Unraveling the Role of CHRNA6, the Neuronal α6 Nicotinic Acetylcholine Receptor Subunit
Abstract
1. Introduction
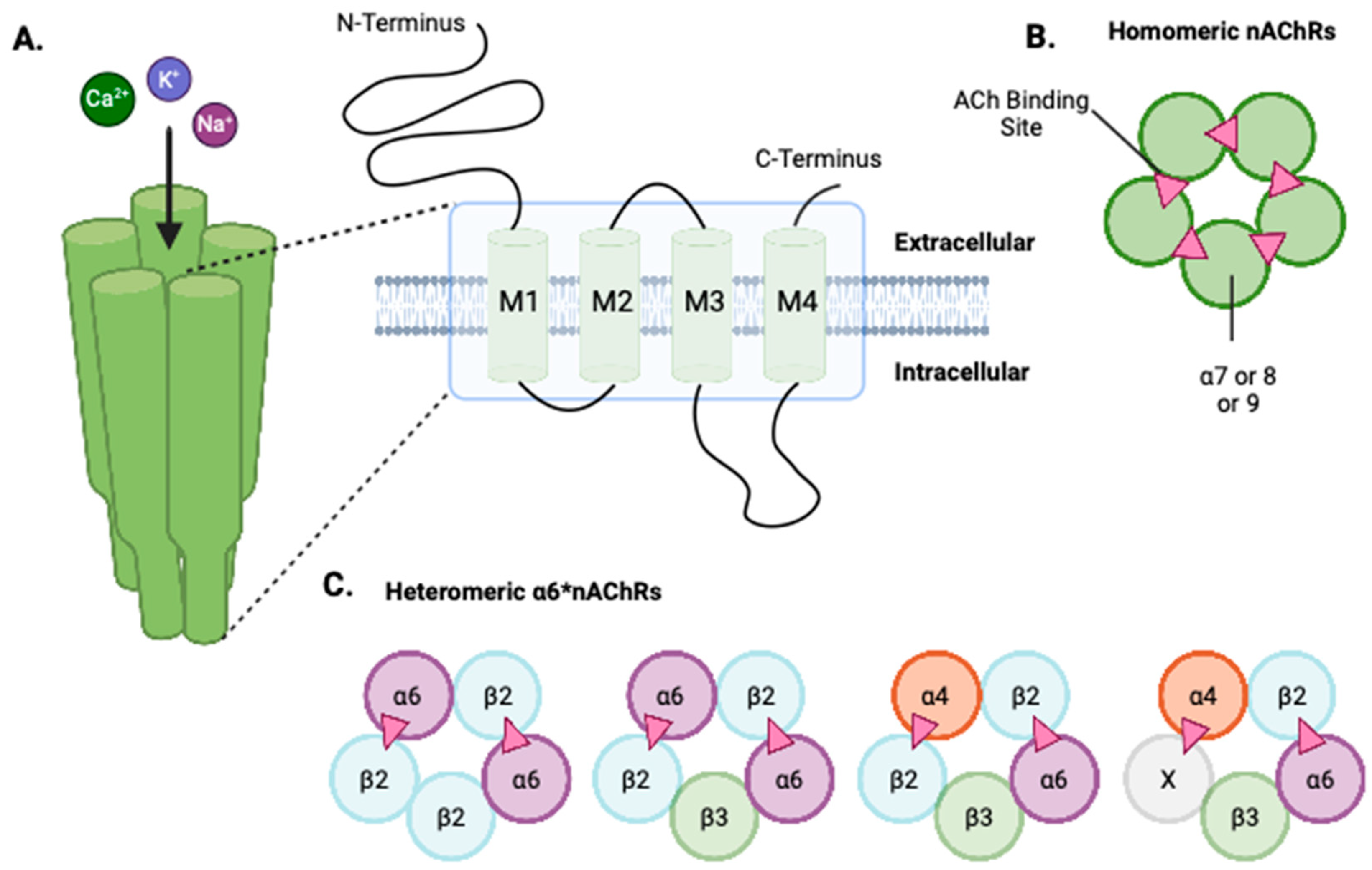
2. Structure and Distribution of α6-Containing Neuronal nAChRs
2.1. Structure of Neuronal nAChRs
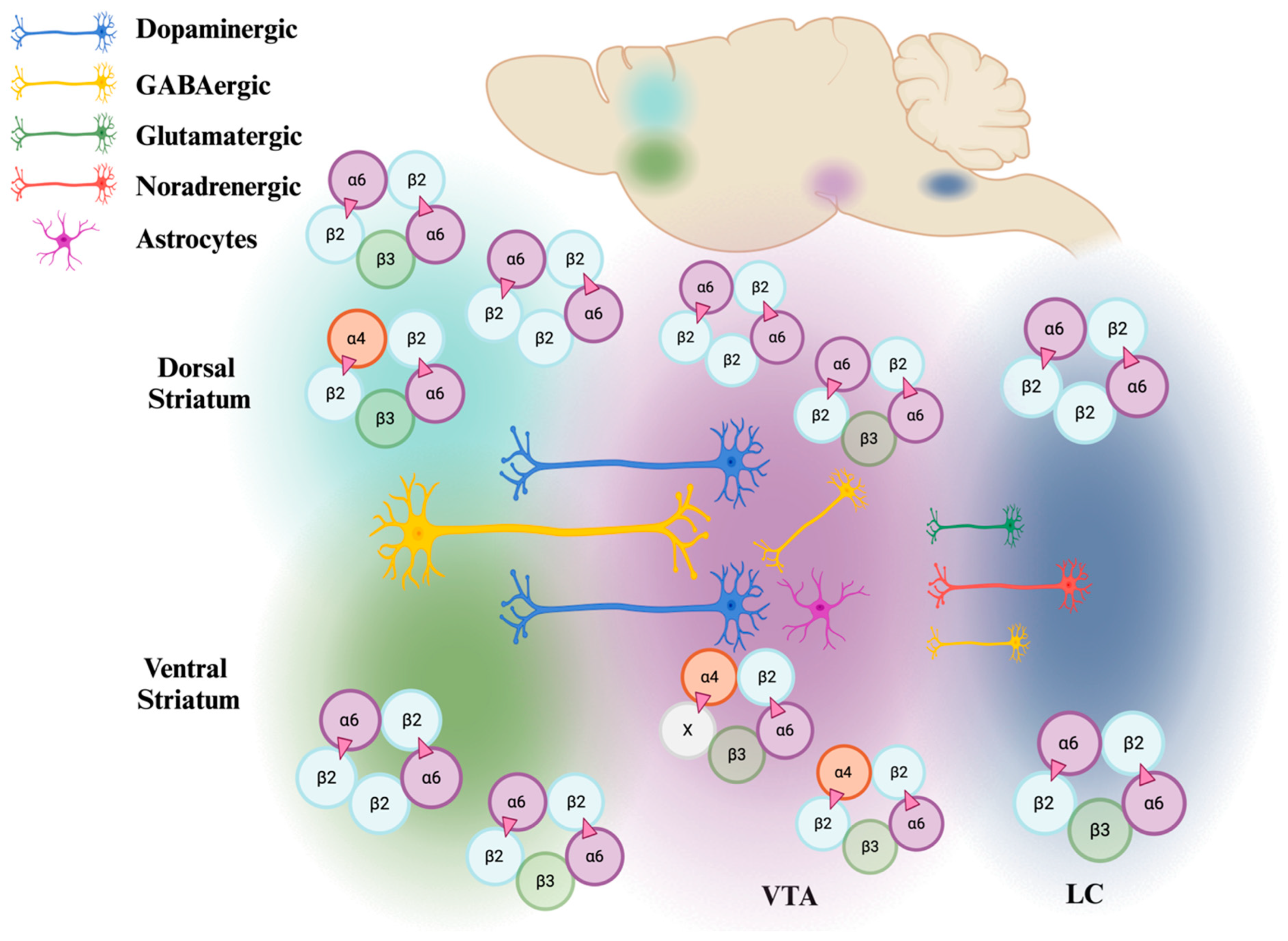
2.2. Distribution of the α6 Subunit
3. Conventional and Novel Strategies in Studying α6
3.1. In Vitro Expression of α6*nAChRs
3.2. Animal Models
3.3. Computational Methods
3.4. Omics Approaches
4. α6*nAChRs: Function and Impact
