Human Stem Cell-Derived Neural Organoids for the Discovery of Antiseizure Agents
Abstract
1. Introduction
2. Methods
2.1. Culture and Neural Differentiation of Stem Cells
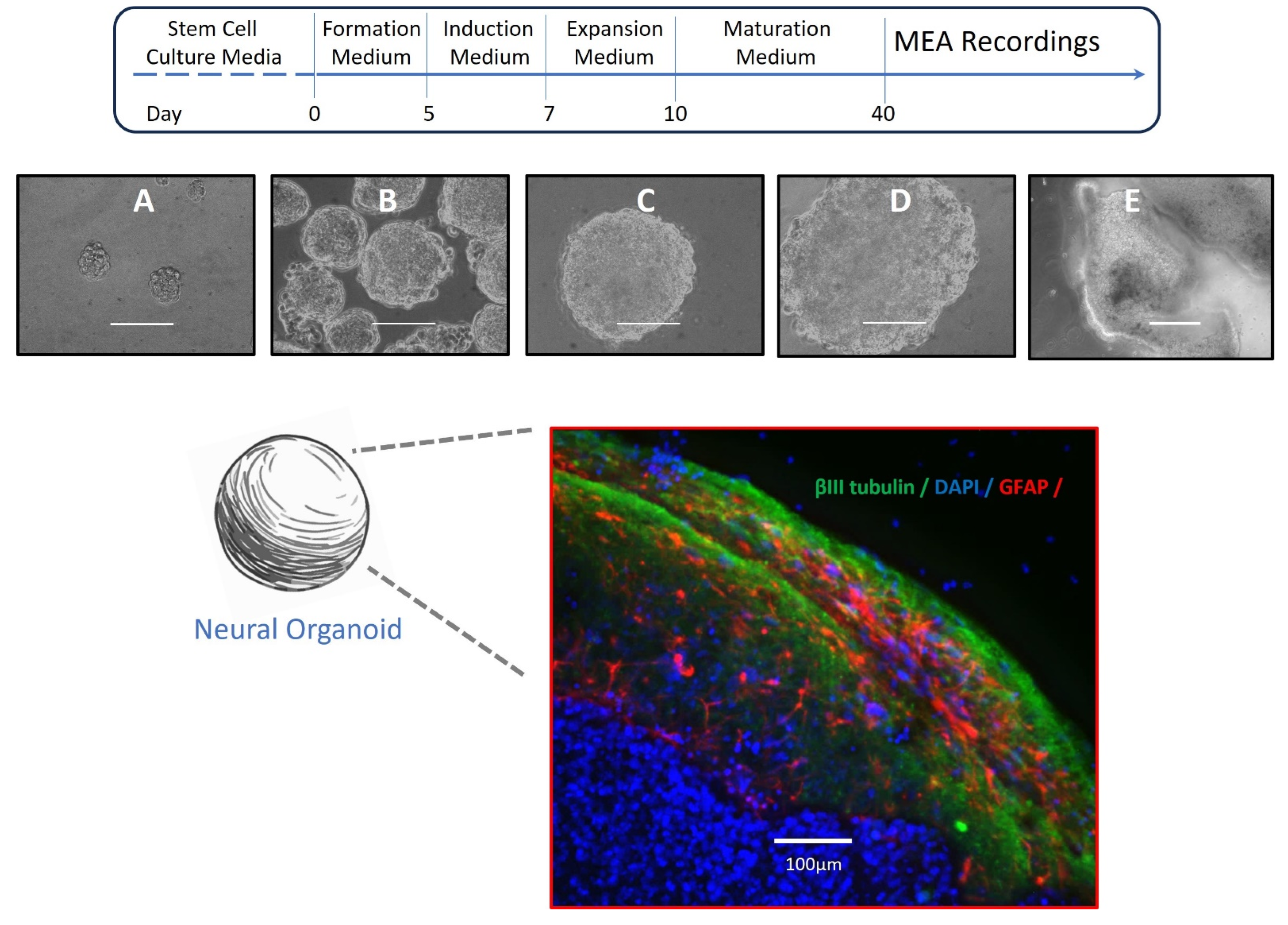
2.2. Formation of Neural Organoids
2.3. Immunocytochemistry
2.4. MEA Electrophysiology
2.5. Drug Solutions and Drug Test Protocol
2.6. Data Analysis
3. Results
3.1. Neural Organoids
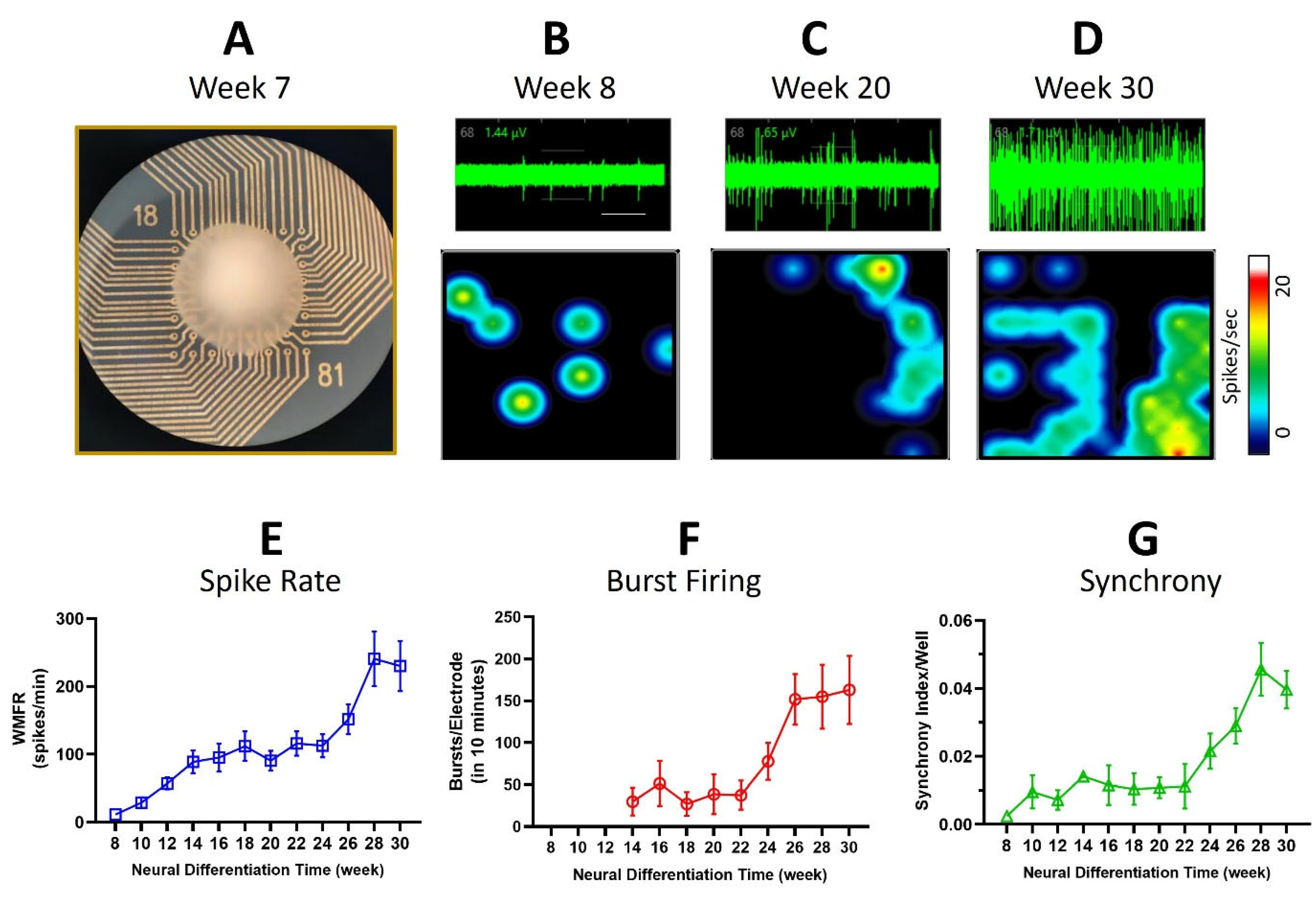
3.2. Multi-Electrode Array Recordings
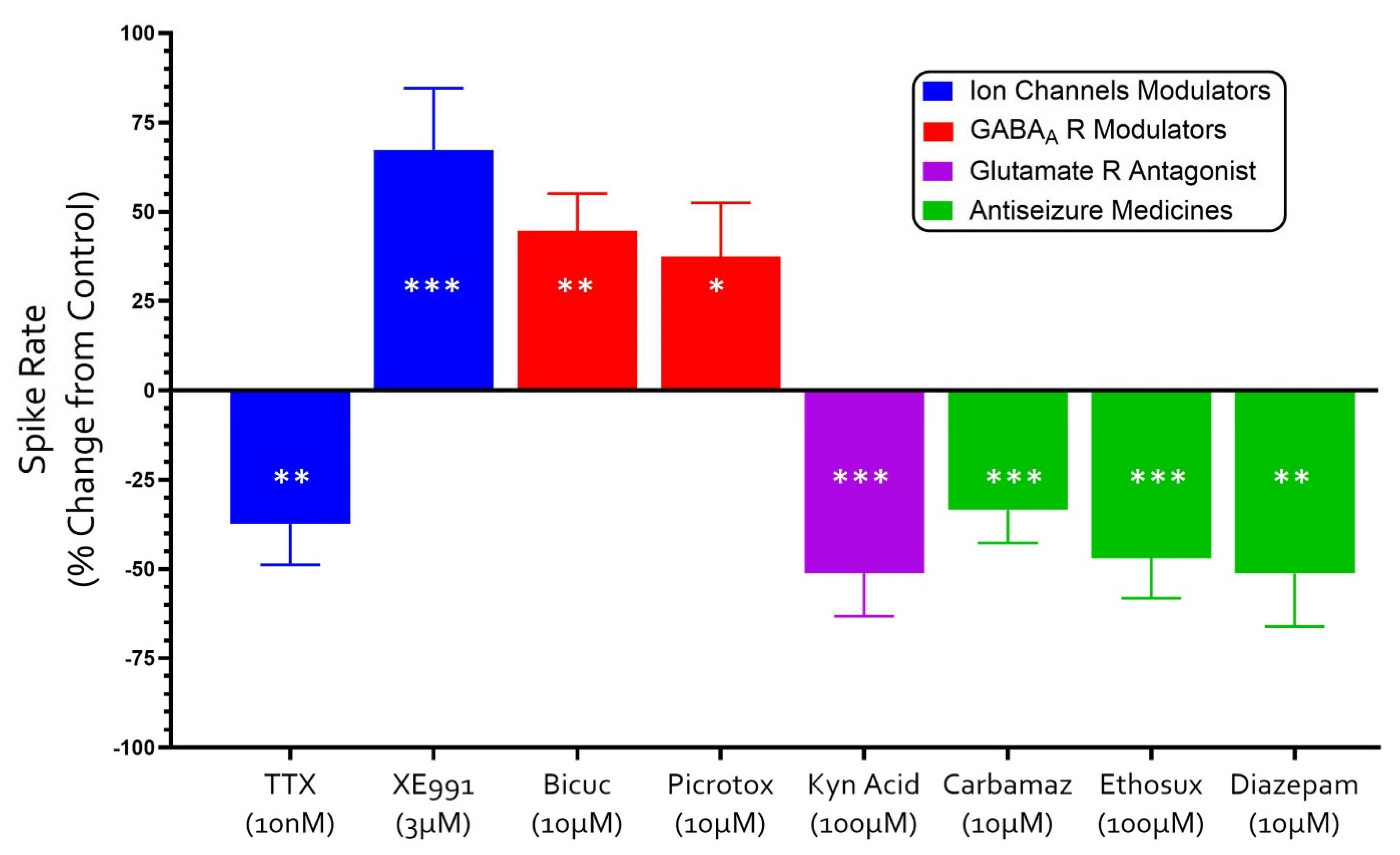
3.3. Neuropharmacology of Electrochemical Signaling in Neural Organoids
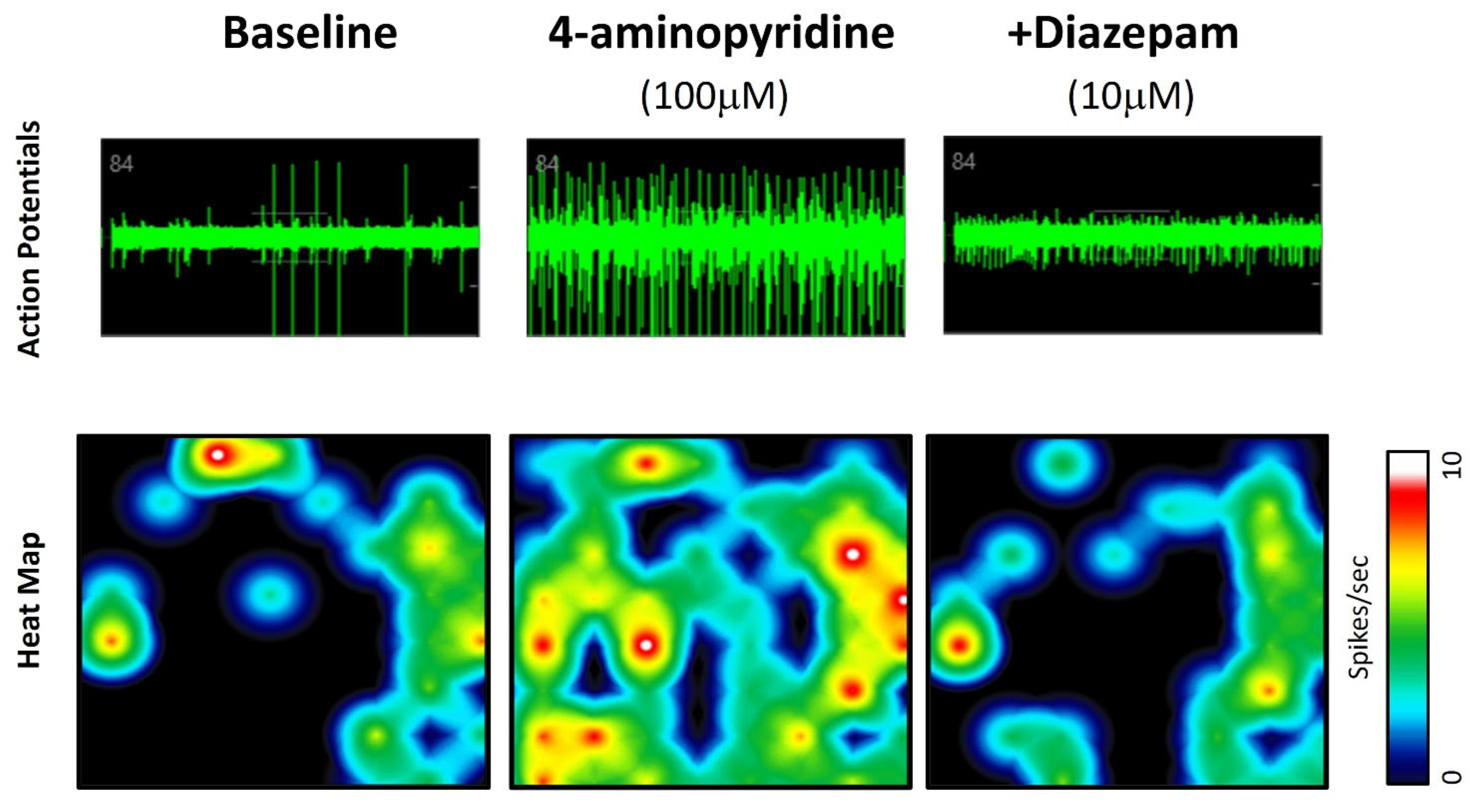
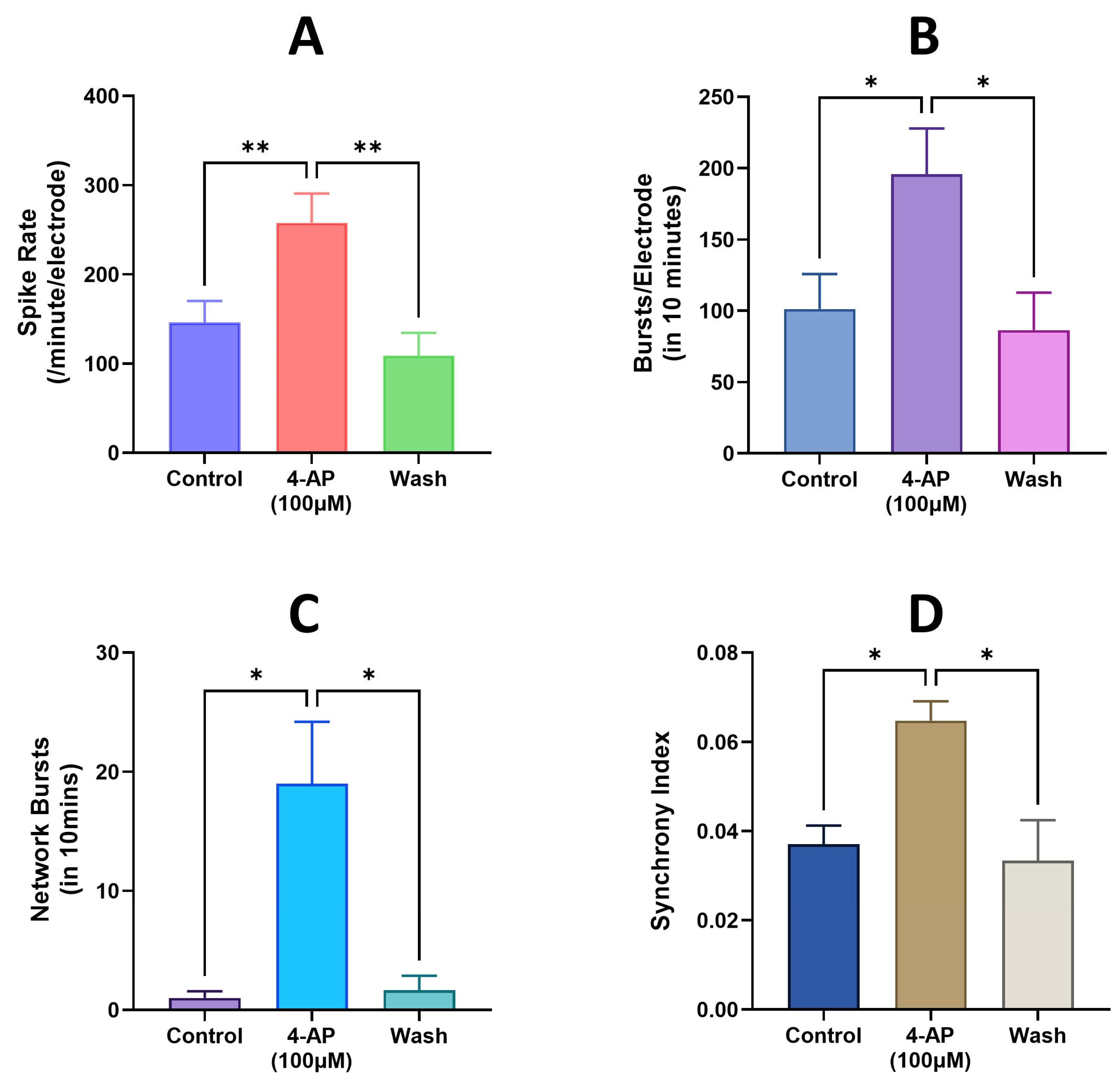
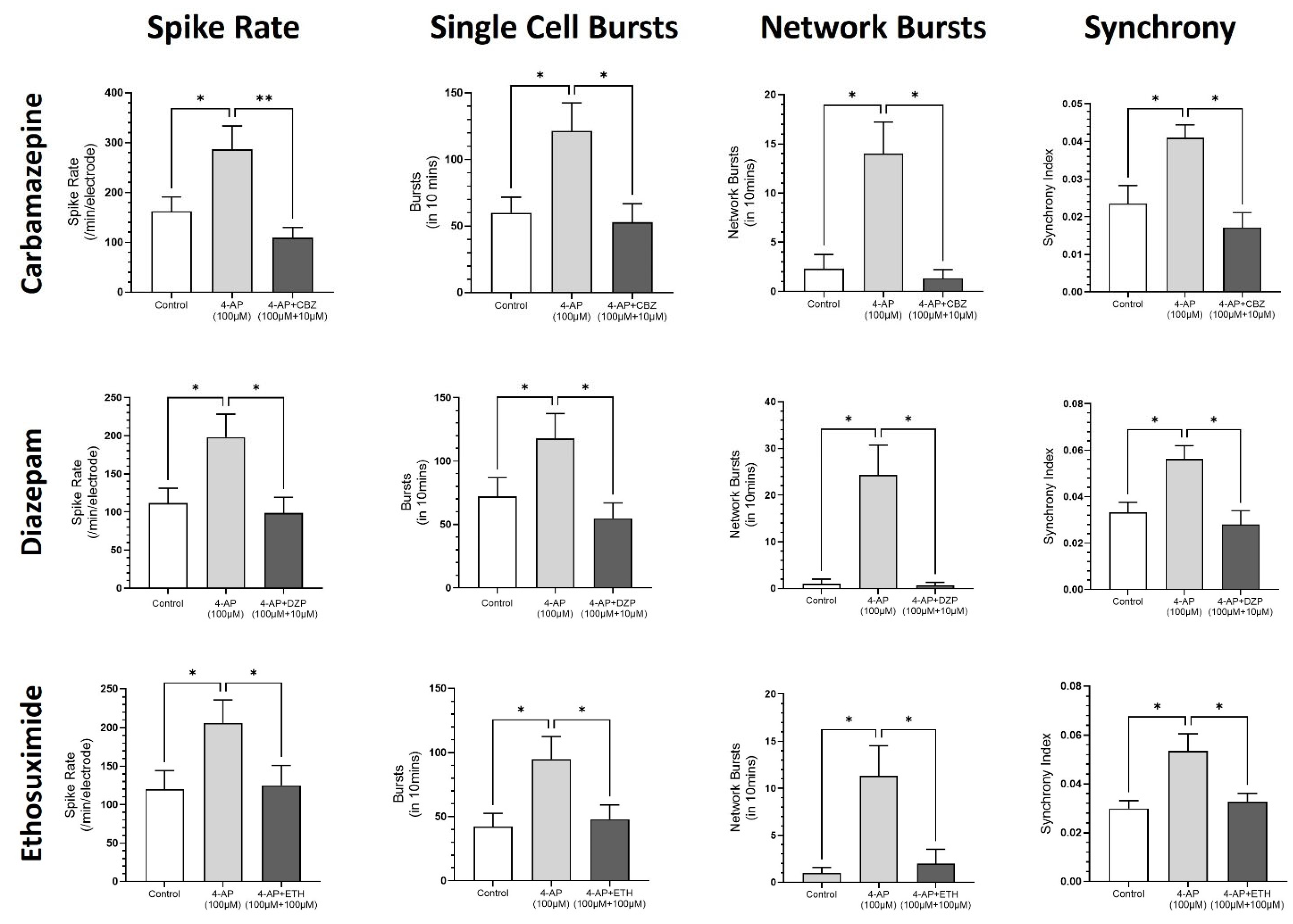
3.4. Modeling Epileptiform Activity in Neural Organoids and the Impact of Anticonvulsants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Bhattacharyya, A. Human Models Are Needed for Studying Human Neurodevelopmental Disorders. Am. J. Hum. Genet. 2018, 103, 829–857. