Glutamate Receptor Signaling in Retina Müller Cells: Plausible Role in Neurodegeneration
Abstract
1. Introduction
2. Glu-Mediated Neurotransmission in the Retina
3. Role of Müller Glial Cells in the Retina
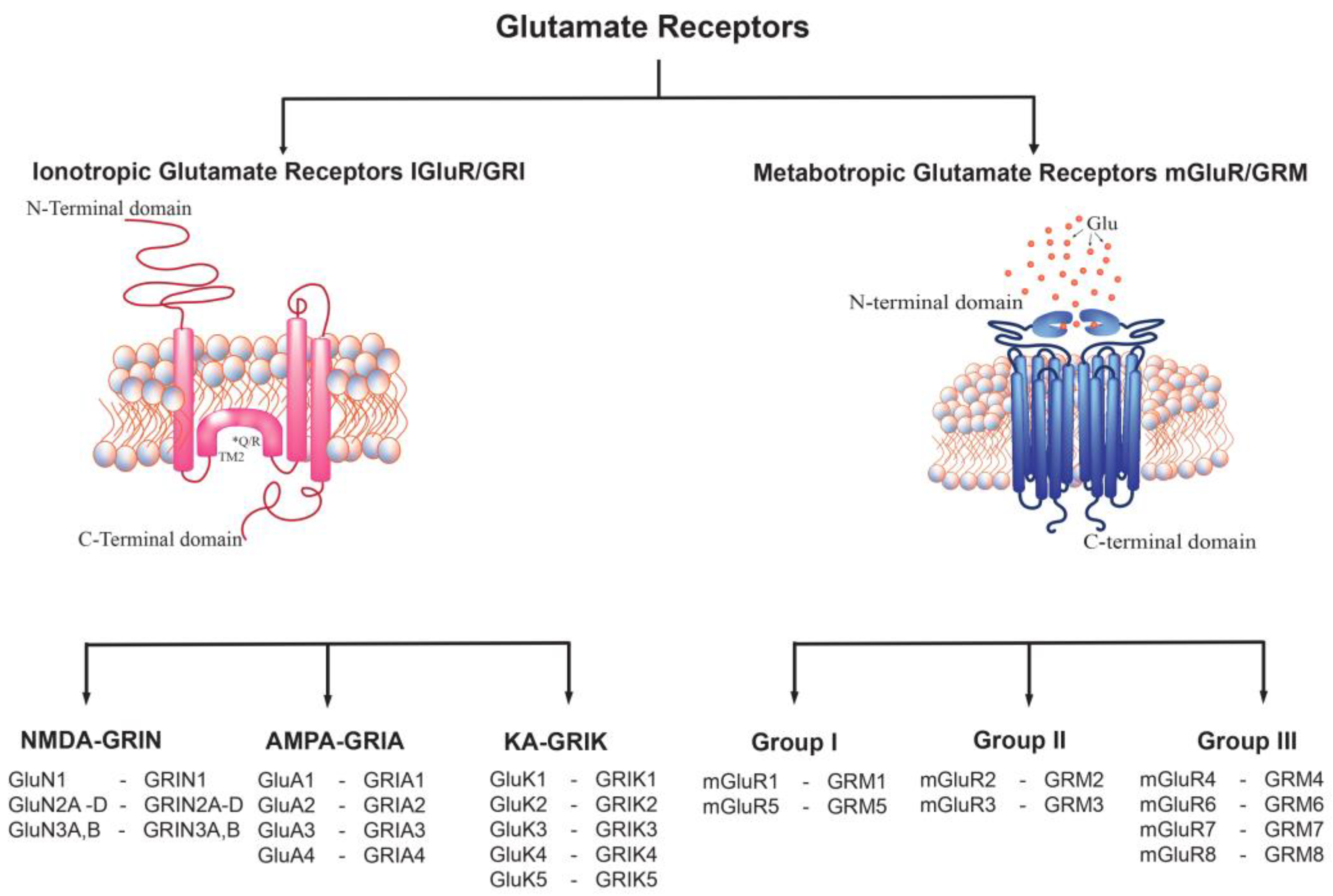
4. Glu Receptor Classification
5. Ionotropic Glu Receptor (GRI)
6. α-amino-3-hydroxy-5-methyl-4-isoxazole Propionate Receptor (GRIA)
7. Kainic Acid Receptor (GRIK)
8. N-methyl-D-Aspartate Receptors (GRIN)
9. Metabotropic Glu Receptors (GRM)
10. Group I GRM
11. Groups II and III mGluRs
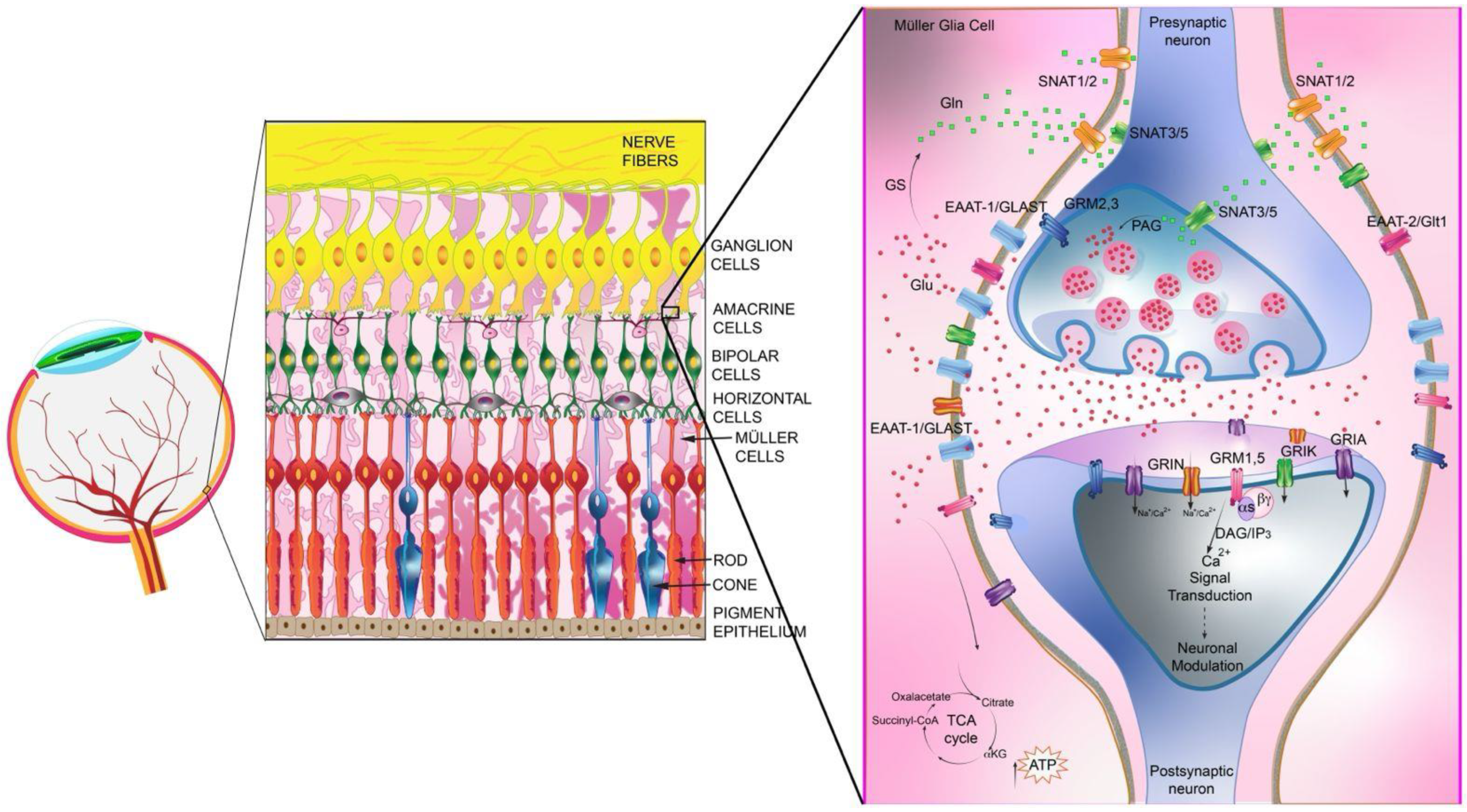
12. Distribution, Expression and Regulation of Glu Receptors in Müller Cells
13. Glu Receptors Localization in Retinal Cells
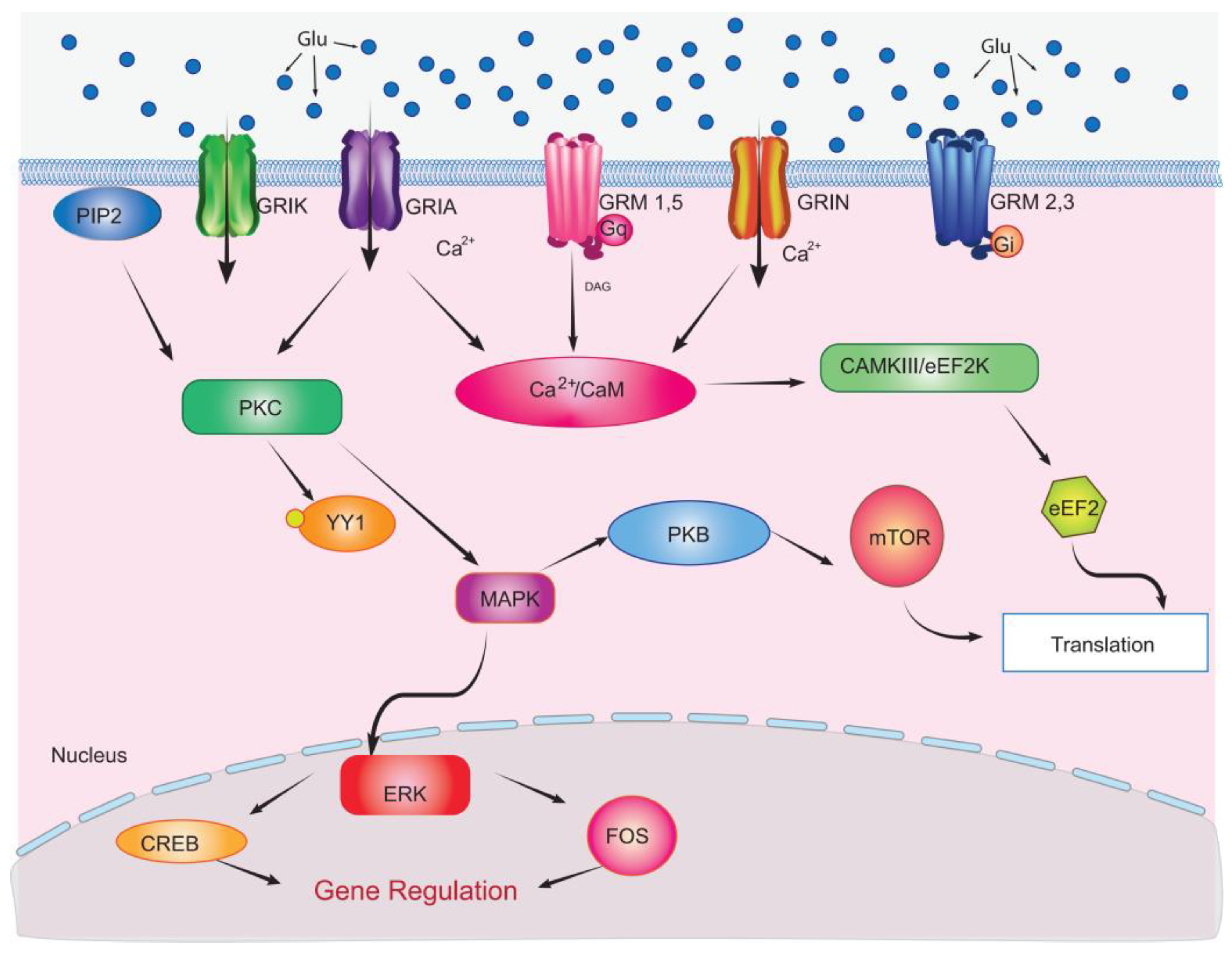
14. Glu Receptor-Mediated Signaling Events in Müller Glial Cells
15. Impact of Glu Receptor Signaling in Excitotoxicity and Retinal Degeneration
16. Role of Müller Glial Cells in Retinal Diseases Prevention
17. Perspectives
18. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Mannu, G.S. Retinal phototransduction. Neurosciences 2014, 19, 275–280. [Google Scholar] [PubMed]
- Ptito, M.; Bleau, M.; Bouskila, J. The Retina: A Window into the Brain. Cells 2021, 10, 3269. [Google Scholar] [CrossRef]
- Rodieck, R.W. The Vertebrate Retina: Principles of Structure and Function; W.H. Freeman & Co Ltd.: San Francisco, CA, USA, 1973. [Google Scholar]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu. Rev. Vis. Sci. 2017, 3, 1–24. [Google Scholar] [CrossRef]
- Baude, A.; Nusser, Z.; Roberts, J.D.B.; Mulvihill, E.; Mcllhinney, R.A.J.; Somogyi, P. The metabotropic glutamate receptor (mGluRlα) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 1993, 11, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto, R.; Kulik, A.; Roberts, J.D.B.; Ohishi, H.; Nusser, Z.; Kaneko, T.; Somogyi, P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 1996, 381, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Franze, K.; Grosche, J.; Skatchkov, S.N.; Schinkinger, S.; Foja, C.; Schild, D.; Uckermann, O.; Travis, K.; Reichenbach, A.; Guck, J. Müller cells are living optical fibers in the vertebrate retina. Proc. Natl. Acad. Sci. USA 2007, 104, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Bringmann, A. New functions of Müller cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.; Osborne, N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Faude, F.; Enzmann, V.; Bringmann, A.; Pannicke, T.; Francke, M.; Biedermann, B.; Kuhrt, H.; Stolzenburg, J.-U.; Skatchkov, S.N.; et al. The Müller (Glial) Cell in normal and diseased retina: A case for single-cell electrophysiology. Ophthalmic Res. 1997, 29, 326–340. [Google Scholar] [CrossRef]
- Tsunoda, S. Organization of Photoreceptor Signaling Complexes. In Handbook of Cell Signaling, 2nd ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 1, pp. 373–377. [Google Scholar] [CrossRef]
- Yin, L.; Smith, R.G.; Sterling, P.; Brainard, D.H. Chromatic properties of horizontal and ganglion cell responses follow a dual gradient in cone opsin expression. J. Neurosci. 2006, 26, 12351–12361. [Google Scholar] [CrossRef] [PubMed]
- Borghuis, B.G.; Ratliff, C.P.; Smith, R.G. Impact of light-adaptive mechanisms on mammalian retinal visual encoding at high light levels. J. Neurophysiol. 2018, 119, 1437–1449. [Google Scholar] [CrossRef]
- Moini, J.; LaGalbo, A.; Ahangari, R. The visual system. In Foundations of the Mind, Brain, and Behavioral Relationships: Understanding Physiological Psychology; Elsevier: London, UK, 2023; Chapter 8; pp. 125–142. [Google Scholar]
- Remington, L.A.; Goodwin, D. Retina. In Clinical Anatomy and Physiology of the Visual System, 4th ed.; Elsevier: St. Louis, MO, USA, 2022; Chapter 8; pp. 111–139. [Google Scholar]
- Jo, A.; Deniz, S.; Xu, J.; Duvoisin, R.M.; DeVries, S.H.; Zhu, Y. A sign-inverted receptive field of inhibitory interneurons provides a pathway for ON-OFF interactions in the retina. Nat. Commun. 2023, 14, 5937. [Google Scholar] [CrossRef] [PubMed]
- Dieck, S.T.; Brandstätter, J.H. Ribbon synapses of the retina. Cell Tissue Res. 2006, 326, 339–346. [Google Scholar] [CrossRef]
- Reichenbach, A.; Henke, A.; Eberhardt, W.; Reichelt, W.; Dettmer, D. K+ ion regulation in retina. Can. J. Physiol. Pharmacol. 1992, 70, S239–S247. [Google Scholar] [CrossRef] [PubMed]
- Fykse, E.M.; Fonnum, F. Amino acid neurotransmission: Dynamics of vesicular uptake. Neurochem. Res. 1996, 21, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.B. Phototransduction. In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 687–692. [Google Scholar] [CrossRef]
- Thoreson, W.B.; Witkovsky, P. Glutamate receptors and circuits in the vertebrate retina. Prog. Retin. Eye Res. 1999, 18, 765–810. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.C.; Miller, R.F. N-methyl-D-aspartate receptors of ganglion cells in rabbit retina. J. Neurophysiol. 1990, 63, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.C. Cell types using glutamate as a neurotransmitter in the vertebrate retina. Prog. Retin. Res. 1990, 9, 399–425. [Google Scholar] [CrossRef]