4.1. Nicotine
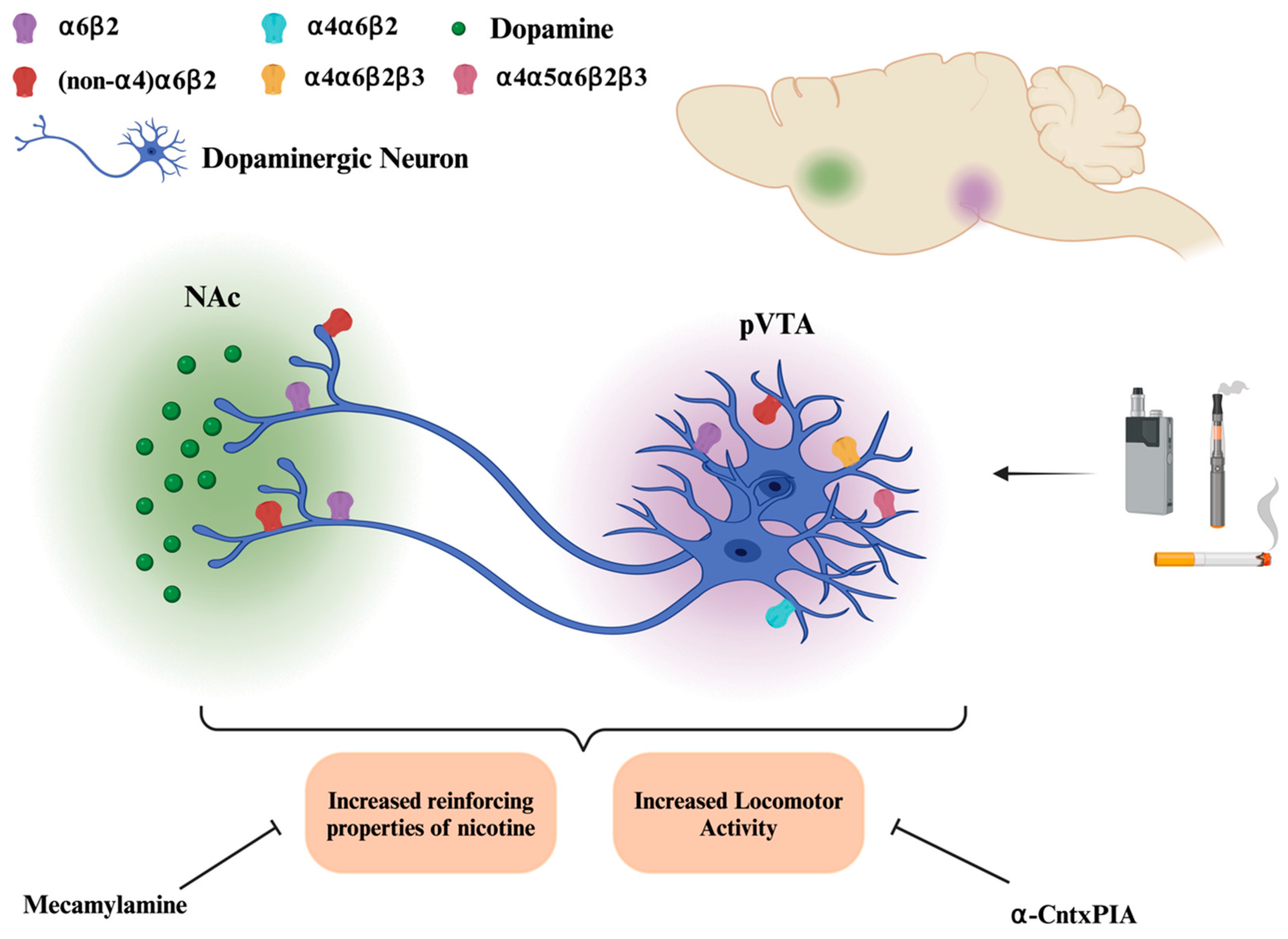
4.1.1. A: α6*nAChRs and Dopamine Release
4.1.2. B: α6*nAChRs and Locomotor Activity
4.1.3. C: α6*nAChRs and Self-Administration
4.1.4. D: Clinical and Pre-Clinical Studies Investigating CHRNA6 Encoding for the α6 Subunit
4.2. Alcohol
4.3. α6 Subunit in Parkinson’s Disease
4.4. α6 Subunit in Pain
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Available online: https://www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/index.html (accessed on 2 January 2025).
