Simulation of Plasma Level Changes in Cerivastatin and Its Metabolites, Particularly Cerivastatin Lactone, Induced by Coadministration with CYP2C8 Inhibitor Gemfibrozil, CYP3A4 Inhibitor Itraconazole, or Both, Using the Metabolite-Linked Model
Abstract
1. Introduction
2. Theory
2.1. Magnitude of DDI: AUCR and Overall Inhibitory Activity
2.2. UGT–CYP2C8 Interplay Model
2.3. Mechanistic Expression for Ai,overall in the UGT–CYP2C8 Model
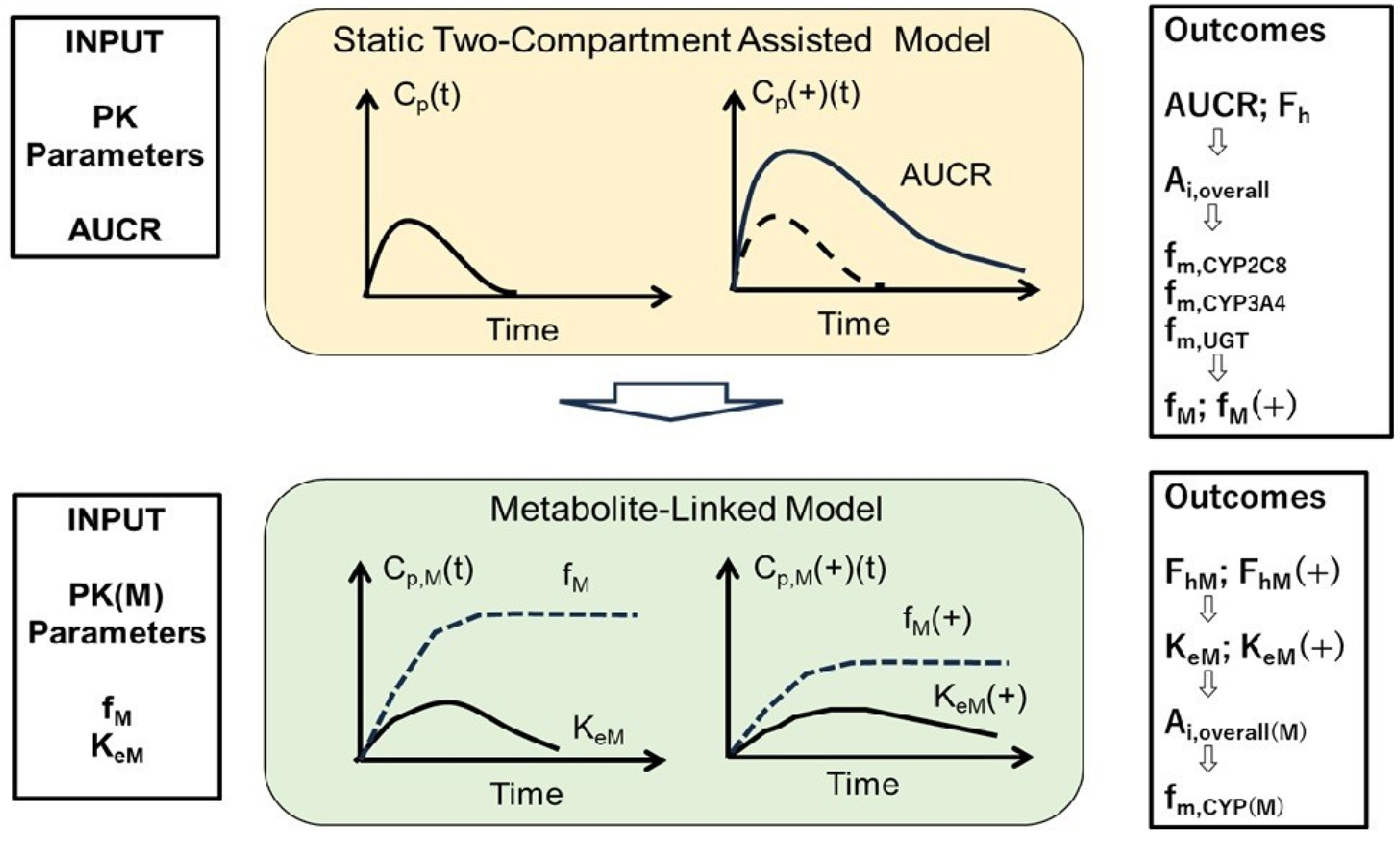
2.4. Static 2-Compartment Model for Parent Drug Simulation
2.5. Metabolite-Linked Model for Time-Dependent Metabolite Levels
3. Results
3.1. Simulated Cp(t) and Cp(t)(+) in Cer + Gem and Cer + Itr DDIs, PK Parameters for Cer, and Ai,overall
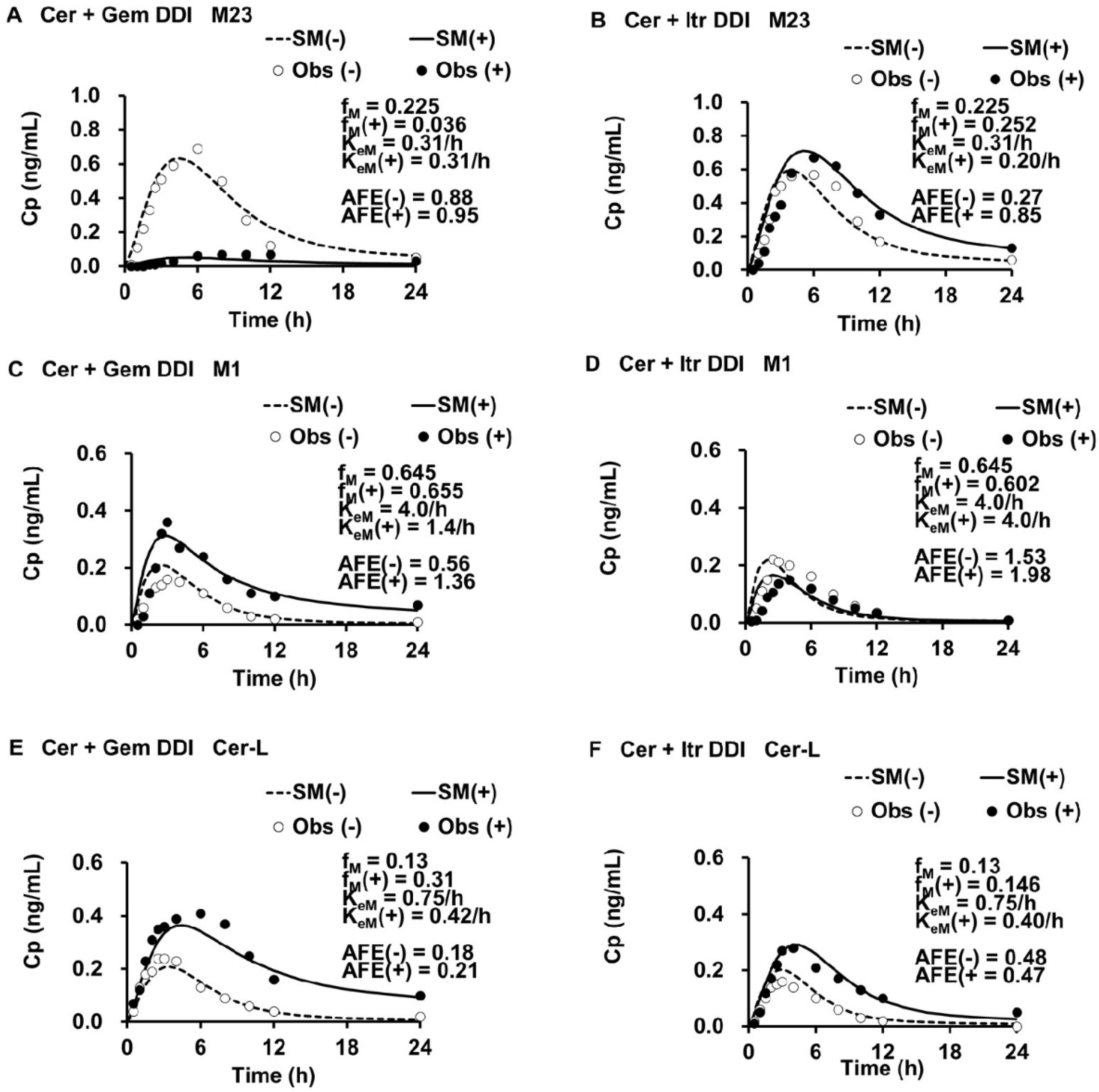
3.2. PK Parameters for M23, M1, and Cer-L
3.3. Simulated Metabolite Cp,M(t), Ratios of fM(+)/fM and KeM(+)/KeM, and Ai,overall(M) in DDIs
3.4. Enzyme Contributions to the Metabolism of M23, M1, and Cer-L
3.5. Sensitivity Analyses: fm,CYP3A4, r, and pAi,UGT(d)
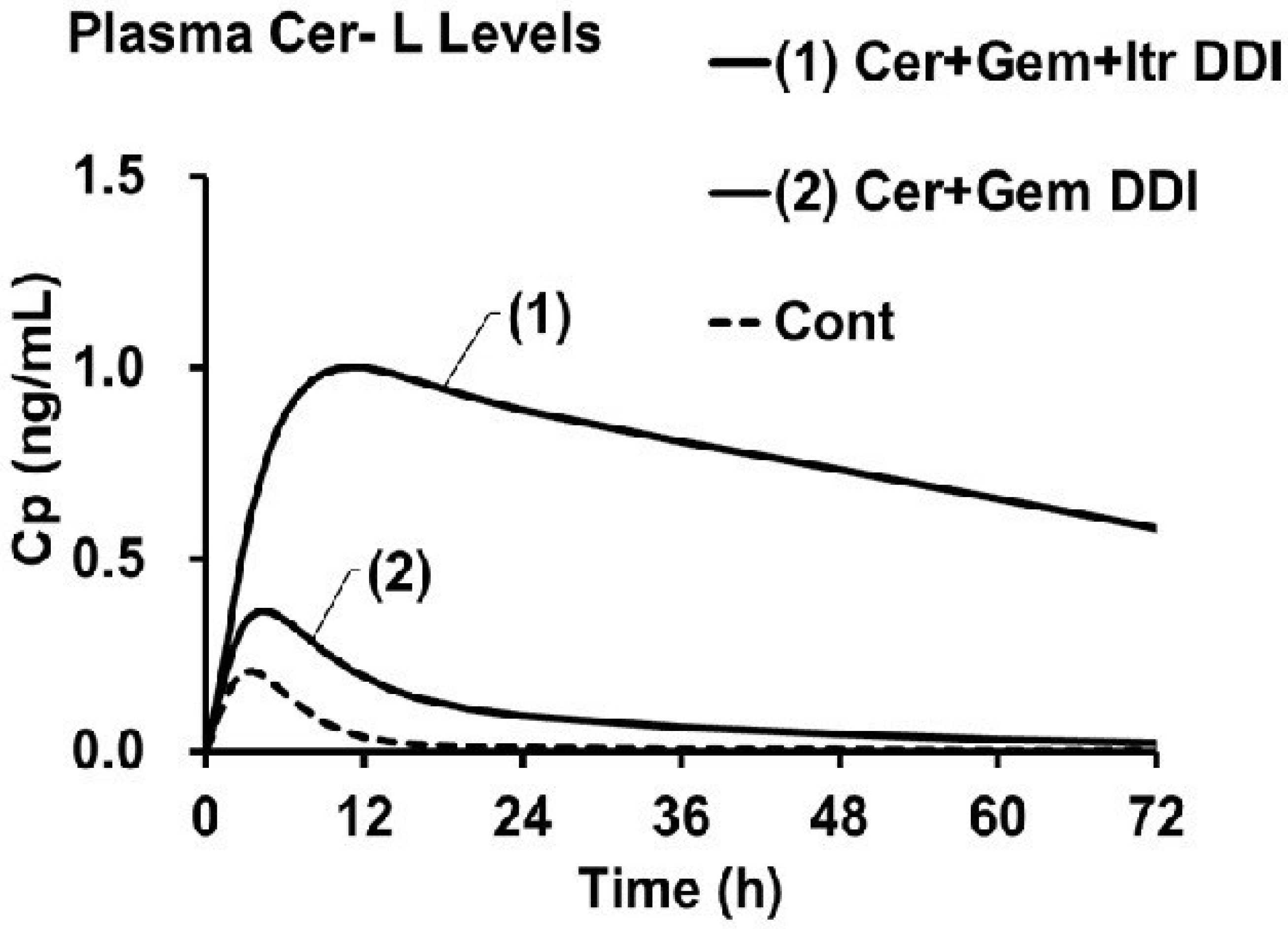
3.6. Prediction of fM(+)/fM and KeM(+)/KeM in the Cer + Gem + Itr DDI
3.7. Predicted AUCR(M) for Metabolites in the Cer + Gem + Itr DDI
4. Discussion
5. Methods
5.1. Data Acquisition for Cer DDIs with Gem and Itr
5.2. Sequential PK Modeling Framework for Parent Drug and Metabolites
5.3. PK Analysis
5.3.1. Modeling of the Parent Drug (Cer)
5.3.2. Modeling of Cer Metabolites
5.4. Software for PK Simulations
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | The area under the plasma drug level curve |
| AUCR | AUC ratio (Fold increase in AUC) |
| CYP | Cytochorme P450 |
| DDI | Drug–drug interaction |
| PK | Pharmacokinetics |
| UGT | UDP-glucuronosyltransferase |
References