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, C.L.; Sirven, J.I.; Feyissa, A.M. The evolution of antiseizure medication therapy selection in adults: Is artificial intelligence-assisted antiseizure medication selection ready for prime time? J. Cent. Nerv. Syst. Dis. 2023, 15, 11795735231209209. [Google Scholar] [CrossRef] [PubMed]
- Hakami, T. Neuropharmacology of antiseizure drugs. Neuropsychopharmacol. Rep. 2021, 41, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef]
- Lybrand, Z.R.; Goswami, S.; Hsieh, J. Stem cells: A path towards improved epilepsy therapies. Neuropharmacology 2020, 168, 107781. [Google Scholar] [CrossRef]
- Salmanzadeh, H.; Poojari, A.; Rabiee, A.; Zeitlin, B.D.; Halliwell, R.F. Neuropharmacology of human TERA2.cl.SP12 stem cell-derived neurons in ultra-long-term culture for antiseizure drug discovery. Front. Neurosci. 2023, 17, 1182720. [Google Scholar] [CrossRef]
- Salmanzadeh, H.; Halliwell, R.F. Antiseizure properties of fenamate NSAIDs determined in mature human stem-cell derived neuroglial circuits. Front. Pharmacol. 2024, 15, 1385523. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Birtele, M.; Lancaster, M.; Quadrato, G. Modelling human brain development and disease with organoids. Nat. Rev. Mol. Cell Biol. 2025, 26, 389–412. [Google Scholar] [CrossRef]