- Cornelius, M.E. Tobacco product use among adults–United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Birdsey, J. Tobacco product use among US middle and high school students—National Youth Tobacco Survey 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Cross, S.J.; Loughlin, S.E.; Leslie, F.M. Nicotine and the adolescent brain. J. Physiol. 2015, 593, 3397–3412. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Lotfipour, S. Nicotine gateway effects on adolescent substance use. West. J. Emerg. Med. 2019, 20, 696. [Google Scholar] [CrossRef] [PubMed]
- Leslie, F.M. Unique, long-term effects of nicotine on adolescent brain. Pharmacol. Biochem. Behav. 2020, 197, 173010. [Google Scholar] [CrossRef]
- Xu, X.; Shrestha, S.S.; Trivers, K.F.; Neff, L.; Armour, B.S.; King, B.A. US healthcare spending attributable to cigarette smoking in 2014. Prev. Med. 2021, 150, 106529. [Google Scholar] [CrossRef]
- Champtiaux, N.; Gotti, C.; Cordero-Erausquin, M.; David, D.J.; Przybylski, C.; Léna, C.; Clementi, F.; Moretti, M.; Rossi, F.M.; Le Novere, N.; et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci. 2003, 23, 7820–7829. [Google Scholar] [CrossRef]
- Azam, L.; Chen, Y.; Leslie, F. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience 2007, 144, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nat. Rev. Neurosci. 2010, 11, 389–401. [Google Scholar] [CrossRef]
- Zoli, M.; Pistillo, F.; Gotti, C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015, 96, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Moen, J.K.; Lee, A.M. Sex differences in the nicotinic acetylcholine receptor system of rodents: Impacts on nicotine and alcohol reward behaviors. Front. Neurosci. 2021, 15, 745783. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; McQuown, S.C.; Leslie, F.M. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 2009, 122, 125–139. [Google Scholar] [CrossRef]
- Thorpe, H.H.A.; Hamidullah, S.; Jenkins, B.W.; Khokhar, J.Y. Adolescent neurodevelopment and substance use: Receptor expression and behavioral consequences. Pharmacol. Ther. 2020, 206, 107431. [Google Scholar] [CrossRef]
- Engle, S.E.; Shih, P.Y.; McIntosh, J.M.; Drenan, R.M. α4α6β2* nicotinic acetylcholine receptor activation on ventral tegmental area dopamine neurons is sufficient to stimulate a depolarizing conductance and enhance surface AMPA receptor function. Mol. Pharmacol. 2013, 84, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Fattore, L.; Cossu, G.; Tolu, S.; Porcu, E.; McIntosh, J.M.; Changeux, J.P.; Maskos, U.; Fratta, W. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci. 2008, 28, 12318–12327. [Google Scholar] [CrossRef] [PubMed]
- Drenan, R.M.; Grady, S.R.; Whiteaker, P.; McClure-Begley, T.; McKinney, S.; Miwa, J.M.; Bupp, S.; Heintz, N.; McIntosh, J.M.; Bencherif, M.; et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity α6* nicotinic acetylcholine receptors. Neuron 2008, 60, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Guiducci, S.; Tedesco, V.; Corbioli, S.; Zanetti, L.; Moretti, M.; Zanardi, A.; Rimondini, R.; Mugnaini, M.; Clementi, F.; et al. Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area α6β2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J. Neurosci. 2010, 30, 5311–5325. [Google Scholar] [CrossRef] [PubMed]
- Sanjakdar, S.S.; Maldoon, P.P.; Marks, M.J.; Brunzell, D.H.; Maskos, U.; McIntosh, J.M.; Bowers, M.S.; Damaj, M.I. Differential roles of α6β2* and α4β2* neuronal nicotinic receptors in nicotine-and cocaine-conditioned reward in mice. Neuropsychopharmacology 2015, 40, 350–360. [Google Scholar] [CrossRef]
- Dawes, C.T.; Loewen, P.J. The CHRNA6 gene, patience, and voter turnout. Patience Voter Turnout 2009. [Google Scholar] [CrossRef]
- Hoft, N.R.; Corley, R.P.; McQueen, M.B.; Schlaepfer, I.R.; Huizinga, D.; Ehringer, M.A. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology 2009, 34, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Lotfipour, S.; Leonard, G.; Perron, M.; Pike, B.; Richer, L.; Séguin, J.R.; Toro, R.; Veillette, S.; Pausova, Z.; Paus, T. Prenatal exposure to maternal cigarette smoking interacts with a polymorphism in the α6 nicotinic acetylcholine receptor gene to influence drug use and striatum volume in adolescence. Mol. Psychiatry 2010, 15, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D.S.; Mermelstein, R.J.; Hedeker, D.; Coon, H.; Cook, E.H.; McMahon, W.M.; Hamil, C.; Dunn, D.; Weiss, R.B. Effect of neuronal nicotinic acetylcholine receptor genes (CHRN) on longitudinal cigarettes per day in adolescents and young adults. Nicotine Tob. Res. 2014, 16, 137–144. [Google Scholar] [CrossRef]
- Pugach, O.; Cannon, D.S.; Weiss, R.B.; Hedeker, D.; Mermelstein, R.J. Classification tree analysis as a method for uncovering relations between CHRNA5A3B4 and CHRNB3A6 in predicting smoking progression in adolescent smokers. Nicotine Tob. Res. 2017, 19, 410–416. [Google Scholar] [PubMed]
- Gotti, C.; Clementi, F.; Fornari, A.; Gaimarri, A.; Guiducci, S.; Manfredi, I.; Moretti, M.; Pedrazzi, P.; Pucci, L.; Zoli, M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 2009, 78, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar]
- Corringer, P.J.; Sallette, J.; Changeux, J.P. Nicotine enhances intracellular nicotinic receptor maturation: A novel mechanism of neural plasticity? J. Physiol.-Paris 2006, 99, 162–171. [Google Scholar] [CrossRef]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Gotti, C.; Zoli, M.; Clementi, F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef]
- Changeux, J.P. The acetylcholine receptor: An allosteric membrane protein. Biochem. Soc. Trans. 1981, 23, 195–205. [Google Scholar] [CrossRef]
- Changeux, J.P.; Devillers-Thiéry, A.; Chemouilli, P. Acetylcholine receptor: An allosteric protein. Science 1984, 225, 1335–1345. [Google Scholar] [CrossRef]
- Wills, L.; Ables, J.L.; Braunscheidel, K.M.; Caligiuri, S.P.; Elayouby, K.S.; Fillinger, C.; Ishikawa, M.; Moen, J.K.; Kenny, P.J. Neurobiological mechanisms of nicotine reward and aversion. Pharmacol. Rev. 2022, 74, 271–310. [Google Scholar] [CrossRef]
- Le Novere, N.; Zoli, M.; Changeux, J.P. Neuronal nicotinic receptor a6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur. J. Neurosci. 1996, 8, 2428–2439. [Google Scholar] [CrossRef]
- Azam, L.; Winzer-Serhan, U.H.; Chen, Y.; Leslie, F.M. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol. 2002, 444, 260–274. [Google Scholar] [CrossRef]
- Champtiaux, N.; Han, Z.-Y.; Bessis, A.; Rossi, F.M.; Zoli, M.; Marubio, L.; McIntosh, J.M.; Changeux, J.-P. Distribution and pharmacology of α6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J. Neurosci. 2002, 22, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Moretti, M.; Zanardi, A.; Gaimarri, A.; Champtiaux, N.; Changeux, J.P.; Whiteaker, P.; Marks, M.J.; Clementi, F.; Zoli, M. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: Identification of a new native nAChR subtype α3β2 (α5 or β3) enriched in retinocollicular afferents. Mol. Pharmacol. 2005, 68, 1162–1171. [Google Scholar]
