Therapeutic Potential of Mineralocorticoid Receptors in Skeletal Muscle Aging
Abstract
1. Introduction
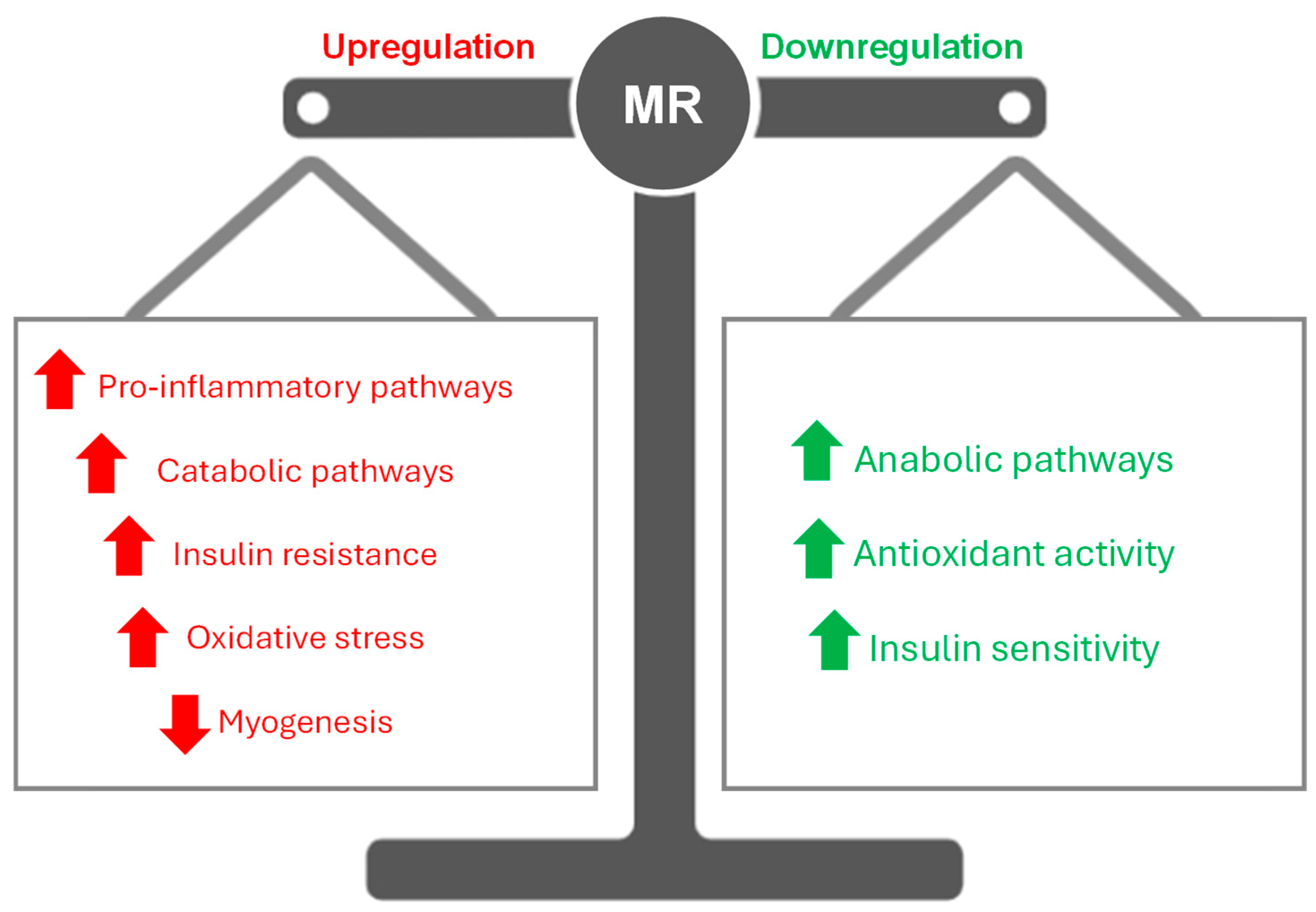
2. Role of Mineralocorticoid Receptors in Skeletal Muscle
2.1. Skeletal Muscle Aging: Insights into the Drivers of Sarcopenia
2.2. MR-Driven Ion Transport in Skeletal Muscle
2.3. The Intersection of MRs and Insulin Resistance
2.4. Linking MRs to Inflammation and Oxidative Stress
3. Counteracting Muscle Deterioration in Aging by Tackling MRs
3.1. Harnessing MR Antagonists for Therapy
3.2. Exercise as a Modulator of MRs
3.3. Future Perspectives
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef]
- Kamel, H.K. Sarcopenia and Aging. Nutr. Rev. 2003, 61, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef]
- Dirks, A.J.; Hofer, T.; Marzetti, E.; Pahor, M.; Leeuwenburgh, C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res. Rev. 2006, 5, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Buglio, A.L.; Vendemiale, G. Oxidative stress and sarcopenia. In Aging; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–103. [Google Scholar] [CrossRef]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Álvarez-Bustos, A.; Rodríguez-Sánchez, B.; Carnicero-Carreño, J.A.; Sepúlveda-Loyola, W.; Garcia-Garcia, F.J.; Rodríguez-Mañas, L. Healthcare cost expenditures associated to frailty and sarcopenia. BMC Geriatr. 2022, 22, 747. [Google Scholar] [CrossRef]
- Tian, D.; Meng, J. Exercise for Prevention and Relief of Cardiovascular Disease: Prognoses, Mechanisms, and Approaches. Oxid. Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Chadwick, J.A.; Hauck, J.S.; Lowe, J.; Shaw, J.J.; Guttridge, D.C.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; Rafael-Fortney, J.A. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J. 2015, 29, 4544–4554. [Google Scholar] [CrossRef]
- Howard, Z.M.; Gomatam, C.K.; Rabolli, C.P.; Lowe, J.; Piepho, A.B.; Bansal, S.S.; Accornero, F.; Rafael-Fortney, J.A. Mineralocorticoid receptor antagonists and glucocorticoids differentially affect skeletal muscle inflammation and pathology in muscular dystrophy. JCI Insight 2022, 7, e159875. [Google Scholar] [CrossRef] [PubMed]
- Howard, Z.M.; Gomatam, C.K.; Piepho, A.B.; Rafael-Fortney, J.A. Mineralocorticoid Receptor Signaling in the Inflammatory Skeletal Muscle Microenvironments of Muscular Dystrophy and Acute Injury. Front. Pharmacol. 2022, 13, 942660. [Google Scholar] [CrossRef]
- Nguyen, E.T.; Berman, S.; Streicher, J.; Estrada, C.M.; Caldwell, J.L.; Ghisays, V.; Ulrich-Lai, Y.; Solomon, M.B. Effects of combined glucocorticoid/mineralocorticoid receptor modulation (CORT118335) on energy balance, adiposity, and lipid metabolism in male rats. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E337–E349. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The Multifaceted Mineralocorticoid Receptor. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 965–994. [Google Scholar] [CrossRef]
- Zennaro, M.-C.; Caprio, M.; Fève, B. Mineralocorticoid receptors in the metabolic syndrome. Trends Endocrinol. Metab. 2009, 20, 444–451. [Google Scholar] [CrossRef]
- Young, M.J. Mechanisms of mineralocorticoid receptor-mediated cardiac fibrosis and vascular inflammation. Curr. Opin. Nephrol. Hypertens. 2008, 17, 174–180. [Google Scholar] [CrossRef]