- Iga, K. Use of three-compartment physiologically based pharmacokinetic modeling to predict hepatic blood levels of fluvoxamine relevant for drug-drug interactions. J. Pharm. Sci. 2015, 104, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Iga, K. Simulation of metabolic drug-drug interactions perpetrated by fluvoxamine using hybridized two-compartment hepatic drug-pool-based tube modeling and estimation of in vivo inhibition constants. J. Pharm. Sci. 2015, 104, 3565–3577. [Google Scholar] [CrossRef] [PubMed]
- Iga, K. Dynamic and static simulations of fluvoxamine-perpetrated drug-drug interactions using multiple cytochrome P450 inhibition modeling, and determination of perpetrator-specific CYP isoform inhibition constants and relative CYP isoform contributions to victim clearance. J. Pharm. Sci. 2016, 105, 1307–1317. [Google Scholar]
- Iga, K.; Kiriyama, A. Simulations of cytochrome P450 3A4-mediated drug-drug interactions by simple two-compartment model-assisted static method. J. Pharm. Sci. 2017, 106, 1426–1438. [Google Scholar] [CrossRef]
- Iga, K.; Kiriyama, A. Usefulness of two-compartment model-assisted and static overall inhibitory-activity method for prediction of drug-drug interaction. Biol. Pharm. Bull. 2017, 40, 2024–2037. [Google Scholar] [CrossRef][Green Version]
- Iga, K.; Kiriyama, A. Interplay of UDP-glucuronosyltransferase and CYP2C8 for CYP2C8 mediated drug oxidation and its impact on drug-drug interaction produced by standardized CYP2C8 inhibitors, clopidogrel and gemfibrozil. Clin. Pharmacokinet. 2024, 63, 43–56. [Google Scholar] [CrossRef]
- Friedman, M.A.; Woodcock, J.; Lumpkin, M.M.; Shuren, J.E.; Hass, A.E.; Thompson, L.J. The safety of newly approved medicines: Do recent market removals mean there is a problem? JAMA 1999, 281, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Smogorzewski, M. The myopathy of statins. J. Ren. Nutr. 2005, 15, 87–93. [Google Scholar] [CrossRef]