- Naito, S.; Ueda, T. Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J. Biol. Chem. 1983, 258, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Tabb, J.S.; Ueda, T. Phylogenetic studies on the synaptic vesicle glutamate transport system. J. Neurosci. 1991, 11, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- DeVries, S.H. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 2000, 28, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Borghuis, B.G.; Looger, L.L.; Tomita, S.; Demb, J.B. Kainate Receptors Mediate Signaling in Both Transient and Sustained OFF Bipolar Cell Pathways in Mouse Retina. J. Neurosci. 2014, 34, 6128–6139. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Habib, S. On and off signaling pathways in the retina and the visual system. Front. Ophthalmol. 2022, 2, 989002. [Google Scholar] [CrossRef] [PubMed]
- Puthussery, T.; Percival, K.A.; Venkataramani, S.; Gayet-Primo, J.; Grünert, U.; Taylor, W.R. Kainate receptors mediate synaptic input to transient and sustained OFF visual pathways in primate retina. J. Neurosci. 2014, 34, 7611–7621. [Google Scholar] [CrossRef]
- Nakajima, Y.; Iwakabe, H.; Akazawa, C.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J. Biol. Chem. 1993, 268, 11868–11873. [Google Scholar] [CrossRef]
- Ichinose, T.; Hellmer, C.B. Differential signalling and glutamate receptor compositions in the OFF bipolar cell types in the mouse retina. J. Physiol. 2016, 594, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Popova, E. ON-OFF Interactions in the Retina: Role of Glycine and GABA. Curr. Neuropharmacol. 2015, 12, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.; Fuchs, P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Aizenman, E.; Frosch, M.P.; Lipton, S.A. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J. Physiol. 1988, 396, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kaneko, A. L-Glutamate-induced responses in OFF-type bipolar cells of the cat retina. Vis. Res. 1996, 36, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Bañuelos, T.N.; Chí-Castañeda, D.; Ortega, A. Glutamate transporters: Gene expression regulation and signaling properties. Neuropharmacology 2019, 161, 107550. [Google Scholar] [CrossRef]
- Kanai, Y.; Hediger, M.A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 1992, 360, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Pines, G.; Danbolt, N.C.; Bjørås, M.; Zhang, Y.; Bendahan, A.; Eide, L.; Koepsell, H.; Storm-Mathisen, J.; Seeberg, E.; Kanner, B.I. Cloning and expression of a rat brain L-glutamate transporter. Nature 1992, 360, 464–467. [Google Scholar] [CrossRef]
- Schultz, K.; Stell, W.K. Immunocytochemical localization of the high-affinity glutamate transporter, EAAC1, in the retina of representative vertebrate species. Neurosci. Lett. 1996, 211, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Campuzano, A.G.; Ortega, A. Glutamate transporters: Critical components of glutamatergic transmission. Neuropharmacology 2021, 192, 108602. [Google Scholar] [CrossRef]
- Ward, M.M.; Jobling, A.I.; Puthussery, T.; Foster, L.E.; Fletcher, E.L. Localization and expression of the glutamate transporter, excitatory amino acid transporter 4, within astrocytes of the rat retina. Cell Tissue Res. 2004, 315, 305–310. [Google Scholar] [CrossRef]
- Fyk-Kolodziej, B.; Qin, P.; Dzhagaryan, A.; Pourcho, R.G. Differential cellular and subcellular distribution of glutamate transporters in the cat retina. Vis. Neurosci. 2004, 21, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Arriza, J.L.; Eliasof, S.; Kavanaugh, M.P.; Amara, S.G. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. USA 1997, 94, 4155–4160. [Google Scholar] [CrossRef]
- Schneider, N.; Cordeiro, S.; Machtens, J.P.; Braams, S.; Rauen, T.; Fahlke, C. Functional properties of the retinal glutamate transporters GLT-1c and EAAT5. J. Biol. Chem. 2014, 289, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Brew, H.; Attwell, D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 1987, 327, 707–709. [Google Scholar] [CrossRef]
- Eliasof, S.; Werblin, F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. J. Neurosci. 1993, 13, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—What is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Shigeri, Y.; Seal, R.P.; Shimamoto, K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res. Rev. 2004, 45, 250–265. [Google Scholar] [CrossRef]
- Hayashi, M.K. Structure-Function Relationship of Transporters in the Glutamate-Glutamine Cycle of the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 1177. [Google Scholar] [CrossRef] [PubMed]
- Sarthy, V.P.; Pignataro, L.; Pannicke, T.; Weick, M.; Reichenbach, A.; Harada, T.; Tanaka, K.; Marc, R. Glutamate transport by retinal Müller cells in glutamate/aspartate transporter-knockout mice. Glia 2005, 49, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Magi, S.; Piccirillo, S.; Amoroso, S.; Lariccia, V. Excitatory amino acid transporters (Eaats): Glutamate transport and beyond. Int. J. Mol. Sci. 2019, 20, 5674. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Swanson, R.A. Astrocyte Glutamate Transport: Review of Properties, Regulation, and Physiological Functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef]