- Pascual-Le Tallec, L.; Lombès, M. The Mineralocorticoid Receptor: A Journey Exploring Its Diversity and Specificity of Action. Mol. Endocrinol. 2005, 19, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Gaudenzi, C.; Mifsud, K.R.; Reul, J.M.H.M. Insights into isoform-specific mineralocorticoid receptor action in the hippocampus. J. Endocrinol. 2023, 258, e220293. [Google Scholar] [CrossRef]
- Le Menuet, D.; Viengchareun, S.; Muffat-Joly, M.; Zennaro, M.-C.; Lombès, M. Expression and function of the human mineralocorticoid receptor: Lessons from transgenic mouse models. Mol. Cell. Endocrinol. 2004, 217, 127–136. [Google Scholar] [CrossRef]
- Ibarrola, J.; Lu, Q.; Zennaro, M.-C.; Jaffe, I.Z. Mechanism by Which Inflammation and Oxidative Stress Induce Mineralocorticoid Receptor Gene Expression in Aging Vascular Smooth Muscle Cells. Hypertension 2023, 80, 111–124. [Google Scholar] [CrossRef]
- Kuhn, E.; Lombès, M. The mineralocorticoid receptor: A new player controlling energy homeostasis. Horm. Mol. Biol. Clin. Investig. 2013, 15, 59–69. [Google Scholar] [CrossRef]
- Hulse, J.L.; Habibi, J.; Igbekele, A.E.; Zhang, B.; Li, J.; Whaley-Connell, A.; Sowers, J.R.; Jia, G. Mineralocorticoid Receptors Mediate Diet-Induced Lipid Infiltration of Skeletal Muscle and Insulin Resistance. Endocrinology 2022, 163, bqac145. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Privitera, G.; Simili, V.; Wohlgemuth, S.E.; Aulisa, L.; Pahor, M.; Leeuwenburgh, C. Multiple Pathways to the Same End: Mechanisms of Myonuclear Apoptosis in Sarcopenia of Aging. Sci. World J. 2010, 10, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.A.; McMurdo, M.E.T.; Struthers, A.D. Mineralocorticoid antagonism: A novel way to treat sarcopenia and physical impairment in older people? Clin. Endocrinol. 2011, 75, 725–729. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Alway, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of Satellite Cell Function in Sarcopenia. Front. Aging Neurosci. 2014, 6, 246. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Devi Rajeswari, V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: A perspective review. Mol. Cell. Biochem. 2023, 478, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- McPhee, J.S.; Cameron, J.; Maden-Wilkinson, T.; Piasecki, M.; Yap, M.H.; Jones, D.A.; Degens, H. The Contributions of Fiber Atrophy, Fiber Loss, In Situ Specific Force, and Voluntary Activation to Weakness in Sarcopenia. J. Gerontol. Ser. A 2018, 73, 1287–1294. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Jia, G.; Lockette, W.; Sowers, J.R. Mineralocorticoid receptors in the pathogenesis of insulin resistance and related disorders: From basic studies to clinical disease. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 320, R276–R286. [Google Scholar] [CrossRef]
- Kuster, G.M.; Kotlyar, E.; Rude, M.K.; Siwik, D.A.; Liao, R.; Colucci, W.S.; Sam, F. Mineralocorticoid Receptor Inhibition Ameliorates the Transition to Myocardial Failure and Decreases Oxidative Stress and Inflammation in Mice With Chronic Pressure Overload. Circulation 2005, 111, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.G.; Kapoor, S.C. Potassium supplementation ameliorates mineralocorticoid-induced sodium retention. Kidney Int. 1993, 43, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Jurkat-Rott, K.; Weber, M.-A.; Fauler, M.; Guo, X.-H.; Holzherr, B.D.; Paczulla, A.; Nordsborg, N.; Joechle, W.; Lehmann-Horn, F. K+ -dependent paradoxical membrane depolarization and Na + overload, major and reversible contributors to weakness by ion channel leaks. Proc. Natl. Acad. Sci. USA 2009, 106, 4036–4041. [Google Scholar] [CrossRef]