- Nieto-Estévez, V.; Hsieh, J. Human Brain Organoid Models of Developmental Epilepsies. Epilepsy Curr. 2020, 20, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.; Hartung, T. The Promise and Potential of Brain Organoids. Adv. Healthc. Mater. 2024, 13, e2302745. [Google Scholar] [CrossRef]
- Danačíková, Š.; Straka, B.; Daněk, J.; Kořínek, V.; Otáhal, J. In vitro human cell culture models in a bench-to-bedside approach to epilepsy. Epilepsia Open 2024, 9, 865–890. [Google Scholar] [CrossRef]
- Tukker, A.M.; Westerink, R.H.S. Novel test strategies for in vitro seizure liability assessment. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.; Knoblich, J. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Przyborski, S.A. Isolation of human embryonal carcinoma stem cells by immunomagnetic sorting. Stem Cells 2001, 19, 500–504. [Google Scholar] [CrossRef]
- Andrews, P.W. Human pluripotent stem cells: Tools for regenerative medicine. Biomater. Transl. 2021, 2, 294–300. [Google Scholar]
- Stewart, R.; Coyne, L.; Lako, M.; Halliwell, R.F.; Przyborski, S.A. Human embryonal carcinoma stem cells expressing green fluorescent protein form functioning neurons in vitro: A research tool for co-culture studies. Stem Cells Dev. 2004, 13, 646–657. [Google Scholar] [CrossRef]
- Coyne, L.; Shan, M.; Przyborski, S.A.; Hirakawa, R.; Halliwell, R.F. Neuropharmacological properties of neurons derived from human stem cells. Neurochem. Int. 2011, 59, 404–412. [Google Scholar] [CrossRef]
- Kandel, E.R.; Koester, J.D.; Mack, S.H.; Siegelbaum, S.A. (Eds.) Principles of Neural Science, 6th ed.; McGraw Hill: New York, NY, USA, 2021. [Google Scholar]
- Halliwell, R.F. Electrophysiological properties of neurons derived from human stem cells and iNeurons in vitro. Neurochem. Int. 2017, 106, 37–47. [Google Scholar] [CrossRef]
- Ventura-Mejía, C.; Nuñez-Ibarra, B.H.; Medina-Ceja, L. An update of 4-aminopyride as a useful model of generalized seizures for testing antiseizure drugs: In vitro and in vivo studies. Acta Neurobiol. Exp. 2023, 83, 63–70. [Google Scholar] [CrossRef]
