A Multifaceted Exploration of Shirakiopsis indica (Willd) Fruit: Insights into the Neuropharmacological, Antipyretic, Thrombolytic, and Anthelmintic Attributes of a Mangrove Species
Abstract
1. Introduction
2. Results
2.1. Acute Toxicity Study
2.2. Neuropharmacological Activity
2.2.1. Anxiolytic Activity
Elevated Plus Maze Test
Hole Board Test
Light–Dark Box Test
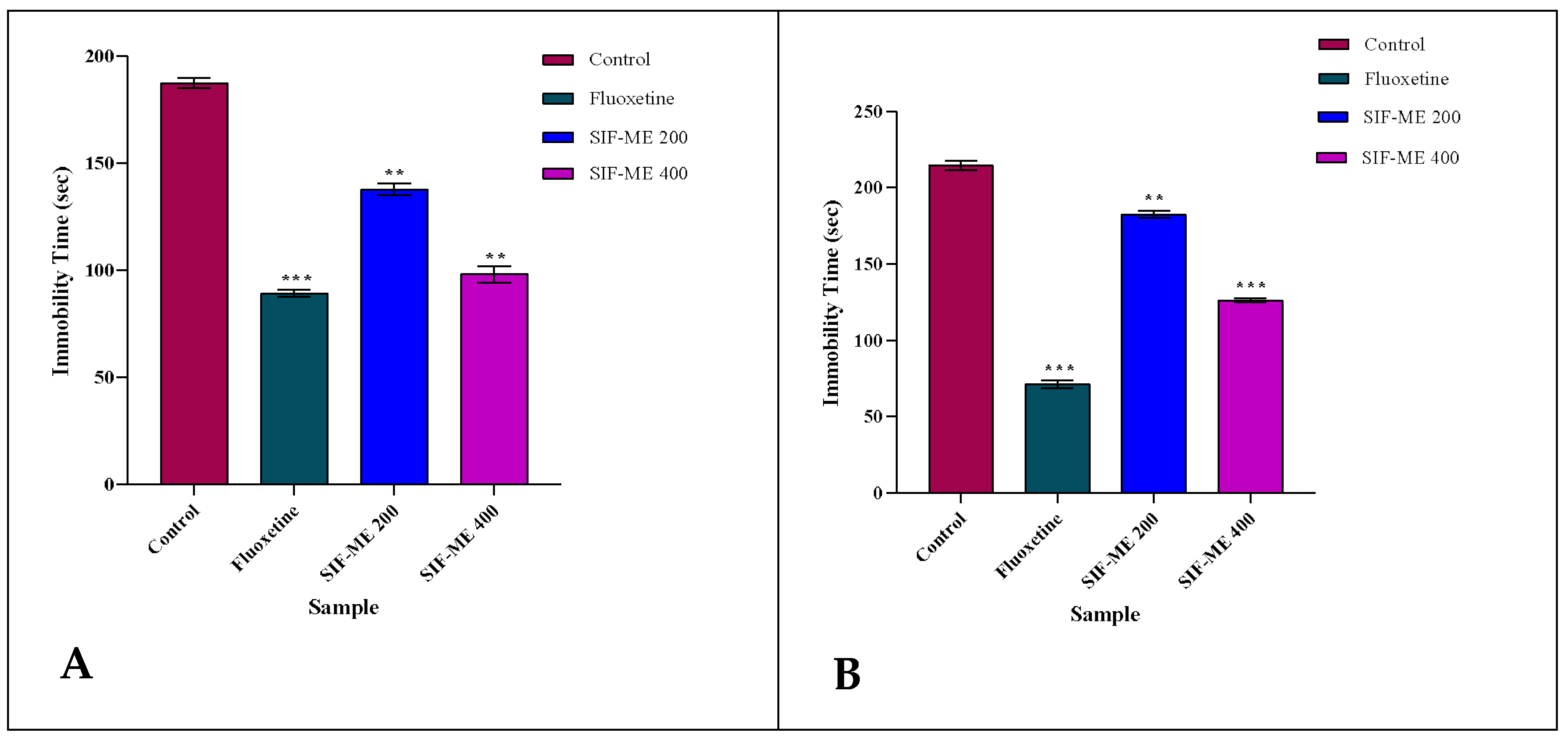
2.2.2. Antidepressant Activity
Forced Swimming Test
Tail Suspension Test
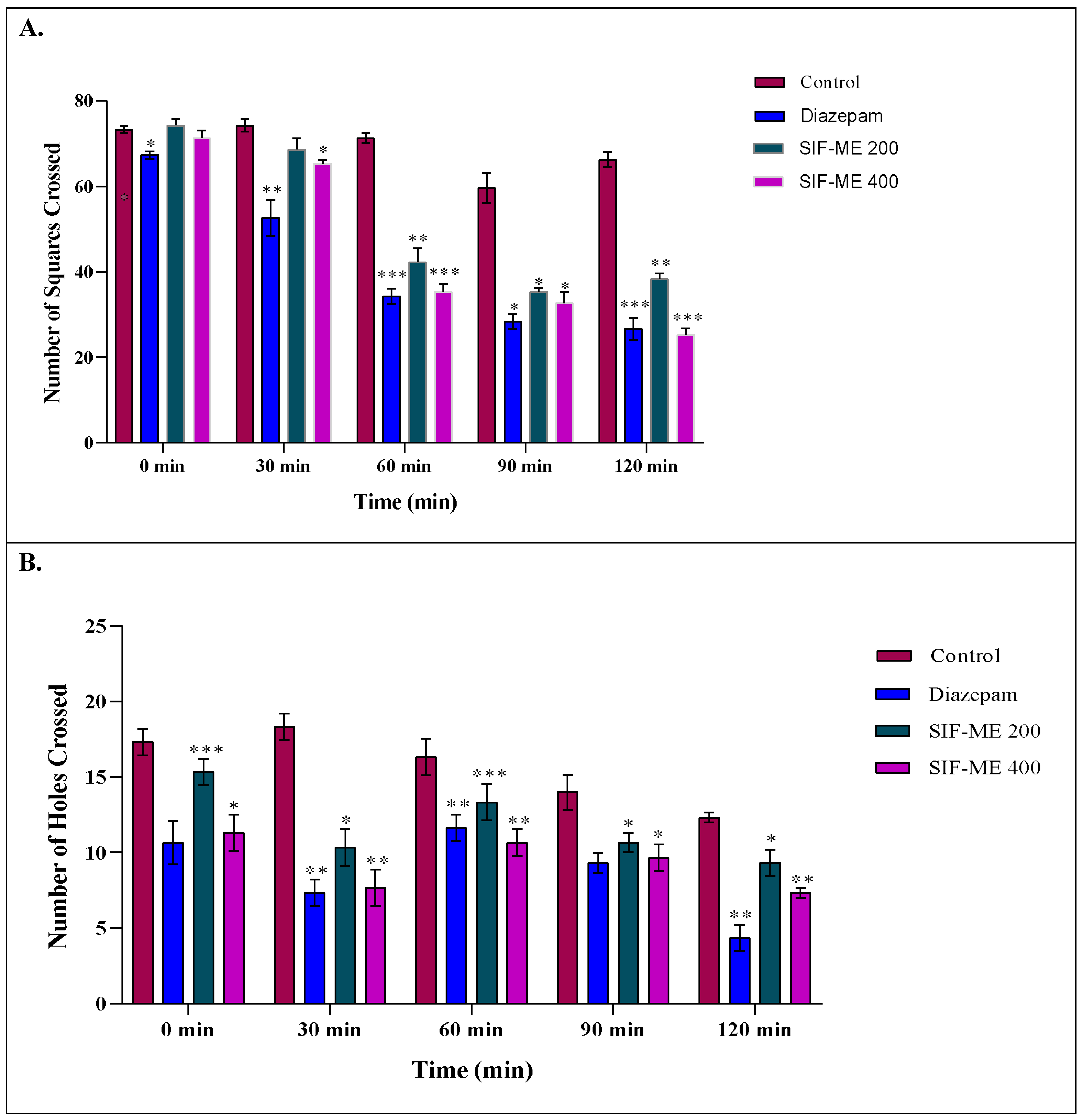
2.2.3. Locomotor Activity
Open Field Test
Hole Cross Test
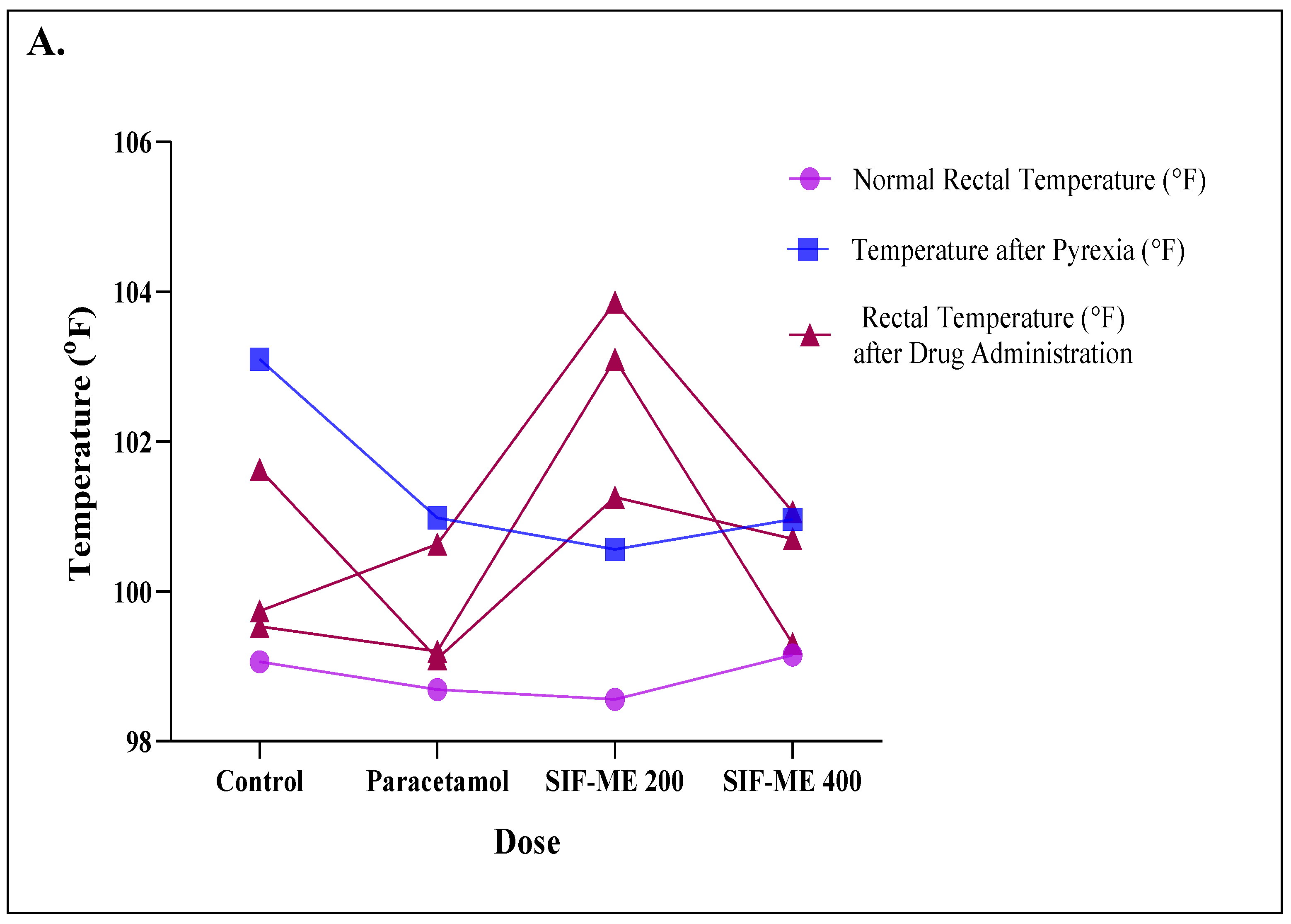
2.3. Antipyretic Activity
Yeast-Induced Pyrexia Method
2.4. Thrombolytic Activity
Human Blood Clot Lysis Method
2.5. Anthelmintic Activity
In Vitro Anthelmintic Test on Tubifex tubifex
2.6. PASS Prediction Study
2.7. In Silico Study
2.7.1. ADME/T Study
2.7.2. Molecular Docking Study
3. Discussion
4. Phytochemistry Reported from Previous Works
5. Materials and Methods
5.1. Plant Collection and Identification
5.2. Extraction Methods
5.3. Chemicals and Reagents
5.4. Experimental Animals and Ethics Statement
5.5. Experimental Design
5.6. Neuropharmacological Activity
5.6.1. Anxiolytic Activity
Elevated Plus Maze Method
Hole Board Test (HBT)
Light–Dark Box Test (LDT)
5.6.2. Antidepressant Activity
Forced Swimming Test (FST)
Tail Suspension Test (TST)
5.6.3. Locomotor Activity
Open Field Method
Hole Cross Method
5.7. Study of Antipyretic Activity
Yeast-Induced Pyrexia in Mice
5.8. Thrombolytic Activity
5.8.1. Blood Clot Lysis Method
Streptokinase (SK) Solution Preparation
5.8.2. Specimen for the Thrombolytic Test
5.9. Anthelmintic Activity
5.10. In Silico Molecular Docking Study
5.10.1. Toxicity Prediction by AdmetSAR
5.10.2. Prediction of Activity Spectra for Substances (PASS)
5.10.3. Software Tools
5.10.4. Selection of Ligands
5.10.5. Validation of the Ligands as Potential Therapeutic Agents
5.10.6. Protein Preparation and Active Site Determination
5.10.7. Validation of the Proteins as Potential Therapeutic Targets
5.10.8. Molecular Docking and Post-Docking Analysis
5.11. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fajemiroye, J.O.; da Silva, D.M.; de Oliveira, D.R.; Costa, E.A. Treatment of Anxiety and Depression: Medicinal Plants in Retrospect. Fundam. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef]
- Michael, T.; Zetsche, U.; Margraf, J. Epidemiology of Anxiety Disorders. Psychiatry 2007, 6, 136–142. [Google Scholar] [CrossRef]
- Klein, D.N.; Goldstein, B.L.; Finsaas, M. Depressive Disorders. In Child and Adolescent Psychopathology, 3rd ed.Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 610–641. [Google Scholar]
- Thibaut, F. Anxiety Disorders: A Review of Current Literature. Dialogues Clin. Neurosci. 2017, 19, 87–88. [Google Scholar] [CrossRef]
- Kenda, M.; Kočevar Glavač, N.; Nagy, M.; Sollner Dolenc, M. Medicinal Plants Used for Anxiety, Depression, or Stress Treatment: An Update. Molecules 2022, 27, 6021. [Google Scholar] [CrossRef]
- Bandelow, B.; Sher, L.; Bunevicius, R.; Hollander, E.; Kasper, S.; Zohar, J.; Möller, H.-J.; WFSBP Task Force on Mental Disorders in Primary Care; WFSBP Task Force on Anxiety Disorders, OCD and PTSD. Guidelines for the Pharmacological Treatment of Anxiety Disorders, Obsessive–Compulsive Disorder and Posttraumatic Stress Disorder in Primary Care. Int. J. Psychiatry Clin. Pract. 2012, 16, 77–84. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Papakostas, G.I. Experimental Medication Treatment Approaches for Depression. Transl. Psychiatry 2017, 7, e1068. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications; Cambridge University Press: Singapore, 2021; ISBN 110883857X. [Google Scholar]