- Eisenhut, M.; Wallace, H. Ion channels in inflammation. Pflüg. Arch. Eur. J. Physiol. 2011, 461, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Vijayan, M.M. The Mineralocorticoid Receptor Functions as a Key Glucose Regulator in the Skeletal Muscle of Zebrafish. Endocrinology 2022, 163, bqac149. [Google Scholar] [CrossRef]
- Feraco, A.; Marzolla, V.; Scuteri, A.; Armani, A.; Caprio, M. Mineralocorticoid Receptors in Metabolic Syndrome: From Physiology to Disease. Trends Endocrinol. Metab. 2020, 31, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.B.; McGraw, A.P.; Jaffe, I.Z.; Sowers, J.R. Mineralocorticoid Receptor–Mediated Vascular Insulin Resistance. Diabetes 2013, 62, 313–319. [Google Scholar] [CrossRef]
- Korol, S.; Mottet, F.; Perreault, S.; Baker, W.L.; White, M.; De Denus, S. A systematic review and meta-analysis of the impact of mineralocorticoid receptor antagonists on glucose homeostasis. Medicine 2017, 96, e8719. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Michelucci, A.; Liang, C.; Protasi, F.; Dirksen, R.T. Altered Ca2+ Handling and Oxidative Stress Underlie Mitochondrial Damage and Skeletal Muscle Dysfunction in Aging and Disease. Metabolites 2021, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, E.; Coleman, R.; Reznick, A.Z. The biochemistry of aging muscle. Exp. Gerontol. 2002, 37, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Gorini, S.; Kim, S.K.; Infante, M.; Mammi, C.; La Vignera, S.; Fabbri, A.; Jaffe, I.Z.; Caprio, M. Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging. Front. Endocrinol. 2019, 10, 584. [Google Scholar] [CrossRef]
- Fuller, P.J.; Young, M.J. Mechanisms of Mineralocorticoid Action. Hypertension 2005, 46, 1227–1235. [Google Scholar] [CrossRef]
- Muñoz-Durango, N.; Vecchiola, A.; Gonzalez-Gomez, L.M.; Simon, F.; Riedel, C.A.; Fardella, C.E.; Kalergis, A.M. Modulation of Immunity and Inflammation by the Mineralocorticoid Receptor and Aldosterone. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Li, Y.-P. Tumor necrosis factor-α and muscle wasting: A cellular perspective. Respir. Res. 2001, 2, 269. [Google Scholar] [CrossRef]
- Clavel, S.; Coldefy, A.-S.; Kurkdjian, E.; Salles, J.; Margaritis, I.; Derijard, B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech. Ageing Dev. 2006, 127, 794–801. [Google Scholar] [CrossRef]
- Langen, R.C.J.; Velden, J.L.J.; Schols, A.M.W.J.; Kelders, M.C.J.M.; Wouters, E.F.M.; Janssen-Heininger, Y.M.W. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J. 2004, 18, 227–237. [Google Scholar] [CrossRef]
- Chantong, B.; Kratschmar, D.V.; Nashev, L.G.; Balazs, Z.; Odermatt, A. Mineralocorticoid and glucocorticoid receptors differentially regulate NF-kappaB activity and pro-inflammatory cytokine production in murine BV-2 microglial cells. J. Neuroinflamm. 2012, 9, 260. [Google Scholar] [CrossRef]
- Thangaraj, S.S.; Oxlund, C.S.; Fonseca, M.P.D.; Svenningsen, P.; Stubbe, J.; Palarasah, Y.; Ketelhuth, D.F.J.; Jacobsen, I.A.; Jensen, B.L. The mineralocorticoid receptor blocker spironolactone lowers plasma interferon-γ and interleukin-6 in patients with type 2 diabetes and treatment-resistant hypertension. J. Hypertens. 2022, 40, 153–162. [Google Scholar] [CrossRef]