- Mackey, E.D.; Engle, S.E.; Kim, M.R.; O’Neill, H.C.; Wageman, C.R.; Patzlaff, N.E.; Wang, Y.; Grady, S.R.; McIntosh, J.M.; Marks, M.J.; et al. α6* nicotinic acetylcholine receptor expression and function in a visual salience circuit. J. Neurosci. 2012, 32, 10226–10237. [Google Scholar] [CrossRef]
- Shih, P.Y.; Engle, S.E.; Oh, G.; Deshpande, P.; Puskar, N.L.; Lester, H.A.; Drenan, R.M. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J. Neurosci. 2014, 34, 9789–9802. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.N.; Engle, S.E.; McIntosh, J.M.; Drenan, R.M. α6-Containing nicotinic acetylcholine receptors in midbrain dopamine neurons are poised to govern dopamine-mediated behaviors and synaptic plasticity. Neuroscience 2015, 304, 161–175. [Google Scholar] [CrossRef]
- Barloscio, D.; Cerri, E.; Domenici, L.; Longhi, R.; Dallanoce, C.; Moretti, M.; Vilella, A.; Zoli, M.; Gotti, C.; Origlia, N. In vivo study of the role of α6-containing nicotinic acetylcholine receptor in retinal function using subtype-specific RDP-MII (E11R) toxin. FASEB J. 2017, 31, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Schneider, A.M.; Mulloy, S.M.; Lee, A.M. Expression pattern of nicotinic acetylcholine receptor subunit transcripts in neurons and astrocytes in the ventral tegmental area and locus coeruleus. Eur. J. Neurosci. 2024, 59, 2225–2239. [Google Scholar] [CrossRef] [PubMed]
- Charpantier, E.; Barnéoud, P.; Moser, P.; Besnard, F.; Sgard, F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport 1998, 9, 3097–3101. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, S.C.; Shin, S.I.; Nelson, A.C.; Pistorius, S.S.; Williams, S.B.; Woodward, T.J.; Park, H.J.; Friend, L.; Gao, M.; Gao, F.; et al. α6 subunit-containing nicotinic receptors mediate low-dose ethanol effects on ventral tegmental area neurons and ethanol reward. Addict. Biol. 2018, 23, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, H.A.; Anderson, E.Q.; Williams, B.M.; Ronström, J.W.; Moen, J.K.; Lee, A.M.; McIntosh, J.M.; Wu, J.; Yorgason, J.T.; Steffensen, S.C. Role of α6-nicotinic receptors in alcohol-induced GABAergic synaptic transmission and plasticity to cholinergic interneurons in the nucleus accumbens. Mol. Neurobiol. 2023, 60, 3113–3129. [Google Scholar] [CrossRef]
- Yang, K.; Buhlman, L.; Khan, G.M.; Nichols, R.A.; Jin, G.; McIntosh, J.M.; Whiteaker, P.; Lukas, R.J.; Wu, J. Functional nicotinic acetylcholine receptors containing α6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. J. Neurosci. 2011, 31, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D. Exploring the role of astroglial glutamate release and association with synapses in neuronal function and behavior. Biol. Psychiatry 2018, 84, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Linker, K.E.; Cross, S.J.; Leslie, F.M. Glial mechanisms underlying substance use disorders. Eur. J. Neurosci. 2019, 50, 2574–2589. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, L.L.; Chandran, K.; Figueroa, L.A.; Barker, J.M. Astrocyte modulation of extinction impairments in ethanol-dependent female mice. Neuropharmacology 2020, 179, 108272. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W. Astrocytes as cellular mediators of cue reactivity in addiction. Curr. Opin. Pharmacol. 2021, 56, 1–6. [Google Scholar] [CrossRef]
- Tizabi, Y.; Getachew, B.; Hauser, S.R.; Tsytsarev, V.; Manhães, A.C.; da Silva, V.D.A. Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction. Brain Sci. 2024, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, N.A.; Tetteh-Quarshie, S.; Henderson, B.J. Neuronal excitability in the medial habenula and ventral tegmental area is differentially modulated by nicotine dosage and menthol in a sex-specific manner. eNeuro 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Verplaetse, T.L.; Morris, E.D.; McKee, S.A.; Cosgrove, K.P. Sex differences in the nicotinic acetylcholine and dopamine receptor systems underlying tobacco smoking addiction. Curr. Opin. Behav. Sci. 2018, 23, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, D.; Ma, X.; Sudweeks, S.; Yorgason, J.T.; Gao, M.; Turner, D.; Eaton, J.B.; McIntosh, J.M.; Lukas, R.J.; et al. Alpha6-containing nicotinic acetylcholine receptor is a highly sensitive target of alcohol. Neuropharmacology 2019, 149, 45–54. [Google Scholar] [CrossRef]
- Azam, L.; McIntosh, J.M. Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol. Sin. 2009, 30, 771–783. [Google Scholar] [CrossRef]
- Grady, S.R.; Salminen, O.; Laverty, D.C.; Whiteaker, P.; McIntosh, J.M.; Collins, A.C.; Marks, M.J. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem. Pharmacol. 2007, 74, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Grady, S.R.; Moretti, M.; Zoli, M.; Marks, M.J.; Zanardi, A.; Pucci, L.; Clementi, F.; Gotti, C. Rodent habenulo–interpeduncular pathway expresses a large variety of uncommon nAChR subtypes 2009, but only the α3β4 and α3β3β4 subtypes mediate acetylcholine release. J. Neurosci. 2009, 29, 2272–2282. [Google Scholar] [CrossRef]
- Letchworth, S.R.; Whiteaker, P. Progress and challenges in the study of α6-containing nicotinic acetylcholine receptors. Biochem. Pharmacol. 2011, 82, 862–872. [Google Scholar] [CrossRef]
- Le Novere, N.; Changeux, J.P. Molecular evolution of the nicotinic acetylcholine receptor: An example of multigene family in excitable cells. J. Mol. Evol. 1995, 40, 155–172. [Google Scholar] [CrossRef]
- Cardenas, A.; Elabd, M.; Lotfipour, S. Specificity of a rodent alpha (α) 6 nicotinic acetylcholine receptor subunit antibody. Psychopharmacology 2020, 237, 283–285. [Google Scholar] [CrossRef]
- Lebbe, E.K.; Peigneur, S.; Wijesekara, I.; Tytgat, J. Conotoxins targeting nicotinic acetylcholine receptors: An overview. Mar. Drugs 2014, 12, 2970–3004. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; Gajewiak, J.; Christensen, S.; Lindstrom, J.; McIntosh, J.M. α-Conotoxin PeIA [S9H, V10A, E14N] potently and selectively blocks α6β2β3 versus α6β4 nicotinic acetylcholine receptors. Mol. Pharmacol. 2012, 82, 972–982. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.M.; Azam, L.; Staheli, S.; Dowell, C.; Lindstrom, J.M.; Kuryatov, A.; Garrett, J.E.; Marks, M.J.; Whiteaker, P. Analogs of α-conotoxin MII are selective for α6-containing nicotinic acetylcholine receptors. Mol. Pharmacol. 2004, 65, 944–952. [Google Scholar] [CrossRef]
- Kulak, J.M.; Nguyen, T.A.; Olivera, B.M.; McIntosh, J.M. α-Conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J. Neurosci. 1997, 17, 5263–5270. [Google Scholar] [CrossRef] [PubMed]
- Exley, R.; Clements, M.A.; Hartung, H.; McIntosh, J.M.; Cragg, S.J. α6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology 2008, 33, 2158–2166. [Google Scholar] [CrossRef] [PubMed]