- Emon, N.U.; Alam, S.; Rudra, S.; Riya, S.R.; Paul, A.; Hossen, S.M.M.; Kulsum, U.; Ganguly, A. Antidepressant, Anxiolytic, Antipyretic, and Thrombolytic Profiling of Methanol Extract of the Aerial Part of Piper Nigrum: In Vivo, in Vitro, and in Silico Approaches. Food Sci. Nutr. 2021, 9, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gutiérrez, S.L.; Balderas, J.; Aguilar, A.; Navarrete, A. Sedative Activity of Some Plants Used in Mexico to Treat Insomnia. Rev. Latinoam. Quím 2009, 37, 243–251. [Google Scholar]
- Bum, E.N.; Taiwe, G.S.; Nkainsa, L.A.; Moto, F.C.O.; Etet, P.F.S.; Hiana, I.R.; Bailabar, T.; Seyni, P.; Rakotonirina, A.; Rakotonirina, S.V. Validation of Anticonvulsant and Sedative Activity of Six Medicinal Plants. Epilepsy Behav. 2009, 14, 454–458. [Google Scholar] [CrossRef]
- Muhammad, A.; Khan, B.; Iqbal, Z.; Khan, A.Z.; Khan, I.; Khan, K.; Alamzeb, M.; Ahmad, N.; Khan, K.; Lal Badshah, S. Viscosine as a Potent and Safe Antipyretic Agent Evaluated by Yeast-Induced Pyrexia Model and Molecular Docking Studies. ACS Omega 2019, 4, 14188–14192. [Google Scholar] [CrossRef]
- Rashed-Al-Qayum, R.-A.-Q.; Khan, M.D.; Moghal, M.M.R.; Amin, M.N.; Hossain, M.S.; Hossain, M.D. Analgesic and Antipyretic Activities of Two Medicinal Plants-Salvinia Minima and Dactyloctenium Australe in Experimental Animal Models. Der Pharm. Sin. 2013, 4, 183–187. [Google Scholar]
- Hossain, M.M.; Ahamed, S.K.; Dewan, S.M.R.; Hassan, M.M.; Istiaq, A.; Islam, M.S.; Moghal, M.M.R. In Vivo Antipyretic, Antiemetic, in Vitro Membrane Stabilization, Antimicrobial, and Cytotoxic Activities of Different Extracts from Spilanthes Paniculata Leaves. Biol. Res. 2014, 47, 1–9. [Google Scholar] [CrossRef]
- Emon, N.U.; Kaiser, M.; Islam, M.; Kabir, M.F.I.; Jamir, M.; Uddin, M.A.J.; Islam, M.N. Anxiolytic and Thrombolytic Investigation of Methanol Extract of Piper Nigrum L. Fruits and Sesamum Indicum L. Seeds. J. Adv. Biotechnol. Exp. Ther. 2020, 3, 158–164. [Google Scholar] [CrossRef]
- Emon, N.U.; Jahan, I.; Sayeed, M.A. Investigation of Antinociceptive, Anti-Inflammatory and Thrombolytic Activity of Caesalpinia Digyna (Rottl.) Leaves by Experimental and Computational Approaches. Adv. Tradit. Med. 2020, 20, 451–459. [Google Scholar] [CrossRef]
- Zishan, S.A.; Uddin, M.M.; Mohammad, M.; Uddin, M.E.; Azad, S.M.; Naima, J.; Ibban, S.S. In Vivo and In Vitro Initiatives for the Examination of Pharmacological Properties of Brassaiopsis Hainla Leaves. Asian J. Res. Bot. 2023, 9, 15–28. [Google Scholar]
- MacDonald, A.S.; Araujo, M.I.; Pearce, E.J. Immunology of Parasitic Helminth Infections. Infect. Immun. 2002, 70, 427–433. [Google Scholar] [CrossRef]
- Spiegler, V.; Liebau, E.; Hensel, A. Medicinal Plant Extracts and Plant-Derived Polyphenols with Anthelmintic Activity against Intestinal Nematodes. Nat. Prod. Rep. 2017, 34, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Sunita, K.; Kumar, P.; Khan, M.A.; Husain, S.A.; Singh, D.K. Anthelminthic/Larvicidal Activity of Some Common Medicinal Plants. Eur. J. Biol. Res. 2017, 7, 324–336. [Google Scholar]
- Hossen, S.M.M.; Islam, M.J.; Hossain, M.R.; Barua, A.; Uddin, M.G.; Emon, N.U. CNS Anti-Depressant, Anxiolytic and Analgesic Effects of Ganoderma Applanatum (Mushroom) along with Ligand-Receptor Binding Screening Provide New Insights: Multi-Disciplinary Approaches. Biochem. Biophys. Rep. 2021, 27, 101062. [Google Scholar] [CrossRef]
- Tareq, A.M.; Farhad, S.; Neshar Uddin, A.B.M.; Hoque, M.; Nasrin, M.S.; Uddin, M.M.R.; Hasan, M.; Sultana, A.; Munira, M.S.; Lyzu, C.; et al. Chemical Profiles, Pharmacological Properties, and in Silico Studies Provide New Insights on Cycas Pectinata. Heliyon 2020, 6, e04061. [Google Scholar] [CrossRef]
- Majid, M.; Khan, M.R.; Shah, N.A.; Haq, I.U.; Farooq, M.A.; Ullah, S.; Sharif, A.; Zahra, Z.; Younis, T.; Sajid, M. Studies on Phytochemical, Antioxidant, Anti-Inflammatory and Analgesic Activities of Euphorbia Dracunculoides. BMC Complement. Altern. Med. 2015, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, T.; Gupta, R.; Siddiqui, Z.A. A Review on Herbal Plants Showing Antidepressant Activity. Int. J. Pharm. Sci. Res. 2011, 2, 3051. [Google Scholar]