- Sønder, S.U.S.; Mikkelsen, M.; Rieneck, K.; Hedegaard, C.J.; Bendtzen, K. Effects of spironolactone on human blood mononuclear cells: Mineralocorticoid receptor independent effects on gene expression and late apoptosis induction. Br. J. Pharmacol. 2006, 148, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Li, Y.-L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 2023, 14, 1133355. [Google Scholar] [CrossRef] [PubMed]
- Beenakker, K.G.M.; Ling, C.H.; Meskers, C.G.M.; De Craen, A.J.M.; Stijnen, T.; Westendorp, R.G.J.; Maier, A.B. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res. Rev. 2010, 9, 431–436. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 94, pp. 155–218. [Google Scholar]
- Lightfoot, A.P.; Cooper, R.G. The role of myokines in muscle health and disease. Curr. Opin. Rheumatol. 2016, 28, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Fiebeler, A.; Luft, F.C. The Mineralocorticoid Receptor and Oxidative Stress. Heart Fail. Rev. 2005, 10, 47–52. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive Oxygen Species: Impact on Skeletal Muscle. In Comprehensive Physiology, 1st ed.; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 941–969. [Google Scholar] [CrossRef]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Burniston, J.; Saini, A.; Tan, L.; Goldspink, D. Aldosterone induces myocyte apoptosis in the heart and skeletal muscles of rats in vivo. J. Mol. Cell. Cardiol. 2005, 39, 395–399. [Google Scholar] [CrossRef]
- Lefranc, C.; Friederich-Persson, M.; Palacios-Ramirez, R.; Nguyen Dinh Cat, A. Mitochondrial oxidative stress in obesity: Role of the mineralocorticoid receptor. J. Endocrinol. 2018, 238, R143–R159. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, D.A.; Choi, E.; Lee, Y.S.; Park, S.J.; Kim, B.-J. Aldosterone Inhibits In Vitro Myogenesis by Increasing Intracellular Oxidative Stress via Mineralocorticoid Receptor. Endocrinol. Metab. 2021, 36, 865–874. [Google Scholar] [CrossRef]
- Lefranc, C.; Friederich-Persson, M.; Braud, L.; Palacios-Ramirez, R.; Karlsson, S.; Boujardine, N.; Motterlini, R.; Jaisser, F.; Nguyen Dinh Cat, A. MR (Mineralocorticoid Receptor) Induces Adipose Tissue Senescence and Mitochondrial Dysfunction Leading to Vascular Dysfunction in Obesity. Hypertension 2019, 73, 458–468. [Google Scholar] [CrossRef]
- Marzetti, E.; Wohlgemuth, S.E.; Lees, H.A.; Chung, H.-Y.; Giovannini, S.; Leeuwenburgh, C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech. Ageing Dev. 2008, 129, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lastra, G.; Whaley-Connell, A.; Manrique, C.; Habibi, J.; Gutweiler, A.A.; Appesh, L.; Hayden, M.R.; Wei, Y.; Ferrario, C.; Sowers, J.R. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E110–E116. [Google Scholar] [CrossRef]
- Lowe, J.; Kadakia, F.K.; Zins, J.G.; Haupt, M.; Peczkowski, K.K.; Rastogi, N.; Floyd, K.T.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E.; Elnakish, M.T.; et al. Mineralocorticoid Receptor Antagonists in Muscular Dystrophy Mice During Aging and Exercise. J. Neuromuscul. Dis. 2018, 5, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Pieronne-Deperrois, M.; Guéret, A.; Djerada, Z.; Crochemore, C.; Harouki, N.; Henry, J.; Dumesnil, A.; Larchevêque, M.; Do Rego, J.; Do Rego, J.; et al. Mineralocorticoid receptor blockade with finerenone improves heart function and exercise capacity in ovariectomized mice. ESC Heart Fail. 2021, 8, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Wellhoener, P.; Born, J.; Fehm, H.L.; Dodt, C. Elevated Resting and Exercise-Induced Cortisol Levels after Mineralocorticoid Receptor Blockade with Canrenoate in Healthy Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5048–5052. [Google Scholar] [CrossRef]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, S.H.; Kwak, H.-B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef]