- Dowell, C.; Olivera, B.M.; Garrett, J.E.; Staheli, S.T.; Watkins, M.; Kuryatov, A.; Yoshikami, D.; Lindstrom, J.M.; McIntosh, J.M. α-Conotoxin PIA is selective for α6 subunit-containing nicotinic acetylcholine receptors. J. Neurosci. 2003, 23, 8445–8452. [Google Scholar] [CrossRef]
- Pucci, L.; Grazioso, G.; Dallanoce, C.; Rizzi, L.; De Micheli, C.; Clementi, F.; Bertrand, S.; Bertrand, D.; Longhi, R.; De Amici, M.; et al. Engineering of α-conotoxin MII-derived peptides with increased selectivity for native α6β2* nicotinic acetylcholine receptors. FASEB J. 2011, 25, 3775–3789. [Google Scholar] [CrossRef] [PubMed]
- Azam, L.; Maskos, U.; Changeux, J.P.; Dowell, C.D.; Christensen, S.; De Biasi, M.; McIntosh, J.M. α-Conotoxin BuIA [T5A; P6O]: A novel ligand that discriminates between α6β4 and α6β2 nicotinic acetylcholine receptors and blocks nicotine-stimulated norepinephrine release. FASEB J. 2010, 24, 5113. [Google Scholar] [CrossRef] [PubMed]
- Armishaw, C.J. Synthetic α-conotoxin mutants as probes for studying nicotinic acetylcholine receptors and in the development of novel drug leads. Toxins 2010, 2, 1471–1499. [Google Scholar] [CrossRef]
- Millar, N.S.; Harkness, P.C. Assembly and trafficking of nicotinic acetylcholine receptors. Mol. Membr. Biol. 2008, 25, 279–292. [Google Scholar] [CrossRef]
- Kuryatov, A.; Olale, F.; Cooper, J.; Choi, C. Human α6 AChR subtypes: Subunit composition, assembly, and pharmacological responses. Neuropharmacology 2000, 39, 2570–2590. [Google Scholar] [CrossRef]
- Evans, N.M.; Bose, S.; Benedetti, G.; Zwart, R.; Pearson, K.H.; McPhie, G.I.; Craig, P.J.; Benton, J.P.; Volsen, S.G.; Sher, E.; et al. Expression and functional characterisation of a human chimeric nicotinic receptor with α6β4 properties. Eur. J. Pharmacol. 2003, 466, 31–39. [Google Scholar] [CrossRef]
- Gu, S.; Matta, J.A.; Lord, B.; Harrington, A.W.; Sutton, S.W.; Davini, W.B.; Bredt, D.S. Brain α7 nicotinic acetylcholine receptor assembly requires NACHO. Neuron 2016, 89, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.A.; Gu, S.; Davini, W.B.; Lord, B.; Siuda, E.R.; Harrington, A.W.; Bredt, D.S. NACHO mediates nicotinic acetylcholine receptor function throughout the brain. Cell Rep. 2017, 19, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Matta, J.A.; Davini, W.B.; Dawe, G.B.; Lord, B.; Bredt, D.S. α6-Containing nicotinic acetylcholine receptor reconstitution involves mechanistically distinct accessory components. Cell Rep. 2019, 26, 866–874. [Google Scholar] [CrossRef]
- Knowland, D.; Gu, S.; Eckert, W.A.; Dawe, G.B.; Matta, J.A.; Limberis, J.; Wickenden, A.D.; Bhattacharya, A.; Bredt, D.S. Functional α6β4 acetylcholine receptor expression enables pharmacological testing of nicotinic agonists with analgesic properties. J. Clin. Investig. 2023, 130, 6158–6170. [Google Scholar] [CrossRef]
- A Lester, H.; Fonck, C.; Tapper, A.R.; McKinney, S.; Damaj, M.I.; Balogh, S.; Owens, J.; Wehner, J.M.; Collins, A.C.; Labarca, C. Hypersensitive knockin mouse strains identify receptors and pathways for nicotine action. Curr. Opin. Drug Discov. Dev. 2003, 6, 633–639. [Google Scholar]
- Lotfipour, S.; Mojica, C.; Nakauchi, S.; Lipovsek, M.; Silverstein, S.; Cushman, J.; Tirtorahardjo, J.; Poulos, A.; Elgoyhen, A.B.; Sumikawa, K.; et al. α2* Nicotinic acetylcholine receptors influence hippocampus-dependent learning and memory in adolescent mice. Learn. Mem. 2017, 24, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.B.; Yan, Y.; Tapia, M.A.; Tucker, B.R.; Thomas, L.N.; George, B.E.; West, A.M.; Marotta, C.B.; Lester, H.A.; Dougherty, D.A.; et al. β2 nAChR activation on VTA DA neurons is sufficient for nicotine reinforcement in rats. eNeuro 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.C.; Mojica, C.; Lotfipour, S. Hypersensitivity of the nicotinic acetylcholine receptor subunit (CHRNA2L9′ S/L9′ S) in female adolescent mice produces deficits in nicotine-induced facilitation of hippocampal-dependent learning and memory. Neurobiol. Learn. Mem. 2024, 213, 107959. [Google Scholar] [CrossRef]
- Cardenas, A.; Bai, Y.; Hajy Heydary, Y.; Li, J.; Leslie, F.M.; Lotfipour, S. Sex-and genotype-dependent nicotine-induced behaviors in adolescent rats with a human polymorphism (rs2304297) in the 3′-UTR of the CHRNA 6 gene. Int. J. Mol. Sci. 2022, 23, 3145. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.A. Mitigating Risk: Smartphone Notifications, Adaptive Surveying, and Genetics. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2015. [Google Scholar]
- Fletcher, J.M. Why have tobacco control policies stalled? Using genetic moderation to examine policy impacts. PLoS ONE 2012, 7, e50576. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, M.; Labbe, A.; Roy-Gagnon, M.H.; Low, N.C.; Dugas, E.; Engert, J.C.; O’Loughlin, J. The association between CHRN genetic variants and dizziness at first inhalation of cigarette smoke. Addict. Behav. 2014, 39, 316–320. [Google Scholar] [CrossRef]
- Lee, W.; Bergen, A.W.; E Swan, G.; Li, D.; Liu, J.; Thomas, P.; Tyndale, R.F.; Benowitz, N.L.; Lerman, C.; Conti, D.V. Gender-stratified gene and gene–treatment interactions in smoking cessation. Pharmacogenomics J. 2012, 12, 521–532.M. [Google Scholar] [CrossRef] [PubMed]
- Thorgeirsson, T.E.; Gudbjartsson, D.F.; Surakka, I.; Vink, J.M.; Amin, N.; Geller, F.; Sulem, P.; Rafnar, T.; Esko, T.; Walter, S.; et al. Sequence variants at CHRNB3–CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 2010, 42, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, J.S.; Haberstick, B.C.; Schlaepfer, I.; Collins, A.C.; Corley, R.P.; Crowley, T.J.; Hewitt, J.K.; Hopfer, C.J.; Lessem, J.; McQueen, M.B.; et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum. Mol. Genet. 2008, 17, 724–734. [Google Scholar] [CrossRef]
- Saccone, S.F.; Hinrichs, A.L.; Saccone, N.L.; Chase, G.A.; Konvicka, K.; Madden, P.A.; Breslau, N.; Johnson, E.O.; Hatsukami, D.; Pomerleau, O.; et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 2007, 16, 36–49. [Google Scholar] [CrossRef]
- Rigbi, A.; Kanyas, K.; Yakir, A.; Greenbaum, L.; Pollak, Y.; Ben-Asher, E.; Lancet, D.; Kertzman, S.; Lerer, B. Why do young women smoke? V. Role of direct and interactive effects of nicotinic cholinergic receptor gene variation on neurocognitive function. Genes Brain Behav. 2008, 7, 164–172. [Google Scholar] [CrossRef]
- Prochaska, J.J.; Benowitz, N.L. Current advances in research in treatment and recovery: Nicotine addiction. Sci. Adv. 2019, 5, eaay9763. [Google Scholar] [CrossRef]
- Acquah, F.A.; Paramel, M.; Kuta, A.; Hussaini, S.R.; Wallace, D.R.; Mooers, B.H. Simulations of Promising Indolizidine—α6-β2 Nicotinic Acetylcholine Receptor Complexes. Int. J. Mol. Sci. 2021, 22, 7934. [Google Scholar] [CrossRef]