- Vandenberg, R.J.; Ryan, R.M. Mechanisms of glutamate transport. Physiol. Rev. 2013, 93, 1621–1657. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Cameron, R.S.; Rakic, P. Glial cell lineage in the cerebral cortex: A review and synthesis. Glia 1991, 4, 124–137. [Google Scholar] [CrossRef] [PubMed]
- López, T.; López-Colomé, A.M.; Ortega, A. Changes in GluR4 expression induced by metabotropic receptor activation in radial glia cultures. Mol. Brain Res. 1998, 58, 40–46. [Google Scholar] [CrossRef]
- Kettenmann, H. Beyond the neuronal circuitry: Introduction. Trends Neurosci. 1996, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J.; Alvarado, J.A.; Weddell, J.E. Histology of the Human Eye: An Atlas and Textbook, 1st ed.; Saunders: St. Louis, MO, USA, 1971. [Google Scholar]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Jeon, C.J.; Strettoi, E.; Masland, R.H. The major cell populations of the mouse retina. J. Neurosci. 1998, 18, 8936–8946. [Google Scholar] [CrossRef] [PubMed]
- Grosche, J.; Härtig, W.; Reichenbach, A. Expression of glial fibrillary acidic protein (GFAP), glutamine synthetase (GS), and Bcl-2 protooncogene protein by Müller (glial) cells in retinal light damage of rats. Neurosci. Lett. 1995, 185, 119–122. [Google Scholar] [CrossRef]
- Riepe, R.E.; Norenburg, M.D. Müller cell localisation of glutamine synthetase in rat retina. Nature 1977, 268, 654–655. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.A.; Boerkoel, P.; Hirsch-Reinshagen, V.; Mackenzie, I.R.; Hsiung, G.-Y.R.; Charm, G.; To, E.F.; Liu, A.Q.; Schwab, K.; Jiang, K.; et al. Müller cell degeneration and microglial dysfunction in the Alzheimer’s retina. Acta Neuropathol. Commun. 2022, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Malchow, R.P.; Qian, H.; Ripps, H. gamma-Aminobutyric acid (GABA)-induced currents of skate Muller (glial) cells are mediated by neuronal-like GABAA receptors. Proc. Natl. Acad. Sci. USA 1989, 86, 4326–4330. [Google Scholar] [CrossRef] [PubMed]
- Wakakura, M.; Yamamoto, N. Cytosolic calcium transient increase through the AMPA/kainate receptor in cultured Müller cells. Vis. Res. 1994, 34, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Boccuni, I.; Fairless, R. Retinal Glutamate Neurotransmission: From Physiology to Pathophysiological Mechanisms of Retinal Ganglion Cell Degeneration. Life 2022, 12, 638. [Google Scholar] [CrossRef]
- Erecińska, M.; Silver, I.A. Metabolism and role of glutamate in mammalian brain. Prog. Neurobiol. 1990, 35, 245–296. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Biedermann, B.; Francke, M.; Iandiev, I.; Grosche, J.; Wiedemann, P.; Albrecht, J.; Reichenbach, A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem. Int. 2009, 54, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Poitry-Yamate, C.; Gianoncelli, A.; Kaulich, B.; Kourousias, G.; Magill, A.W.; Lepore, M.; Gajdosik, V.; Gruetter, R. Feasibility of direct mapping of cerebral fluorodeoxy-D-glucose metabolism in situ at subcellular resolution using soft X-ray fluorescence. J. Neurosci. Res. 2013, 91, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- López-Bayghen, E.; Ortega, A. Glial glutamate transporters: New actors in brain signaling. IUBMB Life 2011, 63, 816–823. [Google Scholar] [CrossRef]
- Newman, E.; Reichenbach, A. The Müller cell: A functional element of the retina. Trends Neurosci. 1996, 19, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lozada, Z.; Guillem, A.M.; Robinson, M.B. Transcriptional Regulation of Glutamate Transporters: From Extracellular Signals to Transcription Factors. In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2016; Volume 76, pp. 103–145. [Google Scholar] [CrossRef]
- Martínez-Lozada, Z.; Ortega, A. Glutamatergic transmission: A matter of three. Neural Plast. 2015, 2015, 787396. [Google Scholar] [CrossRef]
- Flores-Méndez, M.; Mendez-Flores, O.G.; Ortega, A. Glia plasma membrane transporters: Key players in glutamatergic neurotransmission. Neurochem. Int. 2016, 98, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Linser, P.; Moscona, A.A. Induction of glutamine synthetase in embryonic neural retina: Localization in Müller fibers and dependence on cell interactions. Proc. Natl. Acad. Sci. USA 1979, 76, 6476–6480. [Google Scholar] [CrossRef]
- Lewis, G.P.; Erickson, P.A.; Kaska, D.D.; Fisher, S.K. An immunocytochemical comparison of Müller cells and astrocytes in the cat retina. Exp. Eye Res. 1988, 47, 839–853. [Google Scholar] [CrossRef]
- López-Bayghen, E.; Rosas, S.; Castelán, F.; Ortega, A. Cerebellar Bergmann glia: An important model to study neuron-glia interactions. Neuron Glia Biol. 2007, 3, 155–167. [Google Scholar] [CrossRef]
- Heidinger, V.R.; Dreyfus, H.; Sahel, J.; Christen, Y.; Hicks, D. Excitotoxic Damage of Retinal Glial Cells Depends Upon Normal Neuron-Glial Interactions. Glia 1998, 23, 146–155. [Google Scholar] [CrossRef]
- Connaughton, V. Glutamate and Glutamate Receptors in the Vertebrate Retina. 1995. Available online: https://webvision.med.utah.edu/book/part-v-phototransduction-in-rods-and-cones/glutamate-and-glutamate-receptors-in-the-vertebrate-retina/ (accessed on 12 January 2023).