- Jiko, P.A.; Mohammad, M.; Richi, F.T.; Islam, M.A.; Alam, S.; Taher, M.A.; Shao, C.; Wang, S.; Geng, P.; Mamun, A. Al Anti-Inflammatory, Analgesic and Anti-Oxidant Effects of Shirakiopsis Indica (Willd). Fruit Extract: A Mangrove Species in the Field of Inflammation Research. J. Inflamm. Res. 2024, 17, 5821–5854. [Google Scholar] [PubMed]
- Mokmued, K.; Dechayont, B.; Phuaklee, P.; Liplung, C.; Muangpoolsawad, H.; Nuengchamnong, N.; Prommee, N. Evaluation of Anti-Inflammatory, Cytotoxic, Anti-H. Pylori, Antioxidant Activities, and Phytochemical Compositions of Shirakiopsis Indica (Willd.) Esser. ScienceAsia 2021, 47, 549–555. [Google Scholar] [CrossRef]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Sivakumar, T.; Vamsi, M.L.M. Antitumor Activity and Antioxidant Status of Caesalpinia Bonducella against Ehrlich Ascites Carcinoma in Swiss Albino Mice. J. Pharmacol. Sci. 2004, 94, 177–184. [Google Scholar] [CrossRef]
- Crawley, J.N. Exploratory Behavior Models of Anxiety in Mice. Neurosci. Biobehav. Rev. 1985, 9, 37–44. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Matsumiya, T. Changes in Head-Dipping Behavior in the Hole-Board Test Reflect the Anxiogenic and/or Anxiolytic State in Mice. Eur. J. Pharmacol. 1998, 350, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.; Goodwin, F.K. Preliminary Report of a Simple Animal Behavior Model for the Anxiolytic Effects of Benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Graeff, F.G.; Zangrossi, H., Jr. Animal Models of Anxiety Disorders. Biol. Psychiatry 2002, 877–893. [Google Scholar] [CrossRef]
- Lepicard, E.M.; Joubert, C.; Hagneau, I.; Perez-Diaz, F.; Chapouthier, G. Differences in Anxiety-Related Behavior and Response to Diazepam in BALB/CByJ and C57BL/6J Strains of Mice. Pharmacol. Biochem. Behav. 2000, 67, 739–748. [Google Scholar] [CrossRef]
- Subarnas, A.; Tadano, T.; Nakahata, N.; Arai, Y.; Kinemuchi, H.; Oshima, Y.; Kisara, K.; Ohizumi, Y. A Possible Mechanism of Antidepresant Activity of Beta-Amyrin Palmitate Isolated from Lobelia Inflata Leaves in the Forced Swimming Test. Life Sci. 1993, 52, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Southwick, S.M.; Vythilingam, M.; Charney, D.S. The Psychobiology of Depression and Resilience to Stress: Implications for Prevention and Treatment. Annu. Rev. Clin. Psychol. 2005, 1, 255–291. [Google Scholar] [CrossRef]
- Berton, O.; Nestler, E.J. New Approaches to Antidepressant Drug Discovery: Beyond Monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef]
- Osanloo, N.; Najafi-Abedi, A.; Jafari, F.; Javid, F.; Pirpiran, M.; Jafari, M.-R.M.; Khosravi, S.A.M.; Behzadi, M.R.; Ranjbaran, M.; Sahraei, H. Papaver Rhoeas L. Hydroalcoholic Extract Exacerbates Forced Swimming Test-Induced Depression in Mice. Basic. Clin. Neurosci. 2016, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Faizi, M.; Khazaee, R.; Mojab, F.; Jahani, R. Psychopharmacological Assessment of Antidepressant-like, Anxiolytic, and Sedative-Hypnotic Effects of Tilia Platyphyllos Scop. Extract Using Experimental Animal Models: Antidepressant-like, Anxiolytic, and Sedative-Hypnotic Properties of Tilia Platyphyllos. Iran. J. Pharm. Sci. 2022, 18, 116–127. [Google Scholar]
- Moniruzzaman, M.; Atikur Rahman, M.; Ferdous, A. Evaluation of Sedative and Hypnotic Activity of Ethanolic Extract of Scoparia Dulcis Linn. Evid.-Based Complement. Altern. Med. 2015, 2015, 873954. [Google Scholar] [CrossRef]