- Borghouts, L.B.; Keizer, H.A. Exercise and Insulin Sensitivity: A Review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A.; et al. Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age Ageing 2022, 51, afac003. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Chrousos, G.P. Sleep, the hypothalamic–pituitary–adrenal axis, and cytokines: Multiple interactions and disturbances in sleep disorders. Endocrinol. Metab. Clin. N. Am. 2002, 31, 15–36. [Google Scholar] [CrossRef]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. In Frontiers of Hormone Research; Lanfranco, F., Strasburger, C.J., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 47, pp. 12–26. [Google Scholar]
- Piovezan, R.D.; Abucham, J.; Dos Santos, R.V.T.; Mello, M.T.; Tufik, S.; Poyares, D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res. Rev. 2015, 23, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Valaiyapathi, B.; Calhoun, D.A. Role of Mineralocorticoid Receptors in Obstructive Sleep Apnea and Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 23. [Google Scholar] [CrossRef]
- Dattilo, M.; Antunes, H.K.M.; Medeiros, A.; Mônico Neto, M.; Souza, H.S.; Tufik, S.; De Mello, M.T. Sleep and muscle recovery: Endocrinological and molecular basis for a new and promising hypothesis. Med. Hypotheses 2011, 77, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, B.A.; Neufeld, E.V.; Boland, D.M.; Martin, J.L.; Cooper, C.B. Interrelationship between Sleep and Exercise: A Systematic Review. Adv. Prev. Med. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Rafael-Fortney, J.A.; Chimanji, N.S.; Schill, K.E.; Martin, C.D.; Murray, J.D.; Ganguly, R.; Stangland, J.E.; Tran, T.; Xu, Y.; Canan, B.D.; et al. Early Treatment with Lisinopril and Spironolactone Preserves Cardiac and Skeletal Muscle in Duchenne Muscular Dystrophy Mice. Circulation 2011, 124, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Spirduso, W.W.; Cronin, D.L. Exercise dose-response effects on quality of life and independent living in older adults. Med. Sci. Sports Exerc. 2001, 33, S598–S608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nucci, R.A.B.; Nóbrega, O.d.T.; Jacob-Filho, W. Therapeutic Potential of Mineralocorticoid Receptors in Skeletal Muscle Aging. Receptors 2025, 4, 13. https://doi.org/10.3390/receptors4030013
Nucci RAB, Nóbrega OdT, Jacob-Filho W. Therapeutic Potential of Mineralocorticoid Receptors in Skeletal Muscle Aging. Receptors. 2025; 4(3):13. https://doi.org/10.3390/receptors4030013
Chicago/Turabian StyleNucci, Ricardo Aparecido Baptista, Otávio de Toledo Nóbrega, and Wilson Jacob-Filho. 2025. "Therapeutic Potential of Mineralocorticoid Receptors in Skeletal Muscle Aging" Receptors 4, no. 3: 13. https://doi.org/10.3390/receptors4030013
APA StyleNucci, R. A. B., Nóbrega, O. d. T., & Jacob-Filho, W. (2025). Therapeutic Potential of Mineralocorticoid Receptors in Skeletal Muscle Aging. Receptors, 4(3), 13. https://doi.org/10.3390/receptors4030013