- Heuzeroth, H.; Wawra, M.; Fidzinski, P.; Dag, R.; Holtkamp, M. The 4-Aminopyridine Model of Acute Seizures in vitro Elucidates Efficacy of New Antiepileptic Drugs. Front. Neurosci. 2019, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Song, H.; Ming, G.L. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, H.T.; Smirnova, L. The Future of 3D Brain Cultures in Developmental Neurotoxicity Testing. Front. Toxicol. 2022, 4, 808620. [Google Scholar] [CrossRef] [PubMed]
- Shafique, S. Stem cell-based region-specific brain organoids: Novel models to understand neurodevelopmental defects. Birth Defects Res. 2022, 114, 1003–1013. [Google Scholar] [CrossRef]
- Samarasinghe, R.A.; Miranda, O.A.; Buth, J.E.; Mitchell, S.; Ferando, I.; Watanabe, M.; Allison, T.F.; Kurdian, A.; Fotion, N.N.; Gandal, M.J.; et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 2021, 24, 1488–1500. [Google Scholar] [CrossRef]
- Carvill, G.L.; Dulla, C.G.; Lowenstein, D.H.; Brooks-Kayal, A.R. The path from scientific discovery to cures for epilepsy. Neuropharmacology 2020, 167, 107702. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Rabeling, A.; Goolam, M. Progress and potential of brain organoids in epilepsy research. Stem Cell Res. Ther. 2024, 15, 361. [Google Scholar] [CrossRef]
- Kelava, I.; Lancaster, M.A. Stem Cell Models of Human Brain Development. Cell Stem Cell. 2016, 18, 736–748. [Google Scholar] [CrossRef]
- Kim, S.H.; Chang, M.Y. Application of Human Brain Organoids-Opportunities and Challenges in Modeling Human Brain Development and Neurodevelopmental Diseases. Int. J. Mol. Sci. 2023, 24, 12528. [Google Scholar] [CrossRef]
- Yakoub, A.M.; Sadek, M. Development and Characterization of Human Cerebral Organoids: An Optimized Protocol. Cell Transplant. 2018, 27, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Yakoub, A.M. Cerebral organoids exhibit mature neurons and astrocytes and recapitulate electrophysiological activity of the human brain. Neural Regen. Res. 2019, 14, 757–761. [Google Scholar] [CrossRef]
