt, K.Z.; Heymann, J.; Kopp, J.B. Lessons from APOL1 Animal Models. Front. Med. 2021, 8, 762901. [Google Scholar] [CrossRef]
- Karttunen, H.; Ross, M.J. Editing of APOL1 mRNA Reduces APOL1 Expression and Activation of Innate Immunity. Kidney Int. 2023, 104, 230–233. [Google Scholar] [CrossRef]
- Ekulu, P.M.; Adebayo, O.C.; Decuypere, J.-P.; Bellucci, L.; Elmonem, M.A.; Nkoy, A.B.; Mekahli, D.; Bussolati, B.; Van Den Heuvel, L.P.; Arcolino, F.O.; et al. Novel Human Podocyte Cell Model Carrying G2/G2 APOL1 High-Risk Genotype. Cells 2021, 10, 1914. [Google Scholar] [CrossRef]
- Blazer, A.D.; Clancy, R.M. ApoL1 and the Immune Response of Patients with Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2017, 19, 13. [Google Scholar] [CrossRef]
- Ji, L.; Li, T.; Chen, H.; Yang, Y.; Lu, E.; Liu, J.; Qiao, W.; Chen, H. The Crucial Regulatory Role of Type I Interferon in Inflammatory Diseases. Cell Biosci. 2023, 13, 230. [Google Scholar] [CrossRef]
- Pays, E. The Two Levels of Podocyte Dysfunctions Induced by Apolipoprotein L1 Risk Variants. Kidney Dial. 2024, 4, 126–143. [Google Scholar] [CrossRef]
- Tumlin, J.; Rovin, B.; Anders, H.-J.; Mysler, E.F.; Jayne, D.R.W.; Takeuchi, T.; Lindholm, C.; Weiss, G.; Sorrentino, A.; Woollard, K.; et al. Targeting the Type I Interferon Pathway in Glomerular Kidney Disease: Rationale and Therapeutic Opportunities. Kidney Int. Rep. 2025, 10, 29–39. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Luan, Y.; Li, X.; Meng, X.; Liao, W.; Tang, J.; Wang, Z. cGAS-STING, Inflammasomes and Pyroptosis: An Overview of Crosstalk Mechanism of Activation and Regulation. Cell Commun. Signal 2024, 22, 22. [Google Scholar] [CrossRef]
- Davis, S.E.; Khatua, A.K.; Popik, W. Nucleosomal dsDNA Stimulates APOL1 Expression in Human Cultured Podocytes by Activating the cGAS/IFI16-STING Signaling Pathway. Sci. Rep. 2019, 9, 15485. [Google Scholar] [CrossRef] [PubMed]
- Muehlig, A.K.; Gies, S.; Huber, T.B.; Braun, F. Collapsing Focal Segmental Glomerulosclerosis in Viral Infections. Front. Immunol. 2022, 12, 800074. [Google Scholar] [CrossRef]
- Chen, J.; Chen, P.; Song, Y.; Wei, J.; Wu, F.; Sun, J.; Xu, Z. STING Upregulation Mediates Ferroptosis and Inflammatory Response in Lupus Nephritis by Upregulating TBK1 and Activating NF-κB Signal Pathway. J. Biosci. 2024, 49, 9. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, R.; Pan, Y.; Sun, H. Potential Therapeutic Value of the STING Inhibitors. Molecules 2023, 28, 3127. [Google Scholar] [CrossRef]
- Olabisi, O.A.; Barrett, N.J.; Lucas, A.; Smith, M.; Bethea, K.; Soldano, K.; Croall, S.; Sadeghpour, A.; Chakraborty, H.; Wolf, M. Design and Rationale of the Phase 2 Baricitinib Study in Apolipoprotein L1–Mediated Kidney Disease (JUSTICE). Kidney Int. Rep. 2024, 9, 2677–2684. [Google Scholar] [CrossRef]
- Kopp, J.B.; Nelson, G.W.; Sampath, K.; Johnson, R.C.; Genovese, G.; An, P.; Friedman, D.; Briggs, W.; Dart, R.; Korbet, S.; et al. APOL1 Genetic Variants in Focal Segmental Glomerulosclerosis and HIV-Associated Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 2129–2137. [Google Scholar] [CrossRef]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Uscinski Knob, A.L.; et al. Association of Trypanolytic ApoL1 Variants with Kidney Disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef]
- Freedman, B.I.; Kopp, J.B.; Sampson, M.G.; Susztak, K. APOL1 at 10 Years: Progress and next Steps. Kidney Int. 2021, 99, 1296–1302. [Google Scholar] [CrossRef]
- Gulati, A.; Moxey-Mims, M. Defining Risk in APOL1-Associated Kidney Disease: The Story Is Evolving! Am. J. Kidney Dis. 2024, 84, 388–391. [Google Scholar] [CrossRef]