- Alfirevic, A.; Neely, D.; Armitage, J.; Chinoy, H.; Cooper, R.G.; Laaksonen, R.; Carr, D.F.; Bloch, K.M.; Fahy, J.; Hanson, A.; et al. Phenotype standardization for statin-induced myotoxicity. Clin. Pharmacol. Ther. 2014, 96, 470–476. [Google Scholar] [CrossRef]
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of pharmacokinetic, pharmacogenomic and muscle components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef]
- Backman, J.T.; Kyrklund, C.I.; Neuvonen, M.; Neuvonen, P.J. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin. Pharmacol. Ther. 2002, 72, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Farnier, M. Cerivastatin in the treatment of mixed hyperlipidemia: The RIGHT study. The cerivastatin study group. cerivastatin gemfibrozil hyperlipidemia treatment. Am. J. Cardiol. 1998, 82, 47J–51J. [Google Scholar] [CrossRef] [PubMed]
- Mück, W. Clinical pharmacokinetics of cerivastatin. Clin. Pharmacokinet. 2000, 39, 99–116. [Google Scholar]
- Plosker, G.L.; Dunn, C.I.; Figgitt, D.P. Cerivastatin: A review of its pharmacological properties and therapeutic efficacy in the management of hypercholesterolaemia. Drugs 2000, 60, 1179–1206. [Google Scholar] [CrossRef] [PubMed]
- Generaux, G.T.; Bonomo, F.M.; Johnson, M.; Doan, K.M.M. Impact of SLCO1B1 (OATP1B1) and ABCG2 (BCRP) genetic polymorphisms and inhibition on LDL-C lowering and myopathy of statins. Xenobiotica 2011, 41, 639–651. [Google Scholar] [CrossRef]
- Tornio, A.; Neuvonen, P.J.; Niemi, M.; Backman, J.T. Role of gemfibrozil as an inhibitor of CYP2C8 and membrane transporters. Expert. Opin. Drug. Metab. Toxicol. 2017, 13, 83–95. [Google Scholar] [CrossRef]
- Shitara, Y.; Hirano, M.; Sato, H.; Sugiyama, Y. Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: Analysis of the mechanism of the clinically relevant drug-drug interaction between cerivastatin and gemfibrozil. J. Pharmacol. Exp. Ther. 2004, 311, 228–236. [Google Scholar]