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Hansen, K.B.; Furukawa, H.; Traynelis, S.F. Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol. Pharmacol. 2010, 78, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, K.; Wisden, W.; Sommer, B.; Werner, P.; Herb, A.; Verdoorn, T.A.; Sakmann, B.; Seeburg, P.H. A family of AMPA-selective glutamate receptors. Science 1990, 249, 556–560. [Google Scholar] [CrossRef]
- Sommer, B.; Keinänen, K.; Verdoorn, T.A.; Wisden, W.; Burnashev, N.; Herb, A.; Kohler, M.; Takagi, T.; Sakmann, B.; Seeburg, P.H. Flip and flop: A cell-specific functional switch in glutamate-operated channels of the CNS. Science 1990, 249, 1580–1585. [Google Scholar] [CrossRef]
- Bleakman, D.; Lodge, D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology 1998, 37, 1187–1204. [Google Scholar] [CrossRef]
- Bardoul, M.; Levallois, C.; König, N. Functional AMPA/kainate receptors in human embryonic and foetal central nervous system. J. Chem. Neuroanat. 1998, 14, 79–85. [Google Scholar] [CrossRef]
- Orlandi, C.; Barbon, A.; Barlati, S. Activity regulation of adenosine deaminases acting on RNA (ADARs). Mol. Neurobiol. 2012, 45, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Wulff, B.E.; Nishikura, K. Substitutional A-to-I RNA editing. Wiley Interdiscip. Rev. RNA 2010, 1, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kamalova, A.; Nakagawa, T. AMPA receptor structure and auxiliary subunits. J. Physiol. 2021, 599, 453–469. [Google Scholar] [CrossRef]
- Noda, M.; Nakanishi, H.; Nabekura, J.; Akaike, N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci. 2000, 20, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.D.; Huganir, R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007, 23, 613–643. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.T.; Kamboj, S.K.; Cull-Candy, S.G. Single-Channel Properties of Recombinant AMPA Receptors Depend on RNA Editing, Splice Variation, and Subunit Composition. J. Neurosci. 1997, 17, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, M.; Hartley, M.; Heinemann, S. Ca2+ permeability of KA-AMPA—Gated glutamate receptor channels depends on subunit composition. Science 1991, 252, 851–853. [Google Scholar] [CrossRef]
- Geiger, J.R.P.; Melcher, T.; Koh, D.-S.; Sakmann, B.; Seeburg, P.; Jonas, P.; Monyer, H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 1995, 15, 193–204. [Google Scholar] [CrossRef]
- Tomiyama, M.; Rodríguez-Puertas, R.; Cortés, R.; Pazos, A.; Palacios, J.M.; Mengod, G. Flip and flop splice variants of AMPA receptor subunits in the spinal cord of amyotrophic lateral sclerosis. Synapse 2002, 45, 245–249. [Google Scholar] [CrossRef]
- Borges, K.; Kettenmann, H. Blockade of K+ channels induced by AMPA/kainate receptor activation in mouse oligodendrocyte precursor cells is mediated by NA+ entry. J. Neurosci. Res. 1995, 42, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, S.; Tsuzuki, K.; Yoshida, Y.; Yamada, N.; Hagimura, N.; Okado, H.; Miwa, A.; Kurihara, H.; Nakazato, Y.; Tamura, M.; et al. Blockage of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat. Med. 2002, 8, 971–978. [Google Scholar] [CrossRef]
- Iino, M.; Goto, K.; Kakegawa, W.; Okado, H.; Sudo, M.; Ishiuchi, S.; Miwa, A.; Takayasu, Y.; Saito, I.; Tsuzuki, K.; et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 2001, 292, 926–929. [Google Scholar] [CrossRef]
- Fannon, J.; Tarmier, W.; Fulton, D. Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 2015, 63, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Pircher, B.; Pircher, T.; Feigenspan, A. Ionotropic glutamate receptors in the retina—A bioinformatical meta-analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bahn, S.; Volk, B.; Wisden, W. Kainate receptor gene expression in the developing rat brain. J. Neurosci. 1994, 14, 5525–5547. [Google Scholar] [CrossRef]
- Chittajallu, R.; Vignes, M.; Dev, K.K.; Barnes, J.M.; Collingridge, G.L.; Henley, J.M. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 1996, 379, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.J.; Lerma, J. Metabotropic signaling by kainate receptors. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 399–410. [Google Scholar] [CrossRef]
- Rojas, A.; Wetherington, J.; Shaw, R.; Serrano, G.; Swanger, S.; Dingledine, R. Activation of group I metabotropic glutamate receptors potentiates heteromeric kainate receptors. Mol. Pharmacol. 2013, 83, 106–121. [Google Scholar] [CrossRef]
- Rojas, A.; Gueorguieva, P.; Lelutiu, N.; Quan, Y.; Shaw, R.; Dingledine, R. The prostaglandin EP1 receptor potentiates kainate receptor activation via a protein kinase C pathway and exacerbates status epilepticus. Neurobiol. Dis. 2014, 70, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Moreno, A.; Sihra, T.S. Metabotropic actions of kainate receptors in the CNS. J. Neurochem. 2007, 103, 2121–2135. [Google Scholar] [CrossRef]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Cull-Candy, S.G.; Leszkiewicz, D.N. Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE 2004, 2004, re16. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Ciabarra, A.M.; Sullivan, J.M.; Gahn, L.G.; Pecht, G.; Heinemann, S.; Sevarino, K.A. Cloning and characterization of chi-1: A developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J. Neurosci. 1995, 15, 6498–6508. [Google Scholar] [CrossRef]
- Sucher, N.J.; Akbarian, S.; Chi, C.; Leclerc, C.; Awobuluyi, M.; Deitcher, D.; Wu, M.; Yuan, J.; Jones, E.; Lipton, S. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J. Neurosci. 1995, 15, 6509–6520. [Google Scholar] [CrossRef]
- Chatterton, J.E.; Awobuluyi, M.; Premkumar, L.S.; Takahashi, H.; Talantova, M.; Shin, Y.; Cui, J.; Tu, S.; Sevarino, K.A.; Nakanishi, N.; et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 2002, 415, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Erreger, K.; Chen, P.E.; Wyllie, D.J.A.; Traynelis, S.F. Glutamate receptor gating. Crit. Rev. Neurobiol. 2004, 16, 187–224. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Gouaux, E. Mechanisms of activation, inhibition and specificity: Crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003, 22, 2873–2885. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Dunah, A.W.; Wang, Y.T.; Sheng, M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 2005, 46, 745–760. [Google Scholar] [CrossRef]
- Strack, S.; Colbran, R.J. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 1998, 273, 20689–20692. [Google Scholar] [CrossRef] [PubMed]
- Barria, A.; Malinow, R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 2005, 48, 289–301. [Google Scholar] [CrossRef]
- Lee, H.K. Synaptic plasticity and phosphorylation. Pharmacol. Ther. 2006, 112, 810–832. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cornejo-Córdova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Paquet, M.; Cregan, S.P.; Ferguson, S.G. Group I Metabotropic Glutamate Receptor Signalling and its Implication in Neurological Disease. CNS Neurol. Disord. Drug Targets 2010, 9, 574–595. [Google Scholar] [CrossRef] [PubMed]
- Lamas, M.; Lee-Rivera, I.; Lòpez-Colomè, A.M. Cell-specific expression of N-methyl-D-aspartate receptor subunits in Müller glia and neurons from the chick retina. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- López, T.; López-Colomé, A.M.; Ortega, A. NMDA receptors in cultured radial glia. FEBS Lett. 1997, 405, 245–248. [Google Scholar] [CrossRef]
- López-Colomé, A.M.; López, E.; Mendez-Flores, O.G.; Ortega, A. Glutamate Receptor Stimulation Up-Regulates Glutamate Uptake in Human Müller Glia Cells. Neurochem. Res. 2016, 41, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Pankratov, Y.; Parpura, V.; Verkhratsky, A. Ionotropic receptors in neuronal-astroglial signalling: What is the role of ‘excitable’ molecules in non-excitable cells. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 992–1002. [Google Scholar] [CrossRef]
- Clarke, R.J.; Johnson, J.W. Voltage-dependent gating of NR1/2B NMDA receptors. J. Physiol. 2008, 586 Pt 23, 5727–5741. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Pankratov, Y.; Kirchhoff, F.; North, R.A.; Verkhratsky, A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 2006, 26, 2673–2683. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Chvátal, A. NMDA Receptors in Astrocytes. Neurochem. Res. 2020, 45, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Semyanov, A.; Zorec, R. Physiology of Astroglial Excitability. Function 2020, 1, zqaa016. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, F.; Shigemoto, R. Metabotropic glutamate receptors. Cell Tissue Res. 2006, 326, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, D.J.; Gregory, K.J.; Rook, J.M.; Conn, P.J. Allosteric Modulation of Metabotropic Glutamate Receptors. Adv. Pharmacol. 2011, 62, 37–77. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef] [PubMed]
- Rabeh, N.; Hajjar, B.; Maraka, J.O.; Sammanasunathan, A.F.; Khan, M.; Alkhaaldi, S.M.; Mansour, S.; Almheiri, R.T.; Hamdan, H.; Abd-Elrahman, K.S. Targeting mGluR group III for the treatment of neurodegenerative diseases. Biomed. Pharmacother. 2023, 168, 115733. [Google Scholar] [CrossRef]