- Hossain, M.F.; Talukder, B.; Rana, M.N.; Tasnim, R.; Nipun, T.S.; Uddin, S.M.N.; Hossen, S.M.M. In Vivo Sedative Activity of Methanolic Extract of Stericulia Villosa Roxb. Leaves. BMC Complement. Altern. Med. 2016, 16, 398. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.U.; Khan, I.; Rauf, A.; Muhammad, N.; Shahid, M.; Shah, M.R. Antinociceptive, Muscle Relaxant and Sedative Activities of Gold Nanoparticles Generated by Methanolic Extract of Euphorbia Milii. BMC Complement. Altern. Med. 2015, 15, 160. [Google Scholar] [CrossRef]
- Rudra, S.; Tahamina, A.; Emon, N.U.; Adnan, M.; Shakil, M.; Chowdhury, M.H.U.; Barlow, J.W.; Alwahibi, M.S.; Soliman Elshikh, M.; Faruque, M.O. Evaluation of Various Solvent Extracts of Tetrastigma Leucostaphylum (Dennst.) Alston Leaves, a Bangladeshi Traditional Medicine Used for the Treatment of Diarrhea. Molecules 2020, 25, 4994. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Khan, H. Antipyretic, Analgesic and Anti-Inflammatory Activity of Viola Betonicifolia Whole Plant. BMC Complement. Altern. Med. 2012, 12, 59. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-Inflammatory Drugs and Their Mechanism of Action. Inflamm. Res. 1998, 47, 78–87. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, J.; Zhuang, Z.; Yang, Y.; Lin, L.; Wang, S. Thrombolytic Effects of Douchi Fibrinolytic Enzyme from Bacillus Subtilis LD-8547 in Vitro and in Vivo. BMC Biotechnol. 2012, 12, 36. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. The Phenolic Constituents of Plants and Their Taxonomic Significance. I. Dicotyledons. Bot. J. Linn. Soc. 1962, 58, 95–173. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Barde, S.R. Evaluation of Anthelmintic Activity and in Silico PASS Assisted Prediction of Cordia Dichotoma (Forst.) Root Extract. Anc. Sci. Life 2014, 34, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Gaba, M.; Gaba, P.; Singh, S.; Gupta, G.D. An Overview on Molecular Docking. Int. J. Drug Dev. Res. 2010, 2, 219–231. [Google Scholar]
- Sehim, A.E.; Amin, B.H.; Yosri, M.; Salama, H.M.; Alkhalifah, D.H.; Alwaili, M.A.; Abd Elghaffar, R.Y. GC-MS Analysis, Antibacterial, and Anticancer Activities of Hibiscus Sabdariffa L. Methanolic Extract: In Vitro and In Silico Studies. Microorganisms 2023, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Fatema, K.; Mia, M.A.R.; Nipun, T.S.; Hossen, S.M.M. Phytochemical Profiling and Pharmacological Evaluation of Methanolic Leaf Extract of C. Digyna for Cytotoxic, Anti-inflammatory, Antioxidant, Antiarthritic, and Analgesic Activities. Food Sci. Nutr. 2024, 12, 10231–10241. [Google Scholar] [CrossRef]
- Sunanda, B.P.; Latha, K.; Rammohan, B.; Uma Maheswari, M.S.; Surapaneni, K.M. Evaluation of the Neuroprotective Effects of Curcumin (Turmeric) against Scopolamine Induced Cognitive Impairment in Mice. Int. J. Pharm. Phytochem. Res. 2014, 6, 133–136. [Google Scholar]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of Open: Closed Arm Entries in an Elevated plus-Maze as a Measure of Anxiety in the Rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Miyakawa, T.; Yagi, T.; Kagiyama, A.; Niki, H. Radial Maze Performance, Open-Field and Elevated plus-Maze Behaviors in Fyn-Kinase Deficient Mice: Further Evidence for Increased Fearfulness. Mol. Brain Res. 1996, 37, 145–150. [Google Scholar] [CrossRef]
- File, S.E.; Wardill, A.G. Validity of Head-Dipping as a Measure of Exploration in a Modified Hole-Board. Psychopharmacologia 1975, 44, 53–59. [Google Scholar] [CrossRef]
- Kliethermes, C.L.; Crabbe, J.C. Pharmacological and Genetic Influences on Hole-Board Behaviors in Mice. Pharmacol. Biochem. Behav. 2006, 85, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Shahed-Al-Mahmud, M.; Lina, S.M.M. Evaluation of Sedative and Anxiolytic Activities of Methanol Extract of Leaves of Persicaria Hydropiper in Mice. Clin. Phytoscience 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing Substrates Underlying the Behavioral Effects of Antidepressants Using the Modified Rat Forced Swimming Test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Wang, X.; Lei, K.; Li, G.; Quan, Z. Synthesis and Evaluation of Antidepressant Activities of 5-Aryl-4, 5-Dihydrotetrazolo [1, 5-a] Thieno [2, 3-e] Pyridine Derivatives. Molecules 2019, 24, 1857. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M.; Okpako, D.T.; Evans, F.J. Selection, Preparation and Pharmacological Evaluation of Plant Material, Volume 1; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 1, ISBN 0471942170. [Google Scholar]