- Chen, T.K.; Coresh, J.; Daya, N.; Ballew, S.H.; Tin, A.; Crews, D.C.; Grams, M.E. Race, APOL1 Risk Variants, and Clinical Outcomes among Older Adults: The ARIC Study. J. Am. Geriatr. Soc. 2021, 69, 155–163. [Google Scholar] [CrossRef]
- Woroniecki, R.P.; Ng, D.K.; Limou, S.; Winkler, C.A.; Reidy, K.J.; Mitsnefes, M.; Sampson, M.G.; Wong, C.S.; Warady, B.A.; Furth, S.L.; et al. Renal and Cardiovascular Morbidities Associated with APOL1 Status among African-American and Non-African-American Children with Focal Segmental Glomerulosclerosis. Front. Pediatr. 2016, 4, 122. [Google Scholar] [CrossRef] [PubMed]
- Rheault, M.N.; Alpers, C.E.; Barratt, J.; Bieler, S.; Canetta, P.; Chae, D.-W.; Coppock, G.; Diva, U.; Gesualdo, L.; Heerspink, H.J.L.; et al. Sparsentan versus Irbesartan in Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 2023, 389, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.; Gibson, K.L.; Xie, Y.; Wang, Y.; Eddy, S.; Hartman, J.; Sampson, M.; Cassol, C.; Thomas, D.; Gipson, D.S.; et al. The Association of Low Birthweight and Prematurity on Outcomes in Children and Adults with Nephrotic Syndrome—A NEPTUNE Cohort Study. Pediatr. Nephrol. 2023, 38, 3297–3308. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.K.; Robertson, C.C.; Woroniecki, R.P.; Limou, S.; Gillies, C.E.; Reidy, K.J.; Winkler, C.A.; Hingorani, S.; Gibson, K.L.; Hjorten, R.; et al. APOL1 -Associated Glomerular Disease among African-American Children: A Collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) Cohorts. Nephrol. Dial. Transplant. 2016, 32, 983–990. [Google Scholar] [CrossRef]
- Kallash, M.; Wang, Y.; Smith, A.; Trachtman, H.; Gbadegesin, R.; Nester, C.; Canetta, P.; Wang, C.; Hunley, T.E.; Sperati, C.J.; et al. Rapid Progression of Focal Segmental Glomerulosclerosis in Patients with High-Risk APOL1 Genotypes. Clin. J. Am. Soc. Nephrol. 2023, 18, 344–355. [Google Scholar] [CrossRef]
- Hughson, M.D.; Hoy, W.E.; Mott, S.A.; Puelles, V.G.; Bertram, J.F.; Winkler, C.A.; Kopp, J.B. APOL1 Risk Alleles Are Associated with More Severe Arteriosclerosis in Renal Resistance Vessels with Aging and Hypertension. Kidney Int. Rep. 2016, 1, 10–23. [Google Scholar] [CrossRef]
- Freedman, B.I.; Moxey-Mims, M.M.; Alexander, A.A.; Astor, B.C.; Birdwell, K.A.; Bowden, D.W.; Bowen, G.; Bromberg, J.; Craven, T.E.; Dadhania, D.M.; et al. APOL1 Long-Term Kidney Transplantation Outcomes Network (APOLLO): Design and Rationale. Kidney Int. Rep. 2020, 5, 278–288. [Google Scholar] [CrossRef]
- Olabisi, O.A. APOL1 Channel Blocker Reduces Proteinuria in FSGS. Kidney Int. 2023, 104, 228–230. [Google Scholar] [CrossRef]
- Friedman, D.J.; Pollak, M.R. APOL1 Nephropathy: From Genetics to Clinical Applications. Clin. J. Am. Soc. Nephrol. 2021, 16, 294–303. [Google Scholar] [CrossRef]
- Egbuna, O.; Zimmerman, B.; Manos, G.; Fortier, A.; Chirieac, M.C.; Dakin, L.A.; Friedman, D.J.; Bramham, K.; Campbell, K.; Knebelmann, B.; et al. Inaxaplin for Proteinuric Kidney Disease in Persons with Two APOL1 Variants. N. Engl. J. Med. 2023, 388, 969–979. [Google Scholar] [CrossRef]
- Greasley, P.J.; Agrawal, N.; Althage, M.; Sanchez, J.; Kirk, S.; Egeland, E.J.; Åstrand, M.; Westergren, H.; Sherwood, J.; Mccarthy, M.; et al. #1003 Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Multiple Ascending Doses of AZD2373, an Antisense Oligonucleotide Targeting APOL1. Nephrol. Dial. Transplant. 2024, 39, gfae069-0695-1003. [Google Scholar] [CrossRef]
- Chun, J.; Riella, C.V.; Chung, H.; Shah, S.S.; Wang, M.; Magraner, J.M.; Ribas, G.T.; Ribas, H.T.; Zhang, J.-Y.; Alper, S.L.; et al. DGAT2 Inhibition Potentiates Lipid Droplet Formation To Reduce Cytotoxicity in APOL1 Kidney Risk Variants. J. Am. Soc. Nephrol. 2022, 33, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Gyarmati, G.; Shroff, U.N.; Izuhara, A.; Deepak, S.; Komers, R.; Bedard, P.W.; Peti-Peterdi, J. Sparsentan Improves Glomerular Hemodynamics, Cell Functions, and Tissue Repair in a Mouse Model of FSGS. JCI Insight 2024, 9, e177775. [Google Scholar] [CrossRef] [PubMed]