- Linker, K.E.; Gad, M.; Tawadrous, P.; Cano, M.; Green, K.N.; Wood, M.A.; Leslie, F.M. Microglial activation increases cocaine self-administration following adolescent nicotine exposure. Nat. Commun. 2020, 11, 306. [Google Scholar] [CrossRef]
- Lee, A.M.; Mansuri, M.S.; Wilson, R.S.; Lam, T.T.; Nairn, A.C.; Picciotto, M.R. Sex differences in the ventral tegmental area and nucleus accumbens proteome at baseline and following nicotine exposure. Front. Mol. Neurosci. 2021, 14, 657064. [Google Scholar] [CrossRef]
- López, A.J.; Johnson, A.R.; Euston, T.J.; Wilson, R.; Nolan, S.O.; Brady, L.J.; Thibeault, K.C.; Kelly, S.J.; Kondev, V.; Melugin, P.; et al. Cocaine self-administration induces sex-dependent protein expression in the nucleus accumbens. Commun. Biol. 2021, 4, 883. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Hussmann, J.A.; Weissman, J.S. Ribosome profiling: Global views of translation. Cold Spring Harb. Perspect. Biol. 2019, 11, a032698. [Google Scholar] [CrossRef]
- Jones, S.; Sudweeks, S.; Yakel, J.L. Nicotinic receptors in the brain: Correlating physiology with function. Trends Neurosci. 1999, 22, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Galvin, V.C.; Arnsten, A.F.; Wang, M. Involvement of nicotinic receptors in working memory function. Behav. Pharmacol. Cholinergic Syst. 2020, 45, 89–99. [Google Scholar]
- Koukouli, F.; Changeux, J.P. Do nicotinic receptors modulate high-order cognitive processing? Trends Neurosci. 2020, 43, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Soares, A.R.; Etherington, I.M.; Abdulla, Z.I.; Picciotto, M.R. Pathophysiology of nAChRs: Limbic circuits and related disorders. Pharmacol. Res. 2023, 191, 106745. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Morales, M. The brain on drugs: From reward to addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.; Jerlhag, E.; Liljequist, S.; Engel, J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology 2009, 203, 99–108. [Google Scholar] [CrossRef]
- Brunzell, D.H.; Boschen, K.E.; Hendrick, E.S.; Beardsley, P.M.; McIntosh, J.M. α-Conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology 2010, 35, 665–673. [Google Scholar] [CrossRef]
- Collo, G.; Cavalleri, L.; Zoli, M.; Maskos, U.; Ratti, E.; Merlo Pich, E. Alpha6-containing nicotinic acetylcholine receptors mediate nicotine-induced structural plasticity in mouse and human iPSC-derived dopaminergic neurons. Front. Pharmacol. 2018, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Natividad, L.A.; Buczynski, M.W.; McClatchy, D.B.; Yates, J.R., III. From synapse to function: A perspective on the role of neuroproteomics in elucidating mechanisms of drug addiction. Proteomes 2018, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.N.; Banazadeh, M.; Alitaneh, Z.; Suha, A.J.; Esmaeili, A.; Hasannejad-Asl, B.; Siahposht-Khachaki, A.; Hassanshahi, A.; Bagheri-Mohammadi, S. The distribution of neurotransmitters in the brain circuitry: Mesolimbic pathway and addiction. Physiol. Behav. 2024, 284, 114639. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, M.; Dani, J.A. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 2011, 34, 105–130. [Google Scholar] [CrossRef]
- Azam, L.; McIntosh, J.M. Characterization of nicotinic acetylcholine receptors that modulate nicotine-evoked [3H] norepinephrine release from mouse hippocampal synaptosomes. Mol. Pharmacol. 2006, 70, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Zhao-Shea, R.; Liu, L.; Soll, L.G.; Improgo, M.R.; Meyers, E.E.; McIntosh, J.M.; Grady, S.R.; Marks, M.J.; Gardner, P.D.; Tapper, A.R. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao-Shea, R.; McIntosh, J.M.; Gardner, P.D.; Tapper, A.R. Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing α4 and α6 subunits. Mol. Pharmacol. 2012, 81, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Zoli, M.; Rimondini, R.; Léna, C.; Marubio, L.M.; Pich, E.M.; Fuxe, K.; Changeux, J.-P. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 1998, 391, 173–177. [Google Scholar] [CrossRef]
- Kamens, H.M.; Peck, C.; Garrity, C.; Gechlik, A.; Jenkins, B.C.; Rajan, A. α6β2 nicotinic acetylcholine receptors influence locomotor activity and ethanol consumption. Alcohol 2017, 61, 43–49. [Google Scholar] [CrossRef]
- Jackson, K.J.; McIntosh, J.M.; Brunzell, D.H.; Sanjakdar, S.S.; Damaj, M. The role of α6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J. Pharmacol. Exp. Ther. 2009, 331, 547–554. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Meyer, A.C.; Pivavarchyk, M.; Horton, D.B.; Zheng, G.; Smith, A.M.; Wooters, T.E.; McIntosh, J.M.; Crooks, P.A.; Bardo, M.T.; et al. r-bPiDI, an α6β2* nicotinic receptor antagonist, decreases nicotine-evoked dopamine release and nicotine reinforcement. Neurochem. Res. 2015, 40, 2121–2130. [Google Scholar] [CrossRef]
- Maggio, S.E.; Saunders, M.A.; Nixon, K.; Prendergast, M.A.; Zheng, G.; Crooks, P.A.; Dwoskin, L.P.; Bell, R.L.; Bardo, M.T. An improved model of ethanol and nicotine co-use in female P rats: Effects of naltrexone, varenicline, and the selective nicotinic α6β2* antagonist r-bPiDI. Drug Alcohol Depend. 2018, 193, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Cheng, R.; Ma, J.Z.; Swan, G.E. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 2003, 98, 23–31. [Google Scholar] [CrossRef]
- Maes, H.H.; Sullivan, P.F.; Bulik, C.M.; Neale, M.C.; Prescott, C.A.; Eaves, L.J.; Kendler, K.S. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol. Med. 2004, 34, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.M.; Chen, H. Neurogenetic determinants and mechanisms of addiction to nicotine and smoked tobacco. Eur. J. Neurosci. 2019, 50, 2164–2179. [Google Scholar] [CrossRef]
- Bierut, L.J.; Stitzel, J.A. Genetic Contributions of the α5 Nicotinic Receptor Subunit to Smoking Behavior. In Nicotinic Receptors; Humana Press: New York, NY, USA, 2014; pp. 327–339. [Google Scholar]
- Morel, C.; Fattore, L.; Pons, S.; A Hay, Y.; Marti, F.; Lambolez, B.; De Biasi, M.; Lathrop, M.; Fratta, W.; Maskos, U.; et al. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol. Psychiatry 2014, 19, 930–936. [Google Scholar] [CrossRef]
- Wen, L.; Han, H.; Liu, Q.; Su, K.; Yang, Z.; Cui, W.; Yuan, W.; Ma, Y.; Fan, R.; Chen, J.; et al. Significant association of the CHRNB3-CHRNA6 gene cluster with nicotine dependence in the Chinese Han population. Sci. Rep. 2017, 7, 9745. [Google Scholar] [CrossRef] [PubMed]
- Carreño, D.; Lotfipour, S. Sex-and genotype-dependent nicotine plus cue-primed reinstatement is enhanced in adolescent Sprague Dawley rats containing the human CHRNA 6 3′-UTR polymorphism (rs2304297). Front. Psychiatry 2023, 13, 1064211. [Google Scholar] [CrossRef] [PubMed]
- Carreño, D.; Facundo, A.; Cardenas, A.; Lotfipour, S. Sub-chronic nicotine exposure influences methamphetamine self-administration and dopamine overflow in a sex-and genotype-dependent manner in humanized CHRNA 6 3′-UTR SNP (rs2304297) adolescent rats. Front. Pharmacol. 2024, 15, 1445303. [Google Scholar] [CrossRef] [PubMed]