- Takagi, K.; Watanabe, M.; Saito, H. Studies of the Spontaneous Movement of Animals by the Hole Cross Test; Effect of 2-Dimethyl-Aminoethanol and Its Acyl Esters on the Central Nervous System. Jpn. J. Pharmacol. 1971, 21, 797–810. [Google Scholar] [CrossRef]
- Abena, A.A.; Diatewa, M.; Gakosso, G.; Gbeassor, M.; Hondi-Assah, T.H.; Ouamba, J.M. Analgesic, Antipyretic and Anti-Inflammatory Effects of Essential Oil of Lippia Multiflora. Fitoterapia 2003, 74, 231–236. [Google Scholar] [CrossRef]
- Prasad, S.; Kashyap, R.S.; Deopujari, J.Y.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Development of an in Vitro Model to Study Clot Lysis Activity of Thrombolytic Drugs. Thromb. J. 2006, 4, 1–4. [Google Scholar] [CrossRef]
- Ajaiyeoba, E.O.; Onocha, P.A.; Olarenwaju, O.T. In Vitro Anthelmintic Properties of Buchholzia Coriaceae and Gynandropsis Gynandra Extracts. Pharm. Biol. 2001, 39, 217–220. [Google Scholar] [CrossRef]
- Goel, R.K.; Singh, D.; Lagunin, A.; Poroikov, V. PASS-Assisted Exploration of New Therapeutic Potential of Natural Products. Med. Chem. Res. 2011, 20, 1509–1514. [Google Scholar] [CrossRef]
- Khurana, N.; Ishar, M.P.S.; Gajbhiye, A.; Goel, R.K. PASS Assisted Prediction and Pharmacological Evaluation of Novel Nicotinic Analogs for Nootropic Activity in Mice. Eur. J. Pharmacol. 2011, 662, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Ali, M.L.; Noushin, F.; Azme, E.; Hasan, M.M.; Hoque, N.; Metu, A.F. Marine Natural Compounds as Potential CBP Bromodomain Inhibitors for Treating Cancer: An in-Silico Approach Using Molecular Docking, ADMET, Molecular Dynamics Simulations and MM-PBSA Binding Free Energy Calculations. Silico Pharmacol. 2024, 12, 85. [Google Scholar] [CrossRef]
- Mohammad, M.; Rasel, M.H.; Richi, F.T.; Mamun, M.J.I.; Ekram, M.E.H.; Rabbi, S.A.H.; Hossain, S.; Hasan, M.A.; Sarker, M.F.; Alam, S. Neuropharmacological, Cytotoxic, and Anthelmintic Potentials of Lasia Spinosa (L.) Thwaites Rhizome: In Vivo, in Vitro, and Computational Approach. Pharmacol. Res.-Nat. Prod. 2025, 7, 100254. [Google Scholar] [CrossRef]
- Mohammad, M.; Islam, M.A.; Mamun, M.J.I.; Rasel, M.H.; Masum, M.A.A.; Rabbi, S.A.H.; Khalil, I.; Ali, M.L.; Hossen, S.M. Methanolic Extract of Edible Lasia Spinosa Rhizome: A Potential Natural Source of Analgesic, Diuretic, and Thrombolytic Agents. J. Herbs Spices Med. Plants 2025, 31, 381–406. [Google Scholar] [CrossRef]
- Lionta, E.; Spyrou, G.; K Vassilatis, D.; Cournia, Z. Structure-Based Virtual Screening for Drug Discovery: Principles, Applications and Recent Advances. Curr. Top. Med. Chem. 2014, 14, 1923–1938. [Google Scholar] [CrossRef]


| Treatment (mg/kg) | Time Spent in the Light Box (s) | Time Spent in the Dark Box (s) | Transitions |
|---|---|---|---|
| Control | 92.5 ± 1.69 | 205.83 ± 1.20 | 13.33 ± 1.33 |
| Diazepam | 195.24 ± 3.41 *** | 104.75± 3.41 ** | 5.33 ± 0.88 * |
| SIF-ME 200 | 62.73 ± 2.91 ** | 237.267 ± 2.91 ** | 9.66 ± 0.66 |
| SIF-ME 400 | 132.86 ± 2.05 ** | 167.13 ± 2.05 ** | 8.66 ± 1.20 |
| Treatment Dose (mg/mL) | Number of Holes Crossed | ||||
|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | |
| Control | 17.33 ± 0.88 | 18.33 ± 0.88 | 16.33 ± 1.20 | 14 ± 1.15 | 12.33 ± 0.33 |
| Diazepam | 10.66 ± 1.45 | 7.33 ± 0.88 ** | 11.66 ± 0.88 ** | 9.33 ± 0.66 | 4.33 ± 0.88 ** |
| SIF-ME 200 | 15.33 ± 0.88 *** | 10.33 ± 1.20 * | 13.33 ± 1.20 *** | 10.66 ± 0.66 * | 9.33 ± 0.88 * |