- Izumi, S.; Nozaki, Y.; Maeda, K.; Komori, T.; Takenaka, O.; Kusuhara, H.; Sugiyama, Y. Investigation of the impact of substrate selection on in vitro organic anion transporting polypeptide 1B1 inhibition profiles for the prediction of drug-drug interactions. Drug Metab. Dispos. 2015, 43, 235–247. [Google Scholar] [CrossRef]
- Tátrai, P.; Schweigler, P.; Poller, B.; Domange, N.; Wilde, R.D.; Hanna, I.; Gáborik, Z.; Huth, F. A systematic in vitro investigation of the inhibitor preincubation effect on multiple classes of clinically relevant transporters. Drug Metab. Dispos. 2019, 47, 768–778. [Google Scholar] [CrossRef]
- Kantola, T.; Kivistö, K.T.; Neuvonen, P.J. Effect of itraconazole on cerivastatin pharmacokinetics. Eur. J. Clin. Pharmacol. 1999, 54, 851–855. [Google Scholar] [CrossRef]
- Mück, W.; Ritter, W.; Ochmann, K.; Unger, S.; Ahr, G.; Wingender, W.; Kuhlmann, J. Absolute and relative bioavailability of the HMG-CoA reductase inhibitor cerivastatin. Int. J. Clin. Pharmacol. Ther. 1997, 35, 255–260. [Google Scholar]
- Boberg, M.; Angerbauer, R.; Fey, P.; Kanhai, W.K.; Karl, W.; Kern, A.; Ploschke, J.; Radtke, M. Metabolism of cerivastatin by human liver microsomes in vitro. Characterization of primary metabolic pathways and of cytochrome P450 isozymes involved. Drug Metab. Dispos. 1997, 25, 321–331. [Google Scholar] [PubMed]
- Prueksaritanont, T.; Subramanian, R.; Fang, X.; Ma, B.; Qiu, Y.; Lin, J.H.; Pearson, P.G.; Baillie, T.A. Glucuronidation of statins in animals and humans: A novel mechanism of statin lactonization. Drug Metab. Dispos. 2002, 30, 505–512. [Google Scholar] [CrossRef]
- Schirris, T.J.J.; Ritschel, T.; Bilos, A.; Smeitink, J.A.M.; Russel, F.G.M. Statin lactonization by uridine 5′-diphospho-glucuronosyltransferases (UGTs). Mol. Pharm. 2015, 12, 4048–4055. [Google Scholar] [CrossRef]
