Suzetrigine: A Novel Non-Opioid Analgesic for Acute Pain Management—A Review
Abstract
1. Introduction
2. Current Non-Opioid Practices for Acute Pain
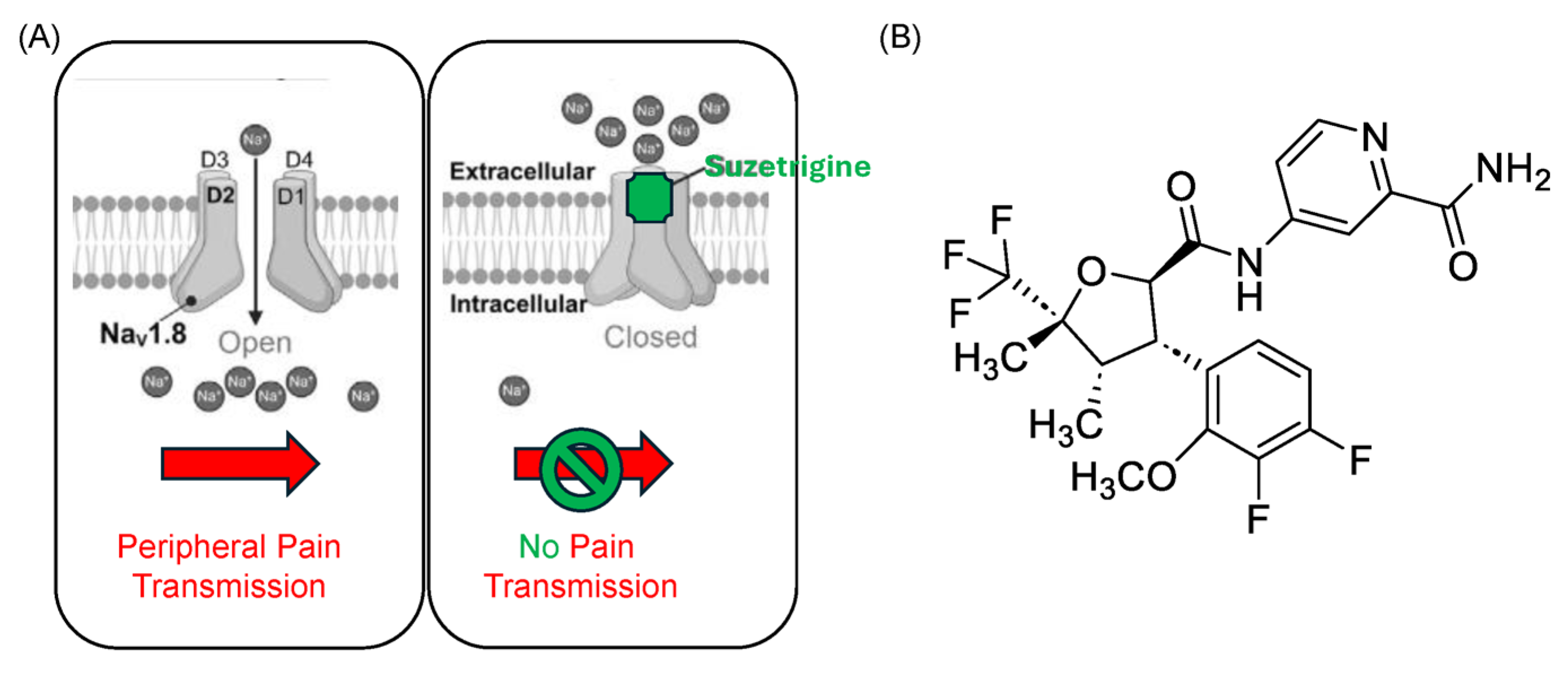
3. The Chemistry of Suzetrigine
4. Mechanism of Action of Suzetrigine: Targeting Peripheral Nociception
5. Pharmacokinetics of Suzetrigine
6. Clinical Efficacy: Evidence from Pivotal Trials
7. Safety and Tolerability
8. Clinical Applications
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Drug Overdose Deaths in the United States, 2001–2021. NCHS Data Brief No. 457. 2022. Available online: https://www.cdc.gov/nchs/products/databriefs/db457.htm#:~:text=Among%20the%20total%20population%2C%20the,%25%2C%20from%2028.3%20to%2032.4 (accessed on 14 April 2025).
- Centers for Disease Control and Prevention. U.S. Overdose Deaths Decrease Almost 27% in 2024. Available online: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2025/20250514.htm?utm_source=chatgpt.com (accessed on 19 May 2025).
- U.S. Food and Drug Administration. Celecoxib (Celebrex) Approval Letter. NDA 20-998. 31 December 1998. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20998AP_appltr.pdf (accessed on 14 April 2025).
- U.S. Food and Drug Administration. Suzetrigine (JOURNAVX) Prescribing Information. NDA 219209. 15 March 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/219209s000lbl.pdf (accessed on 14 April 2025).