| SIF-ME 400 | 11.33 ± 1.20 * | 7.66b ± 1.20 ** | 10.66 ± 0.88 ** | 9.66 ± 0.88 * | 7.33 ± 0.33 ** |
| Treatment | Normal Rectal Temperature (°F) | Temperature After Pyrexia (°F) | Rectal Temperature (°F) After Drug Administration | ||
|---|---|---|---|---|---|
| 60 min | 120 min | 180 min | |||
| Control | 99.06 ± 0.58 | 103.1 ± 1.92 | 99.74 ± 1.08 | 101.63 ± 1.7 | 99.53 ± 0.96 |
| Paracetamol | 98.69 ± 0.42 | 100.98 ± 2.58 | 100.63 ± 2.5 | 99.1 ± 0.6 * | 99.2 ± 1.12 |
| SIF-ME 200 | 98.56 ± 0.15 | 100.56 ± 1.86 | 103.86 ± 1.35 * | 101.26 ± 2.14 | 103.1 ± 0.52 * |
| SIF-ME 400 | 99.15 ± 0.6 | 100.96 ± 1.68 | 101.06 ± 1.56 | 100.7 ± 2.1 | 99.3 ± 0.53 |
| Compounds | Biological Activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiolytic | Antidepressant | Locomotor | Antipyretic | Thrombolytic | Anthelmintic | |||||||
| Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | |
| Butanoic acid, 3-methyl | - | - | - | - | 0.214 | 0.062 | 0.324 | 0.038 | 0.265 | 0.018 | 0.291 | 0.041 |
| Phenol, 2-methoxy | 0.127 | 0.013 | 0.196 | 0.062 | 0.139 | 0.100 | 0.472 | 0.017 | 0.264 | 0.019 | 0.308 | 0.035 |
| 2-Methoxy-4-vinyl phenol | 0.229 | 0.005 | - | - | - | - | 0.427 | 0.022 | 0.200 | 0.073 | 0.291 | 0.041 |
| Phenol, 2,6-dimethoxy | 0.097 | 0.021 | 0.155 | 0.085 | 0.167 | 0.065 | 0.395 | 0.026 | 0.291 | 0.011 | 0.278 | 0.046 |
| Vanillin | 0.073 | 0.042 | - | - | 0.142 | 0.095 | 0.447 | 0.020 | 0.174 | 0.110 | 0.369 | 0.021 |
| Phenol, 2-methoxy-4-propyl | 0.098 | 0.020 | - | - | - | - | 0.492 | 0.014 | 0.258 | 0.021 | 0.236 | 0.065 |
| Phenol, 4-ethenyl-2,6-dimethoxy | 0.157 | 0.009 | - | - | - | - | 0.293 | 0.048 | 0.220 | 0.049 | 0.277 | 0.046 |
| Benzaldehyde, 3-hydroxy-4-methoxy | 0.073 | 0.042 | - | - | 0.142 | 0.095 | 0.447 | 0.020 | 0.174 | 0.110 | 0.369 | 0.021 |
| 5-Methyl-3-phenyl-1,3-oxazolidine | - | - | - | - | - | - | - | - | - | - | 0.161 | 0.135 |
| 2,6-Dimethoxyhydroquinone | 0.111 | 0.016 | - | - | - | - | 0.344 | 0.034 | 0.292 | 0.011 | 0.293 | 0.040 |
| Benzaldehyde, 4-hydroxy-3,5-dimethoxy | - | - | - | - | 0.180 | 0.053 | 0.306 | 0.043 | 0.196 | 0.079 | 0.349 | 0.025 |
| Phenol, 2.6-dimethoxy-4-(2-propenyl) | 0.143 | 0.011 | - | - | - | - | 0.393 | 0.027 | 0.193 | 0.083 | 0.268 | 0.050 |
| 2-Propanone, 1-hydroxy-3-(4-hydroxy-3-methoxyphenyl) | 0.066 | 0.053 | - | - | - | - | 0.334 | 0.036 | 0.209 | 0.062 | 0.325 | 0.086 |
| Retinoic acid | - | - | - | - | - | - | - | - | - | - | 0.306 | 0.035 |
| Octahydro-9-phenanthrene methanol | 0.069 | 0.048 | 0.229 | 0.046 | - | - | 0.275 | 0.056 | - | - | 0.166 | 0.129 |
| Epoxylathyrol | - | - | - | - | - | - | - | - | - | - | 0.259 | 0.054 |
| Beta–Sitosterol | - | - | - | - | - | - | - | - | - | - | - | - |
| 24-Noroleana-3,12-diene | - | - | - | - | - | - | - | - | 0.319 | 0.007 | - | - |
| Compound Name | Absorption | Distribution | Metabolism | Excretion | Toxicity | Drug Likeness | Bioavailability | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Water Solubility (log mol/L) | Intestinal Absorption (Human) (% Absorbed) | VDss (Human) (log L/kg) | BBB Permeability (log BB) | CYP3A4 Substrate | Total Clearance (log mL/min/kg) | AMES Toxicity | Hepatotoxicity | |||
| Butanoic acid, 3-methyl | −0.811 | 88.82 | −0.937 | −0.227 | No | 0.391 | No | No | Yes | 0.85 |
| Phenol, 2-methoxy | −1.264 | 93.374 | 0.174 | −0.226 | No | 0.219 | No | No | Yes | 0.55 |
| 2-Methoxy-4-vinyl phenol | −1.958 | 91.965 | 0.118 | 0.289 | No | 0.233 | Yes | No | Yes | 0.55 |
| Phenol, 2,6-dimethoxy | −1.4 | 93.789 | −0.129 | −0.204 | No | 0.213 | No | No | Yes | 0.55 |
| Vanillin | −1.308 | 84.976 | −0.152 | −0.243 | No | 0.601 | No | No | Yes | 0.55 |
| Phenol, 2-methoxy-4-propyl | −1.628 | 92.829 | 0.36 | 0.387 | No | 0.244 | No | Yes | Yes | 0.55 |
| Phenol, 4-ethenyl-2,6-dimethoxy | −1.925 | 92.945 | 0.206 | 0.396 | No | 0.241 | No | No | Yes | 0.55 |