- Daeppen, J.B.; Smith, T.L.; Danko, G.P.; Gordon, L.; Landi, N.A.; Nurnberger, J.I., Jr.; Bucholz, K.K.; Raimo, E.; Schuckit, M.A. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. Alcohol Alcohol. 2000, 35, 171–175. [Google Scholar] [CrossRef] [PubMed]
- John, U.; Meyer, C.; Rumpf, H.J.; Schumann, A.; Thyrian, J.R.; Hapke, U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003, 38, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Hasin, D.S.; Chou, S.P.; Stinson, F.S.; Dawson, D.A. Nicotine dependence and psychiatric disorders in the united states: Results from the national epidemiologic survey on alcohol and relatedconditions. Arch. Gen. Psychiatry 2004, 61, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.H.; Zhu, J.; Levin, J.; Moeller, S.J.; McKee, S.A.; Goodwin, R.D. Changes in alcohol use by cannabis use status among adolescents and young adults in the United States: Emerging evidence for both substitution and complementarity. Alcohol. Clin. Exp. Res. 2021, 45, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Frie, J.A.; Nolan, C.J.; Murray, J.E.; Khokhar, J.Y. Addiction-related outcomes of nicotine and alcohol co-use: New insights following the rise in vaping. Nicotine Tob. Res. 2022, 24, 1141–1149. [Google Scholar] [CrossRef]
- Cannizzaro, E.; Lavanco, G.; Castelli, V.; Cirrincione, L.; Di Majo, D.; Martines, F.; Argo, A.; Plescia, F. Alcohol and nicotine use among adolescents: An observational study in a Sicilian cohort of high school students. Int. J. Environ. Res. Public Health 2022, 19, 6152. [Google Scholar] [CrossRef]
- Patrick, M.E.; Parks, M.J.; Carroll, D.M.; Mitchell, C. Feasibility of mailed biomarker data collection among US young adults: Saliva-based cotinine and self-reported nicotine use. Drug Alcohol Depend. 2023, 244, 109791. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Morrisett, R.A. Ethanol action on dopaminergic neurons in the ventral tegmental area: Interaction with intrinsic ion channels and neurotransmitter inputs. Int. Rev. Neurobiol. 2010, 91, 235–288. [Google Scholar]
- Rahman, S.; Engleman, E.A.; Bell, R.L. Nicotinic receptor modulation to treat alcohol and drug dependence. Front. Neurosci. 2015, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, O.; Ericson, M.; Engel, J.A.; Söderpalm, B. Accumbal dopamine overflow after ethanol: Localization of the antagonizing effect of mecamylamine. Eur. J. Pharmacol. 1997, 334, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Svensson, L.; Söderpalm, B.; Engel, J.A. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol 2002, 28, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Jerlhag, E.; Svensson, L.; Söderpalm, B.; Engel, J.A. Is an α-conotoxin MII–sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol 2004, 34, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kamens, H.M.; Hoft, N.R.; Cox, R.J.; Miyamoto, J.H.; Ehringer, M.A. The α6 nicotinic acetylcholine receptor subunit influences ethanol-induced sedation. Alcohol 2012, 46, 463–471. [Google Scholar] [CrossRef]
- Feduccia, A.A.; Chatterjee, S.; Bartlett, S.E. Neuronal nicotinic acetylcholine receptors: Neuroplastic changes underlying alcohol and nicotine addictions. Front. Mol. Neurosci. 2012, 5, 83. [Google Scholar] [CrossRef]
- Liu, L.; Zhao-Shea, R.; McIntosh, J.M.; Tapper, A.R. Nicotinic acetylcholine receptors containing the α6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons. Biochem. Pharmacol. 2013, 86, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.S.; Broderick, H.J.; Drenan, R.M.; Chester, J.A. Nicotinic acetylcholine receptors containing α6 subunits contribute to alcohol reward-related behaviours. Genes Brain Behav. 2013, 12, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lindstrom, J. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018, 175, 1805–1821. [Google Scholar] [CrossRef]
- Yang, K.C.; Jin, G.Z.; Wu, J. Mysterious α6-containing nAChRs: Function, pharmacology, and pathophysiology. Acta Pharmacol. Sin. 2009, 30, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Schilaty, N.D.; Hedges, D.M.; Jang, E.Y.; Folsom, R.J.; Yorgason, J.T.; McIntosh, J.M.; Steffensen, S.C. Acute ethanol inhibits dopamine release in the nucleus accumbens via α6 nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2014, 349, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.; Lees, A.; Stern, M. Milestones in Parkinson’s disease—Clinical and pathologic features. Mov. Disord. 2011, 26, 1015–1021. [Google Scholar] [CrossRef]
- Rascol, O.; Lozano, A.; Stern, M.; Poewe, W. Milestones in Parkinson’s disease therapeutics. Mov. Disord. 2011, 26, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Pérez, X.A.; Bordia, T.; McIntosh, J.M.; Quik, M. α6β2* and α4β2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: Relevance to Parkinson’s disease. Mol. Pharmacol. 2010, 78, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Pérez, X.A.; Quik, M. Focus on α4β2* and α6β2* nAChRs for Parkinson’s Disease Therapeutics. Mol. Cell. Pharmacol. 2011, 3, 1. [Google Scholar]
- Hansen, C.A.; Miller, D.R.; Annarumma, S.; Rusch, C.T.; Ramirez-Zamora, A.; Khoshbouei, H. Levodopa-induced dyskinesia: A historical review of Parkinson’s disease, dopamine, and modern advancements in research and treatment. J. Neurol. 2022, 269, 2892–2909. [Google Scholar] [CrossRef] [PubMed]
- Manson, A.; Stirpe, P.; Schrag, A. Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J. Park. Dis. 2012, 2, 189–198. [Google Scholar] [CrossRef] [PubMed]
- LeWitt, P.A. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov. Disord. 2015, 30, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Perez, X.A.; Bordia, T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov. Disord. 2012, 27, 947–957. [Google Scholar] [CrossRef]
- Bordia, T.; Hrachova, M.; Chin, M.; McIntosh, J.M.; Quik, M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J. Pharmacol. Exp. Ther. 2012, 342, 327–334. [Google Scholar] [CrossRef]
- Quik, M.; Perez, X.A.; Grady, S.R. Role of α6 nicotinic receptors in CNS dopaminergic function: Relevance to addiction and neurological disorders. Biochem. Pharmacol. 2011, 82, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; O’Neill, M.; Perez, X.A. Nicotine neuroprotection against nigrostriatal damage: Importance of the animal model. Trends Pharmacol. Sci. 2007, 28, 229–235. [Google Scholar] [CrossRef]
- Bordia, T.; Campos, C.; Huang, L.; Quik, M. Continuous and intermittent nicotine treatment reduces L-3, 4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2008, 327, 239–247. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Zoli, M. Neuroprotection via nAChRs: The role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008, 13, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mallela, A.; Sohn, D.; Carroll, F.I.; Bencherif, M.; Letchworth, S.; Quik, M. Nicotinic Receptor Agonists Reduce l-DOPA–Induced Dyskinesias in a Monkey Model of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2013, 347, 225–234. [Google Scholar] [CrossRef] [PubMed]