- Schwenk, E.S. Nonopioid Pharmacotherapy for Acute Pain in Adults. UpTodate. March 2025. Available online: https://www.uptodate.com/contents/nonopioid-pharmacotherapy-for-acute-pain-in-adults (accessed on 14 April 2025).
- Hyland, S.J.; Wetshtein, A.M.; Grable, S.J.; Jackson, M.P. Acute Pain Management Pearls: A Focused Review for the Hospital Clinician. Healthcare 2022, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Kushner, P.; McCarberg, B.H.; Wright, W.L.; Aldoori, W.; Gao, P.; Iqbal, A.; Petruschke, R. Ibuprofen/acetaminophen fixed-dose combination as an alternative to opioids in management of common pain types. Postgrad Med. 2024, 136, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Sopata, M.; Dragon, E.; Brown, M.T.; Viktrup, L.; West, C.R.; Bao, W.; Agyemang, A. A Randomized Placebo-Controlled Trial of the Anti-Nerve Growth Factor Antibody Tanezumab in Subjects with Cancer Pain Due to Bone Metastasis. Oncologist 2023, 28, e1268–e1278. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C. Process for the Synthesis of Substituted Tetrahydrofuran Modulators of Sodium Channels. WO2022256660A1, 8 December 2022. [Google Scholar]
- Zhu, F.; Qin, H.; Shandong Fuchang Pharmaceutical Co., Ltd. Preparation Method of Suzetigine. CN118772124A, 15 October 2024. [Google Scholar]
- Osteen, J.D.; Herzig, V.; Gilchrist, J.; Emrick, J.J.; Zhang, C.; Wang, X.; Castro, J.; Garcia-Caraballo, S.; Grundy, L.; Rychkov, G.Y.; et al. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 2016, 534, 494–499. [Google Scholar] [CrossRef]
- Osteen, J.D.; Immani, S.; Tapley, T.L.; Indersmitten, T.; Hurst, N.W.; Healey, T.; Aertgeerts, K.; Negulescu, P.A.; Lechner, S.M. Pharmacology and Mechanism of Action of Suzetrigine, a Potent and Selective NaV1.8 Pain Signal Inhibitor for the Treatment of Moderate to Severe Pain. Pain Ther. 2025, 14, 655–674. [Google Scholar] [CrossRef]
- Kingwell, K. NaV1.8 inhibitor poised to provide opioid-free pain relief. Nat. Rev. Drug Discov. 2025, 24, 3–5. [Google Scholar] [CrossRef]
- McCormack, K.; Santos, S.; Chapman, M.L.; Krafte, D.S.; Marron, B.E.; West, C.W.; Krambis, M.J.; Antonio, B.M.; Zellmer, S.G.; Printzenhoff, D.; et al. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proc. Natl. Acad. Sci. USA 2013, 110, E2724–E2732. [Google Scholar] [CrossRef]
- Priest, B.T.; Murphy, B.A.; Lindia, J.A.; Diaz, C.; Abbadie, C.; Ritter, A.M.; Liberator, P.; Iyer, L.M.; Kash, S.F.; Kohler, M.G.; et al. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior. Proc. Natl. Acad. Sci. USA 2005, 102, 9382–9387. [Google Scholar] [CrossRef]
- Jo, S.; Fujita, A.; Osorno, T.; Stewart, R.G.; Vaelli, P.M.; Bean, B.P. Differential state-dependent Nav1.8 inhibition by suzetrigine, LTGO-33, and A-887826. J. Gen. Physiol. 2025, 157, e202413719. [Google Scholar] [CrossRef]
- Vaelli, P.; Fujita, A.; Jo, S.; Zhang, H.B.; Osorno, T.; Ma, X.; Bean, B.P. State-Dependent Inhibition of Nav1.8 Sodium Channels by VX-150 and VX-548. Mol. Pharmacol. 2024, 106, 298–308. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Zang, J.; Jin, X.; Yan, N. Drug discovery targeting Nav1.8: Structural insights and therapeutic potential. Curr. Opin. Chem. Biol. 2024, 83, 102538. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhou, X. Gender difference in the pharmacokinetics and metabolism of VX-548 in rats. Biopharm. Drug Dispos. 2024, 45, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Shanu-Wilson, J. CYP3A-Mediated Metabolism of Suzetrigine. Hypha Discovery. Available online: https://www.hyphadiscovery.com/blog/cyp3a-mediated-metabolism-of-suzetrigine/ (accessed on 14 April 2025).