- Suh, Y.H.; Chang, K.; Roche, K.W. Metabotropic glutamate receptor trafficking. Mol. Cell. Neurosci. 2018, 91, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.C.; Xiao, B.; Naisbitt, S.; Yuan, J.P.; Petralia, R.S.; Brakeman, P.; Doan, A.; Aakalu, V.K.; Lanahan, A.A.; Sheng, M.; et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 1999, 23, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.Y.; Skeberdis, V.A.; Jover, T.; Zheng, X.; Bennett, M.V.L.; Zukin, R.S. Activation of Metabotropic Glutamate Receptor 1 Accelerates NMDA Receptor Trafficking. J. Neurosci. 2001, 21, 6058–6068. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Bailey, C.P.; Henderson, G. Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol. 2008, 153, S379–S388. [Google Scholar] [CrossRef]
- Hanyaloglu, A.C.; Von Zastrow, M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 537–568. [Google Scholar] [CrossRef]
- Nelson, C.P.; Challiss, R.A.J. ‘Phenotypic’ pharmacology: The influence of cellular environment on G protein-coupled receptor antagonist and inverse agonist pharmacology. Biochem. Pharmacol. 2007, 73, 737–751. [Google Scholar] [CrossRef]
- Ramírez, J.L.; Watt, H.L.; Rocheville, M.; Kumar, U. Agonist-induced up-regulation of human somatostatin receptor type 1 is regulated by β-arrestin-1 and requires an essential serine residue in the receptor C-tail. Biochim. Biophys. Acta 2005, 1669, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Achour, L.; Labbé-Jullié, C.; Scott, M.G.H.; Marullo, S. An escort for GPCRs: Implications for regulation of receptor density at the cell surface. Trends Pharmacol. Sci. 2008, 29, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Dhami, G.K.; Ferguson, S.S.G. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol. Ther. 2006, 111, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Valiante, T.A.; Carlen, P.L.; Pennefather, P.S.; Young, S.R.; Bianchi, R.; Wong, R.K.S.; Sekizawa, S.-I.; Bonham, A.C.; Ireland, D.R.; et al. Metabotropic glutamate receptors coupled to IP3 production mediate inhibition of IAHP in rat dentate granule neurons. J. Neurophysiol. 1996, 76, 2691–2700. [Google Scholar] [CrossRef]
- Bhattacharyya, S. Inside story of Group I Metabotropic Glutamate Receptors (mGluRs). Int. J. Biochem. Cell Biol. 2016, 77, 205–212. [Google Scholar] [CrossRef]
- Park, H.S.; Betzenhauser, M.J.; Won, J.H.; Chen, J.; Yule, D.I. The type 2 inositol (1,4,5)-trisphosphate (InsP3) receptor determines the sensitivity of InsP3-induced Ca2+ release to ATP in pancreatic acinar cells. J. Biol. Chem. 2008, 283, 26081–26088. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 2005, 85, 201–279. [Google Scholar] [CrossRef] [PubMed]
- Enz, R. Structure of metabotropic glutamate receptor C-terminal domains in contact with interacting proteins. Front. Mol. Neurosci. 2012, 5, 22883. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wei, F.; Zou, S.; Robbins, M.T.; Sugiyo, S.; Ikeda, T.; Tu, J.-C.; Worley, P.F.; Dubner, R.; Ren, K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation. J. Neurosci. 2004, 24, 9161–9173. [Google Scholar] [CrossRef]
- Heidinger, V.; Manzerra, P.; Wang, X.Q.; Strasser, U.; Yu, S.-P.; Choi, D.W.; Behrens, M.M. Metabotropic Glutamate Receptor 1-Induced Upregulation of NMDA Receptor Current: Mediation through the Pyk2/Src-Family Kinase Pathway in Cortical Neurons. J. Neurosci. 2002, 22, 5452–5461. [Google Scholar] [CrossRef]
- Minakami, R.; Jinnai, N.; Sugiyama, H. Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J. Biol. Chem. 1997, 272, 20291–20298. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chu, X.-P.; Mao, L.-M.; Wang, M.; Lan, H.-X.; Li, M.-H.; Zhang, G.-C.; Parelkar, N.K.; Fibuch, E.E.; Haines, M.; et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron 2006, 52, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Ciruela, F.; Giacometti, A.; McIlhinney, R.A.J. Functional regulation of metabotropic glutamate receptor type 1c: A role for phosphorylation in the desensitization of the receptor. FEBS Lett. 1999, 462, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, A.; Duvoisin, R.M. Opposing effects of protein kinase C and protein kinase a on metabotropic glutamate receptor signaling: Selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc. Natl. Acad. Sci. USA 2000, 97, 6185–6190. [Google Scholar] [CrossRef]
- Martínez-Galán, J.R.; López-Bendito, G.; Luján, R.; Shigemoto, R.; Fairén, A.; Valdeolmillos, M. Cajal-Retzius cells in early postnatal mouse cortex selectively express functional metabotropic glutamate receptors. Eur. J. Neurosci. 2001, 13, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Liu, X.-B.; Jones, E.G. Development of Metabotropic Glutamate Receptors from Trigeminal Nuclei to Barrel Cortex in Postnatal Mouse. J. Comp. Neurol. 1999, 409, 549–566. [Google Scholar] [CrossRef]
- Furuta, A.; Martin, L.J. Laminar Segregation of the Cortical Plate During Corticogenesis Is Accompanied by Changes in Glutamate Receptor Expression. J. Neurobiol. 1999, 39, 67–80. [Google Scholar] [CrossRef]
- Romano, C.; Van Den Pol, A.N.; O’malley, K.L. Enhanced Early Developmental Expression of the Metabotropic Glutamate Receptor mGluR5 in Rat Brain: Protein, mRNA Splice Variants, and Regional Distribution. J. Comp. Neurol. 1996, 367, 403–412. [Google Scholar] [CrossRef]
- Luján, R.; Nusser, Z.; Roberts, J.D.B.; Shigemoto, R.; Somogyi, P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996, 8, 1488–1500. [Google Scholar] [CrossRef]
- Bhave, G.; Karim, F.; Carlton, S.M.; Gereau, R.W., IV. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat. Neurosci. 2001, 4, 417–423. [Google Scholar] [CrossRef]
- Jensen, R.J. Activation of group II metabotropic glutamate receptors reduces directional selectivity in retinal ganglion cells. Brain Res. 2006, 1122, 86–92. [Google Scholar] [CrossRef]
- Gregory, K.J.; Goudet, C. International union of basic and clinical pharmacology. Cxi. pharmacology, signaling, and physiology of metabotropic glutamate receptors. Pharmacol. Rev. 2021, 73, 521–569. [Google Scholar] [CrossRef]
- Mao, L.-M.; Bodepudi, A.; Chu, X.-P.; Wang, J.Q. Group I Metabotropic Glutamate Receptors and Interacting Partners: An Update. Int. J. Mol. Sci. 2022, 23, 840. [Google Scholar] [CrossRef]
- Quraishi, S.; Gayet, J.; Morgans, C.W.; Duvoisin, R.M. Distribution of group-III metabotropic glutamate receptors in the retina. J. Comp. Neurol. 2007, 501, 931–943. [Google Scholar] [CrossRef]
- Hartveit, E.; Brandstätter, J.H.; Enz, R.; Wässle, H. Expression of the mRNA of seven metabotropic glutamate receptors (mGluR1 to 7) in the rat retina. An in situ hybridization study on tissue sections and isolated cells. Eur. J. Neurosci. 1995, 7, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Shigemoto, R.; Nakamura, Y.; Okamoto, N.; Mizuno, N.; Nakanishi, S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell 1994, 77, 361–369. [Google Scholar] [CrossRef]
- Brandstätter, J.H.; Koulen, P.; Wässle, H. Diversity of glutamate receptors in the mammalian retina. Vis. Res. 1998, 38, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dhingra, A.; Fina, M.E.; Koike, C.; Furukawa, T.; Vardi, N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J. Neurophysiol. 2012, 107, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Koike, C.; Obara, T.; Uriu, Y.; Numata, T.; Sanuki, R.; Miyata, K.; Koyasu, T.; Ueno, S.; Funabiki, K.; Tani, A.; et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. USA 2010, 107, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Rampino, M.A.F.; Carroll, R.C.; Nawy, S. G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gβγ dimer. Proc. Natl. Acad. Sci. USA 2012, 109, 8752–8757. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Dallman, R.; Henderson, S.; Chen, C.-K. Gβ5 is required for normal light responses and morphology of retinal ON-bipolar cells. J. Neurosci. 2007, 27, 14199–14204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jeffrey, B.G.; Morgans, C.W.; Burke, N.S.; Haley, T.L.; Duvoisin, R.M.; Brown, R.L. RGS7 and -11 complexes accelerate the ON-bipolar cell light response. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Vardi, N.; Duvoisin, R.; Wu, G.; Sterling, P. Localization of mGluR6 to Dendrites of ON Bipolar Cells in Primate Retina. J. Comp. Neurol. 2000, 423, 402–412. [Google Scholar] [CrossRef]
- Dhingra, A.; Vardi, N. mGlu receptors in the retina. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 641–653. [Google Scholar] [CrossRef]
- Masu, M.; Iwakabe, H.; Tagawa, Y.; Miyoshi, T.; Yamashita, M.; Fukuda, Y.; Sasaki, H.; Hiroi, K.; Nakamura, Y.; Shigemoto, R.; et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 1995, 80, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Liu, W.; Wei, J.; Yan, Z. Regulation of N-methyl-D-aspartic acid (NMDA) receptors by metabotropic glutamate receptor 7. J. Biol. Chem. 2012, 287, 10265–10275. [Google Scholar] [CrossRef]
- Rosenberg, N.; Gerber, U.; Ster, J. Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors. J. Neurosci. 2016, 36, 11521–11531. [Google Scholar] [CrossRef] [PubMed]
- Joffe, M.E.; Santiago, C.I.; Engers, J.L.; Lindsley, C.W.; Conn, P.J. Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol. Psychiatry 2019, 24, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Zhang, X.; Bai, H.M.; Jiang, X.F.; Wang, X.L. Metabotropic glutamate receptor antagonists and agonists: Potential neuroprotectors in diffuse brain injury. J. Clin. Neurosci. 2006, 13, 1023–1027. [Google Scholar] [CrossRef]