- van Hout, M.; Klein, J.; Ahring, P.K.; Brown, D.T.; Thaneshwaran, S.; Dos Santos, A.B.; Jensen, A.A.; Kohlmeier, K.A.; Christophersen, P.; Dyhring, T. Characterization of AN6001, a positive allosteric modulator of α6β2-containing nicotinic acetylcholine receptors. Biochem. Pharmacol. 2020, 174, 113788. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; Loeser, J.D.; Baron, R.; Edwards, R.R. Assessment of chronic pain: Domains, methods, and mechanisms. J. Pain 2016, 17, T10–T20. [Google Scholar] [CrossRef]
- Inoue, K. Nociceptive signaling of P2X receptors in chronic pain states. Purinergic Signal. 2021, 17, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.R.; Zhang, W.J.; Luo, H.L. Association between P2X3 receptors and neuropathic pain: As a potential therapeutic target for therapy. Biomed. Pharmacother. 2022, 150, 113029. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Horenstein, N.A. Therapeutic targeting of α7 nicotinic acetylcholine receptors. Pharmacol. Rev. 2021, 73, 1118–1149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Christensen, S.B.; Dowell, C.; Purushottam, L.; Skalicky, J.J.; McIntosh, J.M.; Chou, D.H.C. Discovery of methylene thioacetal-incorporated α-RgIA analogues as potent and stable antagonists of the human α9α10 nicotinic acetylcholine receptor for the treatment of neuropathic pain. J. Med. Chem. 2021, 64, 9513–9524. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors: Therapeutic targets for novel ligands to treat pain and inflammation. Pharmacol. Res. 2023, 190, 106715. [Google Scholar] [CrossRef]
- Hone, A.J.; Meyer, E.L.; McIntyre, M.; McIntosh, J.M. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the α6β4* subtype. FASEB J. 2012, 26, 917. [Google Scholar] [CrossRef] [PubMed]
- Wieskopf, J.S.; Mathur, J.; Limapichat, W.; Post, M.R.; Al-Qazzaz, M.; Sorge, R.E.; Martin, L.J.; Zaykin, D.V.; Smith, S.B.; Freitas, K.; et al. The nicotinic α6 subunit gene determines variability in chronic pain sensitivity via cross-inhibition of P2X2/3 receptors. Sci. Transl. Med. 2015, 7, ra72–ra287. [Google Scholar] [CrossRef] [PubMed]
- Corongiu, S.; Dessì, C.; Cadoni, C. Adolescence versus adulthood: Differences in basal mesolimbic and nigrostriatal dopamine transmission and response to drugs of abuse. Addict. Biol. 2020, 25, e12721. [Google Scholar] [CrossRef]
- Xue, S.; Behnood-Rod, A.; Wilson, R.; Wilks, I.; Tan, S.; Bruijnzeel, A.W. Rewarding effects of nicotine in adolescent and adult male and female rats as measured using intracranial self-stimulation. Nicotine Tob. Res. 2020, 22, 172–179. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Hoft, N.R.; Ehringer, M.A. The genetic components of alcohol and nicotine co-addiction: From genes to behavior. Curr. Drug Abus. Rev. 2008, 1, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Hoft, N.R.; Corley, R.P.; McQueen, M.B.; Huizinga, D.; Menard, S.; Ehringer, M.A. SNPs in CHRNA6 and CHRNB3 are associated with alcohol consumption in a nationally representative sample. Genes Brain Behav. 2009, 8, 631–637. [Google Scholar] [CrossRef] [PubMed]

| α6*nAChR Subtype | Brain Region | Organism | Supporting Publication | Neuronal Population |
|---|---|---|---|---|
| α6β2 |
| Mouse Rat | [34] [55] [47] [18] [56] | Dopamine GABA Glutamate Norepinephrine |
| α6β4 |
| Mouse | [56] | Dopamine Norepinephrine |
| α6β2β3 |
| Mouse | [34] [55] [40] [57] [38] | Dopamine Norepinephrine |
| α6β3β4 |
| Mouse | [58] | - |
| α4α6β2 |
| Mouse | [18] [56] | Dopamine GABA |
| α4α6β2β3 |
| Mouse | [43] [34] [55] [39] [38] | Dopamine GABA Glutamate |
| α4α5α6β2β3 |
| Mouse | [43] | Dopamine |
| Supporting Publication | Adolescent | Sample Size | % Male | “G” as Risk Allele |
|---|---|---|---|---|
| [27] | Yes | 480 | 40 | Yes |
| [83] | No | 12,507 | 40.9 | No % |
| [23] | Yes | 439 | 41 | Yes |
| [84] | No | 6178 | 48 | No % |
| [85] | Yes | 1293 | 35 | No # |
| [86] | No | 789 | 48.9 | Yes * |
| [87] | No | 76,681 | Not Reported | Yes * |
| [22] | Yes | 423 | 45.4 | Yes |
| [21] | Yes | 1051 | 49.2 | Yes |
| [21] | Yes | 1051 | 49 | No + |
| [20] | Yes | 2674 | 48 | Yes |
| [88] | Yes | 1056 | 58.1 | Yes |
| [89] | No | 1929 | 38 | Yes |
| [90] | No | 244 | 0 | Yes ^ |
| Behavioral Measure | Male Rats α6GG or α6CC | Female Rats α6GG or α6CC |
|---|---|---|
| Locomotor Activity and Anxiolytic Behavior | 1. Increased locomotor activity and anxiolytic behavior in nicotine-treated male α6GG rats compared to saline controls 2. Increased anxiolytic behavior in nicotine-treated α6GG males compared to α6CC males | Increased locomotor activity and anxiolytic behavior in nicotine-treated α6CC rats compared to saline controls as well as nicotine-treated α6GG rats |
| Dopamine Release | Increased dopamine overflow in response to nicotine and methamphetamine in α6GG mice compared to α6CC rats | Increased dopamine overflow in response to nicotine and methamphetamine in α6CC rats compared to α6GG rats |
| Methamphetamine Self-administration | No genotype effect observed in methamphetamine self-administration after exposure to nicotine | Enhanced discrimination between reinforced and non-reinforced response in nicotine-treated α6CC rats and saline-treated α6GG rats |
| Tissue Neurotransmitter Levels | 1. Nicotine + cue-treated adolescent α6GG rats had decreased dopamine levels in the NAc shell compared to other groups 2. Baseline differences: drug-naïve adolescent α6GG rats had greater dopamine levels in the NAc core compared to their adult counterparts and adolescent α6CC rats | No differences were observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajy Heydary, Y.; Castro, E.M.; Lotfipour, S.; Leslie, F.M. Unraveling the Role of CHRNA6, the Neuronal α6 Nicotinic Acetylcholine Receptor Subunit. Receptors 2025, 4, 1. https://doi.org/10.3390/receptors4010001
Hajy Heydary Y, Castro EM, Lotfipour S, Leslie FM. Unraveling the Role of CHRNA6, the Neuronal α6 Nicotinic Acetylcholine Receptor Subunit. Receptors. 2025; 4(1):1. https://doi.org/10.3390/receptors4010001
Chicago/Turabian StyleHajy Heydary, Yasamin, Emily M. Castro, Shahrdad Lotfipour, and Frances M. Leslie. 2025. "Unraveling the Role of CHRNA6, the Neuronal α6 Nicotinic Acetylcholine Receptor Subunit" Receptors 4, no. 1: 1. https://doi.org/10.3390/receptors4010001
APA StyleHajy Heydary, Y., Castro, E. M., Lotfipour, S., & Leslie, F. M. (2025). Unraveling the Role of CHRNA6, the Neuronal α6 Nicotinic Acetylcholine Receptor Subunit. Receptors, 4(1), 1. https://doi.org/10.3390/receptors4010001