- McCoun, J.; Winkle, P.; Solanki, D.; Urban, J.; Bertoch, T.; Oswald, J.; Swisher, M.W.; Taber, L.A.; Healey, T.; Jazic, I.; et al. Suzetrigine, a Non-Opioid NaV1.8 Inhibitor with Broad Applicability for Moderate-to-Severe Acute Pain: A Phase 3 Single-Arm Study for Surgical or Non-Surgical Acute Pain. J. Pain Res. 2025, 18, 1569–1576. [Google Scholar] [CrossRef]
- Bertoch, T.; D’Aunno, D.; McCoun, J.; Solanki, D.; Taber, L.; Urban, J.; Oswald, J.; Swisher, M.W.; Tian, S.; Miao, X.; et al. Suzetrigine, a Non-Opioid NaV1.8 Inhibitor for Treatment of Moderate-to-Severe Acute Pain: Two Phase 3 Randomized Clinical Trials. Anesthesiology 2025, 142, 1085. [Google Scholar] [CrossRef]
- Hu, S.; Lyu, D.; Gao, J. Suzetrigine: The first Nav1.8 inhibitor approved for the treatment of moderate to severe acute pain. Drug Discov. Ther. 2025, 19, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Correll, D.J.; Lechner, S.M.; Jazic, I.; Miao, X.; Shaw, D.; Simard, C.; Osteen, J.D.; Hare, B.; Beaton, A.; et al. Selective Inhibition of NaV1.8 with VX-548 for Acute Pain. N. Engl. J. Med. 2023, 389, 393–405. [Google Scholar] [CrossRef]
- A2074. A Phase 3, Single-Arm Study of Suzetrigine, a Non-Opioid, Pain Signal Inhibitor for Treatment of Acute Pain from Surgical and Non-Surgical Conditions. The Anesthesiology Annual Meeting. Available online: https://www.abstractsonline.com/pp8/#!/20183/presentation/6221 (accessed on 14 April 2025).
- Karri, J.; D’Souza, R.S.; Cohen, S.P. Between promise and peril: Role of suzetrigine as a non-opioid analgesic. BMJ Med. 2025, 4, e001431. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Suzetrigine Clinical Review. NDA 219209; March 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2025/219209Orig1s000ltr.pdf (accessed on 14 April 2025).
- Suzetrigine. Am. J. Health Syst. Pharm. 2025, 82, 635–637. [CrossRef]
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012, 116, 248–273. [Google Scholar] [CrossRef]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Suzetrigine [Package Insert]; Vertex Pharmaceuticals Incorporated: Cambridge, MA, USA, 2025. Available online: https://www.journavx.com/?utm_campaign=b12d_cv23_12d&utm_source=gg41&utm_medium=cp45&utm_term=vertex%20suzetrigine&gad_source=1&gclid=CjwKCAjw5PK_BhBBEiwAL7GTPWA_wbFkRPNNthNxMdNiiZCNSUYFhr6gR-DiZ-nmbA34ndcl_iv4BRoCxxAQAvD_BwE&gclsrc=aw.ds (accessed on 14 April 2025).
- Chou, R.; Wagner, J.; Ahmed, A.Y.; Blazina, I.; Brodt, E.; Buckley, D.I.; Cheney, T.P.; Choo, E.; Dana, T.; Gordon, D.; et al. Treatments for Acute Pain: A Systematic Review [Internet]; Comparative Effectiveness Review, No. 240; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK566506/ (accessed on 12 April 2025).
- ClinicalTrials.gov. Study of Suzetrigine in Painful Diabetic Peripheral Neuropathy (NCT04991178). Available online: https://clinicaltrials.gov/study/NCT05660538 (accessed on 14 April 2025).
- Institute for Clinical and Economic Review. Suzetrigine for Acute Pain: Effectiveness and Value. Final Evidence Report. March 2025. Available online: https://icer.org/wp-content/uploads/2025/03/ICER_Acute-Pain_Final-Report_For-Publication_033125.pdf (accessed on 14 April 2025).
- Bhattacharya, A.; Wickenden, A.D.; Chaplan, S.R. Sodium channel blockers for the treatment of neuropathic pain. Neurotherapeutics 2009, 6, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Vertex Pharmaceuticals. VX-993 Investigator’s Brochure. 2024. Available online: https://investors.vrtx.com/news-releases/news-release-details/vertex-provides-pipeline-and-business-updates-advance-upcoming (accessed on 14 April 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, M.; Demery, A.; Al-Horani, R.A. Suzetrigine: A Novel Non-Opioid Analgesic for Acute Pain Management—A Review. Drugs Drug Candidates 2025, 4, 32. https://doi.org/10.3390/ddc4030032
Jones M, Demery A, Al-Horani RA. Suzetrigine: A Novel Non-Opioid Analgesic for Acute Pain Management—A Review. Drugs and Drug Candidates. 2025; 4(3):32. https://doi.org/10.3390/ddc4030032
Chicago/Turabian StyleJones, Meaghan, Aryanna Demery, and Rami A. Al-Horani. 2025. "Suzetrigine: A Novel Non-Opioid Analgesic for Acute Pain Management—A Review" Drugs and Drug Candidates 4, no. 3: 32. https://doi.org/10.3390/ddc4030032
APA StyleJones, M., Demery, A., & Al-Horani, R. A. (2025). Suzetrigine: A Novel Non-Opioid Analgesic for Acute Pain Management—A Review. Drugs and Drug Candidates, 4(3), 32. https://doi.org/10.3390/ddc4030032