- Fair, S.R.; Julian, D.; Hartlaub, A.M.; Pusuluri, S.T.; Malik, G.; Summerfied, T.L.; Zhao, G.; Hester, A.B.; Ackerman, W.E., 4th; Hollingsworth, E.W.; et al. Electrophysiological Maturation of Cerebral Organoids Correlates with Dynamic Morphological and Cellular Development. Stem Cell Rep. 2020, 15, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Forró, C.; Li, T.L.; Miura, Y.; Zaluska, T.J.; Tsai, C.T.; Kanton, S.; McQueen, J.P.; Chen, X.; Mollo, V.; et al. Kirigami electronics for long-term electrophysiological recording of human neural organoids and assembloids. Nat. Biotechnol. 2024, 42, 1836–1843. [Google Scholar] [CrossRef]
- Trujillo, C.A.; Gao, R.; Negraes, P.D.; Gu, J.; Buchanan, J.; Preissl, S.; Wang, A.; Wu, W.; Haddad, G.G.; Chaim, I.A.; et al. Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 2019, 25, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Gnatkovsky, V.; Li, F.R.; Scalmani, P.; Uva, L.; Avoli, M.; de Curtis, M. Fast activity chirp patterns in focal seizures from patients and animal models. Epilepsia 2025, 66, 621–631. [Google Scholar] [CrossRef]