- Taha, D.A.; Moor, C.H.D.; Barrett, D.A.; Lee, J.B.; Gandhi, R.D.; Hoo, C.W.; Gershkovich, P. The role of acid-base imbalance in statin-induced myotoxicity. Transl. Res. 2016, 174, 140–160. [Google Scholar] [CrossRef] [PubMed]
- Kearney, A.S.; Crawford, L.F.; Mehta, S.C.; Radebaugh, G.W. The interconversion kinetics, equilibrium, and solubilities of the lactone and hydroxyacid forms of the HMG-CoA reductase inhibitor, CI-981. Pharm. Res. 1993, 10, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, O.; Tawfik, D.S. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005, 44, 6371–6382. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.P. Paraoxonase-1 as a regulator of glucose and lipid homeostasis: Impact on the onset and progression of metabolic disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef]
- Skottheim, I.B.; Dahl, A.G.; Hejazifar, S.; Hoel, K.; Asberg, A. Statin induced myotoxicity: The lactone forms are more potent than the acid forms in human skeletal muscle cells in vitro. Eur. J. Pharm. Sci. 2008, 33, 317–325. [Google Scholar] [CrossRef]
- Karonen, T.; Neuvonen, P.J.; Backman, J.T. CYP2C8 but not CYP3A4 is important in the pharmacokinetics of montelukast. Br. J. Clin. Pharmacol. 2012, 73, 257–267. [Google Scholar] [CrossRef]
- Niemi, M.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics and pharmacodynamics of repaglinide: Potentially hazardous interaction between gemfibrozil and repaglinide. Clin. Trial Diabetol. 2003, 46, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, T.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics of pioglitazone. Clin. Pharmacol. Ther. 2005, 77, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P. Drug Distribution to Human Tissues: Prediction and Examination of the Basic Assumption in In Vivo Pharmacokinetics-Pharmacodynamics (PK/PD) Research. J. Pharm. Sci. 2015, 104, 2110–2118. [Google Scholar] [CrossRef] [PubMed]


| Cer + Gem DDI | Cer + Itr DDI | |||
|---|---|---|---|---|