- Folbergrová, J.; Druga, R.; Otáhal, J.; Haugvicová, R.; Mareš, P.; Kubová, H. Seizures induced in immature rats by homocysteic acid and the associated brain damage are prevented by group II metabotropic glutamate receptor agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate. Exp. Neurol. 2005, 192, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Gao, J.; Feng, Y.B.; Pang, Z.Y.; Chi, Z.F. 2R, 4R-APDC decreases cell proliferation in the dentate gyrus of adult rats: The effect of 2R, 4R-APDC on cell proliferation. Neuroreport 2007, 18, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.B.; Lin, Y.-T.; Han, Y.-X.; Pang, Y.-J.; Xu, J.-J.; Xue, Y.; Yao, H. 2R,4R-APDC, a Metabotropic Glutamate Receptor Agonist, Reduced Neuronal Apoptosis by Upregulating MicroRNA-128 in a Rat Model After Seizures. Neurochem. Res. 2018, 43, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Möller, T.; Berger, T.; Schnitzer, J.; Kettenmann, H. Calcium Entry Through Kainate Receptors and Resulting Potassium-Channel Blockade in Bergmann Glial Cells. Science 1992, 256, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Ghiani, C.A. Glutamate receptors in glia: New cells, new inputs and new functions. Trends Pharmacol. Sci. 2000, 21, 252–258. [Google Scholar] [CrossRef]
- Bouvier, M.; Szatkowski, M.; Amato, A.; Attwell, D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature 1992, 360, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Puro, D.G. Calcium Channels of Human Retinal Glial Cells. In Methods in Neurosciences; Elsevier: Amsterdam, The Netherlands, 1994; Volume 19, pp. 68–81. [Google Scholar] [CrossRef]
- Luque, J.M.; Richards, J.G. Expression of NMDA 2B receptor subunit mRNA in bergmann glia. Glia 1995, 13, 228–232. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Kirchhoff, F. NMDA receptors in glia. Neuroscientist 2007, 13, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Puro, D.G.; Yuan, J.P.; Sucher, N.J. Activation of NMDA receptor-channels in human retinal Muller glial cells inhibits inward-rectifying potassium currents. Vis. Neurosci. 1996, 13, 319–326. [Google Scholar] [CrossRef]
- Mao, L.M.; Geosling, R.; Penman, B.; Wang, J.Q. Local substrates of non-receptor tyrosine kinases at synaptic sites in neurons. Sheng Li Xue Bao 2017, 69, 657. [Google Scholar] [PubMed Central]
- Ohnishi, H.; Murata, Y.; Okazawa, H.; Matozaki, T. Src family kinases: Modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 2011, 34, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Rajani, V.; Sengar, A.S.; Salter, M.W. Src and Fyn regulation of NMDA receptors in health and disease. Neuropharmacology 2021, 193, 108615. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Chen, J.; Baudry, M. Calpain-mediated proteolysis of GluR1 subunits in organotypic hippocampal cultures following kainic acid treatment. Brain Res. 1998, 781, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Chang, V.; Molnar, E.; McIlhinney, R.A.J.; Baudry, M. The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience 1996, 73, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Rong, Y.; Chen, J.; Dang, S.; Wang, Z.; Baudry, M. Calpain-mediated regulation of NMDA receptor structure and function. Brain Res. 1998, 790, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Vyklický, L. Calcium-mediated modulation of N-methyl-D-aspartate (NMDA) responses in cultured rat hippocampal neurones. J. Physiol. 1993, 470, 575–600. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, M.D.; Zhang, S.; Bernhardt, J.P.; Huganir, R.L. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 1996, 84, 745–755. [Google Scholar] [CrossRef]
- Marc, R.E. Kainate activation of horizontal, bipolar, amacrine, and ganglion cells in the rabbit retina. J. Comp. Neurol. 1999, 407, 65–76. [Google Scholar] [CrossRef]
- Massey, S.C.; Miller, R.F. Excitatory amino acid receptors of rod- and cone-driven horizontal cells in the rabbit retina. J. Neurophysiol. 1987, 57, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Brandstätter, J.H.; Koulen, P.; Kuhn, R.; Van Der Putten, H.; Wässle, H. Compartmental localization of a metabotropic glutamate receptor (mGluR7): Two different active sites at a retinal synapse. J. Neurosci. 1996, 16, 4749–4756. [Google Scholar] [CrossRef]
- Fletcher, E.L.; Hack, I.; Brandstätter, J.H.; Wa, H.; Wa, W. Synaptic Localization of NMDA Receptor Subunits in the Rat Retina. J. Comp. Neurol. 2000, 420, 98–112. [Google Scholar] [CrossRef]
- Hoffpauir, B.K.; Gleason, E.L. Modulation of synaptic function in retinal amacrine cells. Integr. Comp. Biol. 2005, 45, 658–664. [Google Scholar] [CrossRef]
- Peng, Y.W.; Blackstone, C.D.; Huganir, R.L.; Yau, K.W. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience 1995, 66, 483–497. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, A.R.; Garcia-Román, J.; Gonzlez-Espinosa, C.; Lamas, M. Pharmacological Inhibition of N-Methyl D-Aspartate Receptor Promotes Secretion of Vascular Endothelial Growth Factor in Müller Cells: Effects of Hyperglycemia and Hypoxia. Curr. Eye Res. 2010, 35, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Uchihori, Y.; Puro, D.G. Glutamate as a neuron-to-glial signal for mitogenesis: Role of glial N-methyl-D-aspartate receptors. Brain Res. 1993, 613, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Trebak, M.; Perocchi, F.; Khananshvili, D.; Sekler, I. Crosslink between calcium and sodium signalling. Exp. Physiol. 2018, 103, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Luyt, K.; Váradi, A.; Durant, C.F.; Molnár, E. Oligodendroglial metabotropic glutamate receptors are developmentally regulated and involved in the prevention of apoptosis. J. Neurochem. 2006, 99, 641–656. [Google Scholar] [CrossRef]
- Gerber, U.; Gee, C.E.; Benquet, P. Metabotropic glutamate receptors: Intracellular signaling pathways. Curr. Opin. Pharmacol. 2007, 7, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Ortega, A. AMPA/KA receptor induced AP-1 DNA binding activity in cultured bergmann glia cells. Neuroreport 1994, 5, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K. Calcium Signaling and Gene Expression. In Calcium Signaling; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1131, pp. 537–545. [Google Scholar] [CrossRef]
- Rose, C.R.; Ziemens, D.; Verkhratsky, A. On the special role of NCX in astrocytes: Translating Na+-transients into intracellular Ca2+ signals. Cell Calcium 2020, 86, 102154. [Google Scholar] [CrossRef] [PubMed]
- López-Bayghen, E.; Ortega, A. Glutamate-dependent transcriptional regulation of GLAST: Role of PKC. J. Neurochem. 2004, 91, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Barrera, I.; Flores-Méndez, M.; Hernández-Kelly, L.C.; Cid, L.; Huerta, M.; Zinker, S.; López-Bayghen, E.; Aguilera, J.; Ortega, A. Glutamate regulates eEF1A phosphorylation and ribosomal transit time in Bergmann glial cells. Neurochem. Int. 2010, 57, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lozada, Z.; Hernández-Kelly, L.C.; Aguilera, J.; López-Bayghen, E.; Ortega, A. Signaling through EAAT-1/GLAST in cultured Bergmann glia cells. Neurochem. Int. 2011, 59, 871–879. [Google Scholar] [CrossRef] [PubMed]
- López-Bayghen, E.; Espinoza-Rojo, M.; Ortega, A. Glutamate down-regulates GLAST expression through AMPA receptors in Bergmann glial cells. Mol. Brain Res. 2003, 115, 1–9. [Google Scholar] [CrossRef]
- Aguirre, A.; López-Bayghen, E.; Ortega, A. Glutamate-dependent transcriptional regulation of the chkbp gene: Signaling mechanisms. J. Neurosci. Res. 2002, 70, 117–127. [Google Scholar] [CrossRef]
- Chai, R.; Xing, C.; Gao, D.; Yuan, H.; Zhan, Y.; Wang, S. Remote-Controlling Potassium Channels in Living Cells through Photothermal Inactivation of Calmodulin. Adv. Healthc. Mater. 2018, 7, 1800674. [Google Scholar] [CrossRef]
- Wyttenbach, T.; Grabenauer, M.; Thalassinos, K.; Scrivens, J.H.; Bowers, M.T. The effect of calcium ions and peptide ligands on the relative stabilities of the calmodulin dumbbell and compact structures. J. Phys. Chem. B 2010, 114, 437–447. [Google Scholar] [CrossRef]
- Kazanietz, M.G. Novel ‘nonkinase’ phorbol ester receptors: The C1 domain connection. Mol. Pharmacol. 2002, 61, 759–767. [Google Scholar] [CrossRef]
- Millán, A.; Arias-Montaño, J.A.; Méndez, J.A.; Hernández-Kelly, L.C.R.; Ortega, A. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors signaling complexes in Bergmann glia. J. Neurosci. Res. 2004, 78, 56–63. [Google Scholar] [CrossRef]
- López-Bayghen, E.; Aguirre, A.; Ortega, A. Transcriptional regulation through glutamate receptors: Involvement of tyrosine kinases. J. Neurosci. Res. 2003, 74, 717–725. [Google Scholar] [CrossRef]
- Aguirre, A.; López, T.; López-Bayghen, E.; Ortega, A. Glutamate regulates kainate-binding protein expression in cultured chick Bergmann glia through an activator protein-1 binding site. J. Biol. Chem. 2000, 275, 39246–39253. [Google Scholar] [CrossRef] [PubMed]
- Millán, A.; Aguilar, P.; Méndez, J.A.; Arias-Montaño, J.A.; Ortega, A. Glutamate activates PP125FAK through AMPA/kainate receptors in Bergmann glia. J. Neurosci. Res. 2001, 66, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Rosas, S.; Vargas, M.A.; López-Bayghen, E.; Ortega, A. Glutamate-dependent transcriptional regulation of GLAST/EAAT1: A role for YY1. J. Neurochem. 2007, 101, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Naredo, I.; Angulo, C.; Hernández-Kelly, L.; López-Bayghen, E.; Aguilera, J.; Ortega, A. Insulin-dependent regulation of GLAST/EAAT1 in Bergmann glial cells. Neurosci. Lett. 2009, 451, 134–138. [Google Scholar] [CrossRef] [PubMed]