| Benzaldehyde, 3-hydroxy-4-methoxy | −1.295 | 89.886 | −0.165 | −0.243 | No | 0.599 | No | No | Yes | 0.55 |
| 5-Methyl-3-phenyl-1,3-oxazolidine | −2.304 | 97.289 | 0.259 | 0.498 | No | 0.282 | No | No | Yes | 0.55 |
| 2,6-Dimethoxyhydroquinone | −1.715 | 85.96 | 0.169 | −0.342 | No | 0.63 | No | No | Yes | 0.55 |
| Benzaldehyde, 4-hydroxy-3,5-dimethoxy | −1.481 | 90.106 | −0.042 | −0.281 | No | 0.621 | No | No | Yes | 0.55 |
| Phenol, 2,6-dimethoxy-4-(2-propenyl) | −2.069 | 92.959 | 0.272 | 0.362 | No | 0.293 | No | No | Yes | 0.55 |
| 2-Propanone, 1-hydroxy-3-(4-hydroxy-3-methoxyphenyl) | −1.291 | 83.06 | −0.345 | −0.254 | No | 0.26 | No | No | Yes | 0.55 |
| Retinoic acid | −4.924 | 94.419 | −0.51 | 0.236 | Yes | 1.443 | No | Yes | NF | NF |
| Octahydro-9-phenanthrene methanol | −3.862 | 95.247 | 0.995 | 0.61 | Yes | 1.176 | No | No | Yes | 0.55 |
| Epoxylathyrol | −4.273 | 97.394 | 0.16 | −0.571 | No | 0.666 | No | NO | Yes | 0.55 |
| Beta–Sitosterol | −6.773 | 94.464 | 0.193 | 0.781 | Yes | 0.628 | No | No | Yes | 0.55 |
| 24-Noroleana-3,12-diene | −6.85 | 96.674 | 0.44 | 0.848 | Yes | 0.073 | No | No | Yes | 0.55 |
| SL No. | Compounds | MW | Retention Time | Area % |
|---|---|---|---|---|
| 1 | Butanoic acid, 3-methyl | 102.1 g/mol | 3.904 | 0.15 |
| 2 | Phenol, 2-methoxy | 124.1 g/mol | 6.83 | 0.37 |
| 3 | 2-Methoxy-4-vinyl phenol | 150.1 g/mol | 9.289 | 0.29 |
| 4 | Phenol, 2,6-dimethoxy | 154.1 g/mol | 9.632 | 0.38 |
| 5 | Vanillin | 152.1 g/mol | 10.171 | 0.3 |
| 6 | endo-1,5,6,7-Tetramethylbicyclo[3.2.0]hept-6-en-3-ol | 166.2 g/mol | 10.626 | 0.8 |
| 7 | Phenol, 2-methoxy-4-propyl | 166.2 g/mol | 10.695 | 0.24 |
| 8 | 3(2H)-Benzofuranone, 2.4-dimethyl | 162.1 g/mol | 10.971 | 0.2 |
| 9 | beta.-D-Glucopyranose, 1.6-anhydro | 162.1 g/mol | 11.031 | 0.22 |
| 10 | Guaiacol, 4-butyl | 180.2 g/mol | 11.35 | 0.55 |
| 11 | Phenol, 4-ethenyl-2,6-dimethoxy | 180.2 g/mol | 11.663 | 0.33 |
| 12 | Benzaldehyde, 3-hydroxy-4-methoxy | 152.1 g/mol | 11.934 | 0.28 |
| 13 | 5-Methyl-3-phenyl-1,3-oxazolidine | 163.2 g/mol | 12.375 | 0.41 |
| 14 | (3R,3aS,6S,7R)-3,6,8,8-Tetramethyloctahydro-1H3a,7-methanoazulen-6-o | 222.3 g/mol | 12.425 | 0.17 |
| 15 | 2,6-Dimethoxyhydroquinone | 170.1 g/mol | 12.602 | 0.32 |
| 16 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy | 182.1 g/mol | 12.708 | 0.49 |
| 17 | Phenol, 2,6-dimethoxy-4-(2-propenyl) | 194.2 g/mol | 13.18 | 0.49 |
| 18 | 3-O-Methyl-d-glucose | 194.1 g/mol | 13.251 | 0.29 |
| 19 | 2-Propanone, 1-hydroxy-3-(4-hydroxy3-methoxyphenyl | 196.2 g/mol | 13.566 | 0.93 |
| 20 | 4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenyl | 180.2 g/mol | 13.747 | 8.29 |
| 21 | Dihydroxy-4-methyldodecahydro-2H-benzo[d] oxecin-2-one | 256.3 g/mol | 14.882 | 0.38 |
| 22 | Sinapyl alcohol | 210.2 g/mol | 17.386 | 3.68 |
| 23 | 4-Hydroxy-3,5,5-trimethyl-4-(3-oxobut-1-en-1-yl) cyclohex-2-enone | 222.2 g/mol | 18.661 | 0.25 |
| 24 | 9,11-Octadecadienoic acid, methyl ester | 294.5 g/mol | 18.941 | 2.56 |
| 25 | 9,12,15-Octadecatrienoic acid, methyl ester | 292.5 g/mol | 19.046 | 6.02 |
| 26 | Phytol | 296.5 g/mol | 19.197 | 0.97 |
| 27 | Methyl stearate | 298.5 g/mol | 19.449 | 0.67 |
| 28 | Hexadecanamide | 255.4 g/mol | 20.421 | 0.39 |
| 29 | 9-Octadecenamide | 281.5 g/mol | 23.448 | 7.39 |
| 30 | Z,Z,Z-8,9-Epoxyeicosa-5,11,14-trienoic acid, methyl ester | 334.5 g/mol | 23.558 | 1.54 |
| 31 | Cyclohexanone, 5-ethenyl-5-methyl4-(1-methylethenyl)-2-(1-methylethylidene) | 218.3 g/mol | 23.75 | 0.75 |
| 32 | 4-Cycloocten-1-one, 8-(4-octen-4-yl) | 234.3 g/mol | 23.876 | 0.93 |
| 33 | 5.Beta.,7.beta.H,10.alpha.-Eudesm-11-en-1.alpha.-ol | 222.3 g/mol | 23.99 | 0.71 |
| 34 | Bicyclo[4.1.0]heptane, 1-(3-oxo-4-phenylthiobutyl)-2,2,6-trimethyl | 316.5 g/mol | 25.15 | 0.58 |
| 35 | Diethylene glycol dibenzoate | 314.3 g/mol | 25.397 | 0.39 |