| (−) | (+) | (−) | (+) | |
| CLoral (1/h) | 15.0 | 3.0 | 13.0 | 11.5 |
| CLtotal (1/h) | 9.24 | 2.04 | 7.32 | 6.55 |
| Fa×Fg | 0.7 | 0.7 | 0.62 | 0.62 |
| Ka (1/h) | 0.4 | 1.0 | 0.7 | 0.5 |
| Fh | 0.88 | 0.97 | 0.90 | 0.91 |
| F | 0.61 | 0.68 | 0.56 | 0.56 |
| V0 (L) | 20 | 20 | 20 | 20 |
| Vdss (L) | 45 | 45 | 45 | 45 |
| Kd (1/h) | 0.15 | 0.15 | 0.15 | 0.15 |
| AUCR | 1 | 5.00 | 1 | 1.13 |
| Ai,overall | 1 | 4.76 | 1 | 1.12 |
| fm,CYP2C8 | 0.75 | 0.75 | ||
| fm,UGT | 0.13 | 0.13 | ||
| fm,CYP3A4 | 0.12 | 0.12 | ||
| pAi,CYP2C8 | 1 | 16 | 1 | 1 |
| pAi,UGT(d) | 1 | 2 | 1 | 1 |
| pAi,CYP3A4 | 1 | 1 | 1 | 10 |
| M23 | M1 | Cer-L | |
|---|---|---|---|
| fM | 0.225 [=0.3 a× fm,CYP2C8] | 0.654 [=fm,CYP3A4 + 0.7 a× fm,CYP2C8] | 0.13 [=fm,UGT] |
| KeM (1/h) | 0.31 | 4.0 | 0.75 |
| V0M (L) | 20 | 20 | 20 |
| VdssM (L) | 45 | 45 | 45 |
| KdM (1/h) | 0.15 | 0.15 | 0.15 |
| CLtotM [=KeM × V0M] (L/h) | 6.1 | 80 | 15 |
| FhM | 0.93 | 0 | 0.80 |
| Metabolite | Cer + Gem DDI | Cer + Itr DDI | |
|---|---|---|---|
| M23 | fM(+)/fM | 0.16 | 1.12 |
| KeM(+)/KeM | 1.00 | 0.64 | |
| FhM(+) Ai,overall(M) | 0.93 1.00 | 0.95 1.58 | |
| M1 | fM(+)/fM | 1.01 | 0.93 |
| KeM(+)/KeM | 0.35 | 1.00 | |
| FhM(+) Ai,overall(M) | 0.35 32 | 0 a 1.00 | |
| Cer-L | fM(+)/fM | 2.38 | 1.12 |
| KeM(+)/KeM | 0.56 | 0.53 | |
| FhM(+) Ai,overall(M) | 0.89 1.97 | 0.89 2.11 |
| Enzyme | M23 | M1 | Cer-L |
|---|---|---|---|
| fm,CYP3A4(M) | 0.33 | 0 | 0.58 (from 0.5 to 0.6) |
| fm,CYP2C8(M) | 0 | 1 | 0.50 (from 0.5 to 0.4) |
| fm,UGT(G2)(M) | 0 | 0 | 0 |
| fm,UGT(G1)(M) | 0.67 | 0 | 0 |
| Products | M24 (by CYP3A4) | M24 [by CYP2C8] | M1-L (by CYP3A4) |
| M23-G1 [by UGT(G1)] | M23-L (by CYP2C8) |
| Metabolite | Cer + Gem + Itr DDI | |
|---|---|---|
| M23 | fM(+)/fM | 0.312 |
| KeM(+)/KeM | 0.64 | |
| Fh(M)(+) | 0.95 | |
| Ai,overall(M) | 1.58 | |
| M1 | fM(+)/fM | 0.44 |
| KeM(+)/KeM | 0.35 | |