- López-Colomé, A.M.; Murbartián, J.; Ortega, A. Excitatory amino acid-induced AP-1 DNA binding activity in Müller glia. J. Neurosci. Res. 1995, 41, 179–184. [Google Scholar] [CrossRef] [PubMed]
- López-Colomé, A.M.; Ortega, A.; Romo-De-Vivar, M. Excitatory amino acid-induced phosphoinositide hydrolysis in Müller glia. Glia 1993, 9, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.R.; Esguerra, M.; Kim, P.M.; Newman, E.A.; Snyder, S.H.; Zahs, K.R.; Miller, R.F. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 6789–6794. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.M.; Lee, A.; Björkman, S.T.; Miller, S.M.; Sullivan, R.K.P.; Poronnik, P.; Colditz, P.B.; Pow, D.V. Cytoskeletal anchoring of GLAST determines susceptibility to brain damage: An identified role for GFAP. J. Biol. Chem. 2007, 282, 29414–29423. [Google Scholar] [CrossRef]
- Hellyer, S.; Leach, K.; Gregory, K.J. Neurobiological insights and novel therapeutic opportunities for CNS disorders from mGlu receptor allosteric and biased modulation. Curr. Opin. Pharmacol. 2017, 32, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Durand, D.; Carniglia, L.; Turati, J.; Ramírez, D.; Saba, J.; Caruso, C.; Lasaga, M. Amyloid-beta neurotoxicity and clearance are both regulated by glial group II metabotropic glutamate receptors. Neuropharmacology 2017, 123, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Vieira, L.B.; Pires, R.G.W.; Olmo, R.P.; Ferguson, S.S.G. Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol. Res. 2017, 115, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Molinaro, G.; Battaglia, G.; Giuffrida, M.L.; Riozzi, B.; Traficante, A.; Bruno, V.; Cannella, M.; Merlo, S.; Wang, X.; et al. Targeting Group II Metabotropic Glutamate (mGlu) Receptors for the Treatment of Psychosis Associated with Alzheimer’s Disease: Selective Activation of mGlu2 Receptors Amplifies β-Amyloid Toxicity in Cultured Neurons, Whereas Dual Activation of mGlu2 and mGlu3 Receptors Is Neuroprotective. Mol. Pharmacol. 2011, 79, 618–626. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, J.; Hessler, P.; Tahir, S.K.; Chen, J.; Jin, S.; Souers, A.J.; Leverson, J.D.; Lam, L.T. Genomic analysis and selective small molecule inhibition identifies BCL-XL as a critical survival factor in a subset of colorectal cancer. Mol. Cancer 2015, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Lech, T.; Gołda, S.; Pilc, A.; Lasoń, W. New evidences for a role of mGluR7 in astrocyte survival: Possible implications for neuroprotection. Neuropharmacology 2018, 141, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Gorter, J.A.; Ijlst-Keizers, H.; Rozemuller, A.J.; Yankaya, B.; Leenstra, S.; Troost, D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: Opposite regulation of glutamate transporter proteins. Eur. J. Neurosci. 2003, 17, 2106–2118. [Google Scholar] [CrossRef] [PubMed]
- Gegelashvili, G.; Dehnes, Y.; Danbolt, N.C.; Schousboe, A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem. Int. 2000, 37, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; You, J.R.; Wei, K.C.; Gean, P.W. Stimulating ERK/PI3K/NFκB signaling pathways upon activation of mGluR2/3 restores OGD-induced impairment in glutamate clearance in astrocytes. Eur. J. Neurosci. 2014, 39, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B. Diabetic retinopathy and the NMDA receptor. Drug News Perspect. 2002, 15, 226–232. [Google Scholar] [CrossRef]
- Vorwerk, C.K.; Gorla, M.S.R.; Dreyer, E.B. An Experimental Basis for Implicating Excitotoxicity in Glaucomatous Optic Neuropathy. Surv. Ophthalmol. 1999, 43, S142–S150. [Google Scholar] [CrossRef] [PubMed]
- Gowda, K.; Zinnantif, W.J.; LaNoue, K.F. The influence of diabetes on glutamate metabolism in retinas. J. Neurochem. 2011, 117, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Waggie, K.S.; Kahle, P.J.; Tolwani, R.J. Neurons and mechanisms of neuronal death in neurodegenerative diseases: A brief review. Lab. Anim. Sci. 1999, 49, 358–362. [Google Scholar] [PubMed]
- Opere, C.A.; Heruye, S.; Njie-Mbye, Y.F.; Ohia, S.E.; Sharif, N.A. Regulation of excitatory amino acid transmission in the retina: Studies on neuroprotection. J. Ocul. Pharmacol. Ther. 2018, 34, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, F. Amino Acids Metabolism in Retinopathy: From Clinical and Basic Research Perspective. Metabolites 2022, 12, 1244. [Google Scholar] [CrossRef]
- Berliocchi, L.; Bano, D.; Nicotera, P. Ca2+ signals and death programmes in neurons. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 2255–2258. [Google Scholar] [CrossRef]
- Hartwick, A.T.E.; Hamilton, C.M.; Baldridge, W.H. Glutamatergic calcium dynamics and deregulation of rat retinal ganglion cells. J. Physiol. 2008, 586, 3425–3446. [Google Scholar] [CrossRef]
- Santiago, A.R.; Hughes, J.M.; Kamphuis, W.; Schlingemann, R.O.; Ambrósio, A.F. Diabetes changes ionotropic glutamate receptor subunit expression level in the human retina. Brain Res. 2008, 1198, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Baudry, M.; Bi, X.; Gall, C.; Lynch, G. The biochemistry of memory: The 26 year journey of a ‘new and specific hypothesis’. Neurobiol. Learn. Mem. 2011, 95, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Silva, A.J. The molecular and cellular biology of enhanced cognition. Nat. Rev. Neurosci. 2009, 10, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.K. Calcium: A role for neuroprotection and sustained adaptation. Mol. Interv. 2006, 6, 315–329. [Google Scholar] [CrossRef]
- König, N.; Poluch, S.; Estabel, J.; Durand, M.; Drian, M.J.; Exbrayat, J.M. Synaptic and non-synaptic AMPA receptors permeable to calcium. Jpn. J. Pharmacol. 2001, 86, 1–17. [Google Scholar] [CrossRef]
- Kelly, L.; Brown, C.; Gibbard, A.G.; Jackson, T.; Swinny, J.D. Subunit-specific expression and function of AMPA receptors in the mouse locus coeruleus. Am. J. Anat. 2023, 243, 813–825. [Google Scholar] [CrossRef]
- Gorter, J.A.; Petrozzino, J.J.; Aronica, E.M.; Rosenbaum, D.M.; Opitz, T.; Bennett, M.V.L.; Connor, J.A.; Zukin, R.S. Global ischemia induces downregulation of Glur2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA1 neurons of gerbil. J. Neurosci. 1997, 17, 6179–6188. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.M.; Yokota, H.; Mashiko, T.; Castillo, P.E.; Zukin, R.S.; Bennett, M.V.L. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. USA 2005, 102, 12230–12235. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, Y.Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef] [PubMed]
- Schrammel, A.; Gorren, A.C.F.; Schmidt, K.; Pfeiffer, S.; Mayer, B. S-nitrosation of glutathione by nitric oxide, peroxynitrite, and •NO/O2•−. Free Radic. Biol. Med. 2003, 34, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide 2010, 23, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—Induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Fujio, Y.; Nakayama, H. Caveolae-Specific CaMKII Signaling in the Regulation of Voltage-Dependent Calcium Channel and Cardiac Hypertrophy. Front. Physiol. 2018, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Brewster, A.L.; Marzec, K.; Hairston, A.; Ho, M.; Anderson, A.E.; Lai, Y.-C. Early cardiac electrographic and molecular remodeling in a model of status epilepticus and acquired epilepsy. Epilepsia 2016, 57, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.G.F.; Laabich, A.; Fan, W.; Wang, X. The relationship between neurotrophic factors and CaMKII in the death and survival of retinal ganglion cells. Prog. Brain Res. 2008, 173, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Enkhjargal, B.; Xie, Z.; Sun, C.; Wu, L.; Malaguit, J.; Chen, S.; Tang, J.; Zhang, J.; Zhang, J.H. Dihydrolipoic acid inhibits lysosomal rupture and NLRP3 through lysosome-associated membrane Protein-1/Calcium/Calmodulin-Dependent Protein Kinase II/TAK1 pathways after subarachnoid hemorrhage in rat. Stroke 2018, 49, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xia, T.; Cui, Y.; Chu, S.; Ma, Z.; Gu, X. The role of CaMKII in neuropathic pain and fear memory in chronic constriction injury in rats. Int. J. Neurosci. 2019, 129, 146–154. [Google Scholar] [CrossRef]
- Wang, S.; Liao, L.; Huang, Y.; Wang, M.; Zhou, H.; Chen, D.; Liu, F.; Ji, D.; Xia, X.; Jiang, B.; et al. Pin1 is regulated by CaMKII activation in glutamate-induced retinal neuronal regulated necrosis. Front. Cell. Neurosci. 2019, 13, 276. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Jia, Y.; Jeng, J.M.; Sensi, S.L.; Weiss, J.H. Zn2+ currents are mediated by calcium-permeable AMPA/kainate channels in cultured murine hippocampal neurones. J. Physiol. 2002, 543 Pt 1, 35–48. [Google Scholar] [CrossRef]
- Van Damme, P.; Braeken, D.; Callewaert, G.; Robberecht, W.; Van Den Bosch, L. GluR2 deficiency accelerates motor neuron degeneration in a mouse model of amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2005, 64, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Bogaert, E.; Dewil, M.; Hersmus, N.; Kiraly, D.; Scheveneels, W.; Bockx, I.; Braeken, D.; Verpoorten, N.; Verhoeven, K.; et al. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc. Natl. Acad. Sci. USA 2007, 104, 14825–14830. [Google Scholar] [CrossRef]