- Trachtman, H.; Radhakrishnan, J.; Rheault, M.N.; Alpers, C.E.; Barratt, J.; Heerspink, H.J.L.; Noronha, I.L.; Perkovic, V.; Rovin, B.; Trimarchi, H.; et al. Focal Segmental Glomerulosclerosis Patient Baseline Characteristics in the Sparsentan Phase 3 DUPLEX Study. Kidney Int. Rep. 2024, 9, 1020–1030. [Google Scholar] [CrossRef]
- Rovin, B.H.; Barratt, J.; Heerspink, H.J.L.; Alpers, C.E.; Bieler, S.; Chae, D.-W.; Diva, U.A.; Floege, J.; Gesualdo, L.; Inrig, J.K.; et al. Efficacy and Safety of Sparsentan versus Irbesartan in Patients with IgA Nephropathy (PROTECT): 2-Year Results from a Randomised, Active-Controlled, Phase 3 Trial. Lancet 2023, 402, 2077–2090. [Google Scholar] [CrossRef]
- Gbadegesin, R.; Lane, B. Inaxaplin for the Treatment of APOL1-Associated Kidney Disease. Nat. Rev. Nephrol. 2023, 19, 479–480. [Google Scholar] [CrossRef]
| Study Name | Population Characteristics | Key Findings | Implications | References |
|---|---|---|---|---|
| NEPTUNE cohort | African American children with nephropathy | APOL1 HR variants linked to faster eGFR decline | Highlights need for early intervention | [61] |
| CKiD Cohort | Children with CKD, including APOL1 variant carriers | APOL1 HR associated with faster progression of CKD | Underscores importance of genetic risk stratification | [2] |
| ARIC Study | Older adults, APOL1 genotype association with CKD outcomes | APOL1 HR linked to increased risk of ESKD and cardiovascular events | Emphasizes systemic impact of APOL1 variants | [60] |
| DUPLEX Trial | Adults with biopsy-proven FSGS | Sparsentan reduced proteinuria significantly | Supports dual endothelin–angiotensin blockade | [62] |
| Genetic Epidemiology Study | Global population; APOL1 variant frequencies | G1 and G2 alleles prevalent in African ancestry | Emphasizes health disparities in CKD | [12,15] |
| Therapeutic Approach | Mechanism of Action | Advantages | Limitations | References |
|---|---|---|---|---|
| Small-Molecule Inhibitors | Inhibit APOL1-mediated ion channel activity | Oral administration; promising preclinical data | Limited long-term data; early clinical stage | [68,69,70] |
| Antisense Oligonucleotides | Reduce APOL1 mRNA expression | High specificity; targeted therapy | Requires frequent administration; high cost | [71] |
| Gene-Editing Technologies | Correct APOL1 genetic variants via CRISPR | Potential for permanent correction | Ethical concerns; early-stage research | [43,69] |
| JAK-STAT Pathway Inhibitors | Modulate inflammatory signaling pathways | Addresses downstream effects of APOL1 | Non-specific action on immune signaling | [38,39,55] |
| Lipid Metabolism Modulators | Restore lipid homeostasis disrupted by APOL1 | Broad metabolic benefits | Indirect action on APOL1 mechanisms | [35,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delrue, C.; Speeckaert, M.M. From Gene to Clinic: The Role of APOL1 in Focal Segmental Glomerulosclerosis. Sclerosis 2025, 3, 6. https://doi.org/10.3390/sclerosis3010006
Delrue C, Speeckaert MM. From Gene to Clinic: The Role of APOL1 in Focal Segmental Glomerulosclerosis. Sclerosis. 2025; 3(1):6. https://doi.org/10.3390/sclerosis3010006
Chicago/Turabian StyleDelrue, Charlotte, and Marijn M. Speeckaert. 2025. "From Gene to Clinic: The Role of APOL1 in Focal Segmental Glomerulosclerosis" Sclerosis 3, no. 1: 6. https://doi.org/10.3390/sclerosis3010006
APA StyleDelrue, C., & Speeckaert, M. M. (2025). From Gene to Clinic: The Role of APOL1 in Focal Segmental Glomerulosclerosis. Sclerosis, 3(1), 6. https://doi.org/10.3390/sclerosis3010006