- Yokoi, R.; Shibata, M.; Odawara, A.; Ishibashi, Y.; Nagafuku, N.; Matsuda, N.; Suzuki, I. Analysis of signal components < 500 Hz in brain organoids coupled to microelectrode arrays: A reliable testbed for preclinical seizure liability assessment of drugs and screening of antiepileptic drugs. Biochem. Biophys. Rep. 2021, 28, 101148. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmanzadeh, H.; Halliwell, R.F. Human Stem Cell-Derived Neural Organoids for the Discovery of Antiseizure Agents. Receptors 2025, 4, 12. https://doi.org/10.3390/receptors4030012
Salmanzadeh H, Halliwell RF. Human Stem Cell-Derived Neural Organoids for the Discovery of Antiseizure Agents. Receptors. 2025; 4(3):12. https://doi.org/10.3390/receptors4030012
Chicago/Turabian StyleSalmanzadeh, Hamed, and Robert F. Halliwell. 2025. "Human Stem Cell-Derived Neural Organoids for the Discovery of Antiseizure Agents" Receptors 4, no. 3: 12. https://doi.org/10.3390/receptors4030012
APA StyleSalmanzadeh, H., & Halliwell, R. F. (2025). Human Stem Cell-Derived Neural Organoids for the Discovery of Antiseizure Agents. Receptors, 4(3), 12. https://doi.org/10.3390/receptors4030012