- Santos, A.E.; Duarte, C.B.; Iizuka, M.; Barsoumian, E.L.; Ham, J.; Lopes, M.C.; Carvalho, A.L. Excitotoxicity mediated by Ca2+-permeable GluR4-containing AMPA receptors involves the AP-1 transcription factor. Cell Death Differ. 2006, 13, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Siliprandi, R.; Lipartiti, M.; Fadda, E.; Arban, R.; Kozikowski, A.P.; Manev, H. A derivative of a rigid glutamate analog protects the retina from excitotoxicity. Neuroreport 1994, 5, 1227–1229. [Google Scholar] [CrossRef]
- Hlaváčková, M.; Kožichová, K.; Neckář, J.; Kolář, F.; Musters, R.J.P.; Novák, F.; Nováková, O. Up-regulation and redistribution of protein kinase C-δ in chronically hypoxic heart. Mol. Cell. Biochem. 2010, 345, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Casson, R.J.; Franzco, D. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 2006, 34, 54–63. [Google Scholar] [CrossRef]
- Wang, X.; Mo, X.; Li, D.; Wang, Y.; Fang, Y.; Rong, X.; Miao, H.; Shou, T. Neuroprotective effect of transcorneal electrical stimulation on ischemic damage in the rat retina. Exp. Eye Res. 2011, 93, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Nickells, R.W.; Kerrigan, L.A.; Pease, M.E.; Thibault, D.J.; Zack, D.J. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Investig. Ophthalmol. Vis. Sci. 1995, 36, 774–786. [Google Scholar]
- Schor, N.F. Inactivation of mammalian brain glutamine synthetase by oxygen radicals. Brain Res. 1988, 456, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Prentice, H.; Modi, J.P.; Wu, J.Y. Mechanisms of Neuronal Protection against Excitotoxicity, Endoplasmic Reticulum Stress, and Mitochondrial Dysfunction in Stroke and Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 964518. [Google Scholar] [CrossRef]
- Doyle, K.P.; Simon, R.P.; Stenzel-Poore, M.P. Mechanisms of ischemic brain damage. Neuropharmacology 2008, 55, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Oshima, T.; Attwell, D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 2000, 403, 316–321. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Erecinska, M.; Silver, I.A. ATP and brain function. J. Cereb. Blood Flow Metab. 1989, 9, 2–19. [Google Scholar] [CrossRef]
- Farhat, E.; Devereaux, M.E.M.; Cheng, H.; Weber, J.-M.; Pamenter, M.E. Na+/K+-ATPase activity is regionally regulated by acute hypoxia in naked mole-rat brain. Neurosci. Lett. 2021, 764, 136244. [Google Scholar] [CrossRef]
- Kirdajova, D.B.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Nivison-Smith, L.; Fletcher, E.L.; Trenholm, S.; Awatramani, G.B.; Kalloniatis, M. Early remodeling of Müller cells in the rd/rd mouse model of retinal dystrophy. J. Comp. Neurol. 2013, 521, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef]
- Pow, D.V.; Robinson, S.R. Glutamate in some retinal neurons is derived solely from glia. Neuroscience 1994, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Grosche, A.; Pannicke, T.; Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells. Front. Endocrinol. 2013, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Gorovits, R.; Avidan, N.; Avisar, N.; Shaked, I.; Vardimon, L. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. Proc. Natl. Acad. Sci. USA 1997, 94, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Diabetes mellitus, hypertension, and cardiovascular disease: Which role for oxidative stress? Metabolism 1995, 44, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.Y.; Wang, A.G.; Wei, Y.H. Leber’s hereditary optic neuropathy: A multifactorial disease. Prog. Retin. Eye Res. 2006, 25, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A. Inward-rectifying potassium channels in retinal glial (müller) cells. J. Neurosci. 1993, 13, 3333–3345. [Google Scholar] [CrossRef] [PubMed]
- Vinores, S.A. Breakdown of the Blood–Retinal Barrier. Encycl. Eye 2010, 216–222. [Google Scholar] [CrossRef]
- Fleegal, M.A.; Hom, S.; Borg, L.K.; Davis, T.P. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and posthypoxic reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2012–H2019. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.K.; Alkon, D.L. Activation of protein kinase C isozymes for the treatment of dementias. Adv. Pharmacol. 2012, 64, 273–302. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. In Lipases and Phospholipases; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1835, pp. 69–105. [Google Scholar] [CrossRef]
- Lin, Y.; Jones, B.W.; Liu, A.; Vazquéz-Chona, F.R.; Lauritzen, J.S.; Ferrell, W.D.; Marc, R.E. Rapid glutamate receptor 2 trafficking during retinal degeneration. Mol. Neurodegener. 2012, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Campuzano, A.G.; Hernández-Kelly, L.C.; Ortega, A. DNA Methylation-Dependent Gene Expression Regulation of Glutamate Transporters in Cultured Radial Glial Cells. Mol. Neurobiol. 2022, 59, 1912–1924. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, L.-L.; Cao, M.; Hu, A.; Hu, D.; Luo, Y.; Wang, H.; Zhong, J.-N. DNA methylation plays important roles in retinal development and diseases. Exp. Eye Res. 2021, 211, 108733. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.F.; Ta, J.L.; Fuller, G.; Yung, W.K.A. The excitatory amino acid transporter-2 induces apoptosis and decreases glioma growth in vitro and in vivo. Cancer Res. 2005, 65, 1934–1940. [Google Scholar] [CrossRef]
- Okabe, Y.; Takahashi, T.; Mitsumasu, C.; Kosai, K.I.; Tanaka, E.; Matsuishi, T. Alterations of gene expression and glutamate clearance in astrocytes derived from an mecp2-null mouse model of rett syndrome. PLoS ONE 2012, 7, e35354. [Google Scholar] [CrossRef] [PubMed]
- Dunn, H.G.; MacLeod, P.M. Rett syndrome: Review of biological abnormalities. Can. J. Neurol. Sci. 2001, 28, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Hendrich, B.; Holmes, M.; Martin, J.E.; Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001, 27, 322–326. [Google Scholar] [CrossRef]
- Bruno, V.; Battaglia, G.; Copani, A.; Giffard, R.G.; Raciti, G.; Raffaele, R.; Shinozaki, H.; Nicoletti, F. Activation of Class II or III Metabotropic Glutamate Receptors Protects Cultured Cortical Neurons Against Excitotoxic Degeneration. Eur. J. Neurosci. 1995, 7, 1906–1913. [Google Scholar] [CrossRef]
- Flor, P.J.; Battaglia, G.; Nicoletti, F.; Gasparini, F.; Bruno, V. Neuroprotective Activity of Metabotropic Glutamate Receptor Ligands. 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6556/ (accessed on 6 January 2024).
- Kingston, A.E.; O’Neill, M.J.; Lam, A.; Bales, K.R.; Monn, J.A.; Schoepp, D.D. Neuroprotection by metabotropic glutamate receptor agonists: LY354740, LY379268 and LY389795. Eur. J. Pharmacol. 1999, 377, 155–165. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Copani, A.; Nicoletti, F.; Sortino, M.A.; Caraci, F. Metabotropic glutamate receptors in glial cells: A new potential target for neuroprotection? Front. Mol. Neurosci. 2018, 11, 414. [Google Scholar] [CrossRef]
- Calabresi, P.; Centonze, D.; Cupini, L.M.; Costa, C.; Pisani, F.; Bernardi, G. Ionotropic glutamate receptors: Still a target for neuroprotection in brain ischemia? Insights from in vitro studies. Neurobiol. Dis. 2003, 12, 82–88. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Qin, D.; Chen, H.; Wang, J.; Wang, J.; Song, S.; Wang, C.; Wang, Y.; Liu, S.; et al. The role of brain derived neurotrophic factor in central nervous system. Front. Aging Neurosci. 2022, 14, 986443. [Google Scholar] [CrossRef]
| Cell Type | Identified Receptor Subtypes and Subunits | Organism | Detection Techniques | References |
|---|---|---|---|---|
| Horizontal Cells | GRIN1, GRIA1, GRIA4, GRIK2 | Rat, mouse, rabbit | Immunolabeling, scRNA sequencing | [101,201,202] |
| Bipolar Cells | GRIN1, GRIN2A, GRIN2B, GRIK1, GRM 4, GRM 6, GRM 7 | Rat, rabbit | Immunofluorescence, western blot, immunocytochemistry. | [27,167,203] |
| Amacrine Cells | GRIN1, GRIN2B, GRIA1, GRIA4, GRIK2, GRM1, GRM2, GRM5, GRMR3 | Rat, cat, mouse, chick embryo, Goldfish | Immunolabeling, immunocytochemistry, immunoelectron microscopy | [164,167,204,205] |
| Retinal Ganglion Cells | GRIA1, GRIA4, GRIK2, GRIN1, GRIN 2A, GRIN2B GRMR1 | Mouse, rat | Immunolabeling, immunocytochemistry. | [167,204,206] |
| Müller Cells | GRIA4, GRIN1 | Rat | Immunocytochemistry, Western blot. | [192,206,207,208] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyetayo, B.; Mendoza-Silva, Y.; Subair, T.; Hernández-Kelly, L.C.; Felder-Schmittbuhl, M.-P.; Olivares-Bañuelos, T.N.; Ortega, A. Glutamate Receptor Signaling in Retina Müller Cells: Plausible Role in Neurodegeneration. Receptors 2025, 4, 4. https://doi.org/10.3390/receptors4010004
Oyetayo B, Mendoza-Silva Y, Subair T, Hernández-Kelly LC, Felder-Schmittbuhl M-P, Olivares-Bañuelos TN, Ortega A. Glutamate Receptor Signaling in Retina Müller Cells: Plausible Role in Neurodegeneration. Receptors. 2025; 4(1):4. https://doi.org/10.3390/receptors4010004
Chicago/Turabian StyleOyetayo, Bolaji, Yurixy Mendoza-Silva, Temitayo Subair, Luisa C Hernández-Kelly, Marie-Paule Felder-Schmittbuhl, Tatiana N. Olivares-Bañuelos, and Arturo Ortega. 2025. "Glutamate Receptor Signaling in Retina Müller Cells: Plausible Role in Neurodegeneration" Receptors 4, no. 1: 4. https://doi.org/10.3390/receptors4010004
APA StyleOyetayo, B., Mendoza-Silva, Y., Subair, T., Hernández-Kelly, L. C., Felder-Schmittbuhl, M.-P., Olivares-Bañuelos, T. N., & Ortega, A. (2025). Glutamate Receptor Signaling in Retina Müller Cells: Plausible Role in Neurodegeneration. Receptors, 4(1), 4. https://doi.org/10.3390/receptors4010004