| 36 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | 330.5 g/mol | 25.849 | 0.64 |
| 37 | Bis(2-ethylhexyl) phthalate | 390.6 g/mol | 26.147 | 0.31 |
| 38 | Retinoic acid | 300.4 g/mol | 26.94 | 2.57 |
| 39 | S-Octahydro-9-phenanthrene methanol | 216.3 g/mol | 27.12 | 1.03 |
| 40 | Epoxylathyrol | 350.4 g/mol | 28.065 | 0.61 |
| 41 | 3,3′-Dimethoxy-4,4′-dihydroxystilbene | 272.2 g/mol | 28.195 | 0.6 |
| 42 | Oleic Acid | 354.6 g/mol | 28.38 | 0.39 |
| 43 | Retinol | 286.5 g/mol | 28.44 | 1.34 |
| 44 | Methyl 5,11,14-eicosatrienoate | 320.5 g/mol | 28.636 | 3.35 |
| 45 | 9,12,15-Octadecatrienoic acid, 2.3-dihydroxypropyl ester, (Z,Z,Z)- | 352.5 g/mol | 28.755 | 5.34 |
| 46 | Benzene, 1-[(4-butyl phenyl)ethynyl]-4-ethoxy2-methyl | 292.4 g/mol | 28.83 | 2.59 |
| 47 | 10,13-Dimethyl-3-oxo2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl 2,2,2-trifluoroacetate | 331.5 g/mol | 28.974 | 3.04 |
| 48 | Retinal | 284.4 g/mol | 29.323 | 1.04 |
| 49 | (1S,2E,4S,5R,7E,11E)-Cembra-2,7,11-trien-4,5-diol | 306.5 g/mol | 29.41 | 0.58 |
| 50 | Methyl 1,4a-dimethyl-6-methylidene-5-[2-(5-oxo-2Hfuran-4-yl)ethyl]-3,4,5,7,8,8a-hexahydro-2Hnaphthalene-1-carboxylate | 346.5 g/mol | 29.72 | 1.73 |
| 51 | 9-(Acetyloxy)-4a,7b-dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo1,1a,1b,4,4a,5,7a,7b,8,9-decahydro-9aH-cyclopropa | 560.7 g/mol | 30.861 | 1.7 |
| 52 | Cholest-22-ene-21-ol, 3.5-dehydro-6-methoxy-, pivalate | 498.8 g/mol | 31.266 | 1.96 |
| 53 | 1H-Cyclopropa[3,4]benz[1,2-e]azulene-4a,5,7b,9,9a (1aH)-pentol, 3-[(acetyloxy)methyl]- 1b,4,5,7a,8,9-hexahydro-1,1,6,8-tetramethyl | 492.6 g/mol | 31.842 | 2.44 |
| 54 | (11.xi.)-4,7-Dihydroxy-12,13-epoxytrichothec-9-en8-one | 280.3 g/mol | 33.31 | 0.22 |
| 55 | Spirost-5-en-3-ol, acetate, (3.beta.,25R)- | 456.7 g/mol | 34.002 | 0.24 |
| 56 | Nonacosan-10-ol | 424.8 g/mol | 34.551 | 0.73 |
| 57 | Stigmasterol | 412.7 g/mol | 37.316 | 1.18 |
| 58 | 22-Desoxycarpesterol | 546.8 g/mol | 37.815 | 0.33 |
| 59 | Beta-sitosterol | 414.7 g/mol | 38.573 | 3.5 |
| 60 | 24-Noroleana-3,12-diene | 394.7 g/mol | 39.627 | 5.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, M.; Mamun, M.J.I.; Khatun, M.M.; Rasel, M.H.; Masum, M.A.A.; Suma, K.J.; Haque, M.R.; Rabbi, S.A.H.; Hossain, M.H.; Hasnat, H.; et al. A Multifaceted Exploration of Shirakiopsis indica (Willd) Fruit: Insights into the Neuropharmacological, Antipyretic, Thrombolytic, and Anthelmintic Attributes of a Mangrove Species. Drugs Drug Candidates 2025, 4, 31. https://doi.org/10.3390/ddc4030031
Mohammad M, Mamun MJI, Khatun MM, Rasel MH, Masum MAA, Suma KJ, Haque MR, Rabbi SAH, Hossain MH, Hasnat H, et al. A Multifaceted Exploration of Shirakiopsis indica (Willd) Fruit: Insights into the Neuropharmacological, Antipyretic, Thrombolytic, and Anthelmintic Attributes of a Mangrove Species. Drugs and Drug Candidates. 2025; 4(3):31. https://doi.org/10.3390/ddc4030031
Chicago/Turabian StyleMohammad, Mahathir, Md. Jahirul Islam Mamun, Mst. Maya Khatun, Md. Hossain Rasel, M Abdullah Al Masum, Khurshida Jahan Suma, Mohammad Rashedul Haque, Sayed Al Hossain Rabbi, Md. Hemayet Hossain, Hasin Hasnat, and et al. 2025. "A Multifaceted Exploration of Shirakiopsis indica (Willd) Fruit: Insights into the Neuropharmacological, Antipyretic, Thrombolytic, and Anthelmintic Attributes of a Mangrove Species" Drugs and Drug Candidates 4, no. 3: 31. https://doi.org/10.3390/ddc4030031
APA StyleMohammad, M., Mamun, M. J. I., Khatun, M. M., Rasel, M. H., Masum, M. A. A., Suma, K. J., Haque, M. R., Rabbi, S. A. H., Hossain, M. H., Hasnat, H., Mahjabin, N., & Alam, S. (2025). A Multifaceted Exploration of Shirakiopsis indica (Willd) Fruit: Insights into the Neuropharmacological, Antipyretic, Thrombolytic, and Anthelmintic Attributes of a Mangrove Species. Drugs and Drug Candidates, 4(3), 31. https://doi.org/10.3390/ddc4030031