| Fh(M)(+) | 0.35 | |
| Ai,overall(M) | 32 | |
| Cer-L | fM(+)/fM | 5 |
| KeM(+)/KeM | 0.072~0.080 | |
| Fh(M)(+) | 0.98 | |
| Ai,overall(M) | 13.8~15.2 |
| Cer + Gem DDI | Cer + Itr DDI | Cer + Gem + Itr DDI | |||
|---|---|---|---|---|---|
| Simulated | Observed | Simulated | Observed | Predicted | |
| AUCR(Cer) | 5.0 | 5.0 | 1.1 | 1.1 | 10 |
| AUCR(M23) | 0.16 | 0.17 | 1.7 | 1.3 | 0.48 |
| AUCR(M1) | 2.9 | 4.4 | 0.93 | 0.76 | 1.2 |
| AUCR(Cer-L) | 4.2 | 4.4 | 2.1 | 2.6 | 62~69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iga, K. Simulation of Plasma Level Changes in Cerivastatin and Its Metabolites, Particularly Cerivastatin Lactone, Induced by Coadministration with CYP2C8 Inhibitor Gemfibrozil, CYP3A4 Inhibitor Itraconazole, or Both, Using the Metabolite-Linked Model. Drugs Drug Candidates 2025, 4, 34. https://doi.org/10.3390/ddc4030034
Iga K. Simulation of Plasma Level Changes in Cerivastatin and Its Metabolites, Particularly Cerivastatin Lactone, Induced by Coadministration with CYP2C8 Inhibitor Gemfibrozil, CYP3A4 Inhibitor Itraconazole, or Both, Using the Metabolite-Linked Model. Drugs and Drug Candidates. 2025; 4(3):34. https://doi.org/10.3390/ddc4030034
Chicago/Turabian StyleIga, Katsumi. 2025. "Simulation of Plasma Level Changes in Cerivastatin and Its Metabolites, Particularly Cerivastatin Lactone, Induced by Coadministration with CYP2C8 Inhibitor Gemfibrozil, CYP3A4 Inhibitor Itraconazole, or Both, Using the Metabolite-Linked Model" Drugs and Drug Candidates 4, no. 3: 34. https://doi.org/10.3390/ddc4030034
APA StyleIga, K. (2025). Simulation of Plasma Level Changes in Cerivastatin and Its Metabolites, Particularly Cerivastatin Lactone, Induced by Coadministration with CYP2C8 Inhibitor Gemfibrozil, CYP3A4 Inhibitor Itraconazole, or Both, Using the Metabolite-Linked Model. Drugs and Drug Candidates, 4(3), 34. https://doi.org/10.3390/ddc4030034








