1. Introduction
Type B aortic dissection (TBAD) remains a complex clinical challenge. Although thoracic endovascular aortic repair (TEVAR) improves early outcomes, long-term complications, including persistent false lumen (FL) reperfusion, aortic dilatation, endoleaks, and eventual rupture—are still common. In selected patients, targeted FL occlusion may represent a viable strategy for preventing aneurysmal progression and enhancing aortic remodeling. Different endovascular methods have been developed for this purpose, including the candy-plug, Knickerbocker, and cork-in-the-bottleneck techniques, as well as coil or plug embolization strategies. However, each approach presents limitations in terms of technical feasibility, anatomical suitability, and long-term durability. Here, we report the first documented case of FL exclusion using a custom-made false lumen occlusion device (FLOD) in a patient with chronic TBAD (cTBAD) and aneurysmal degeneration after prior TEVAR.
2. Case Presentation
A 77-year-old male, a former smoker, with a history of arterial hypertension and chronic obstructive pulmonary disease, underwent TEVAR in 2017 for a Stanford type B aortic dissection. The dissection originated just distal to the left subclavian artery (LSA) and extended to the origin of the left renal artery.
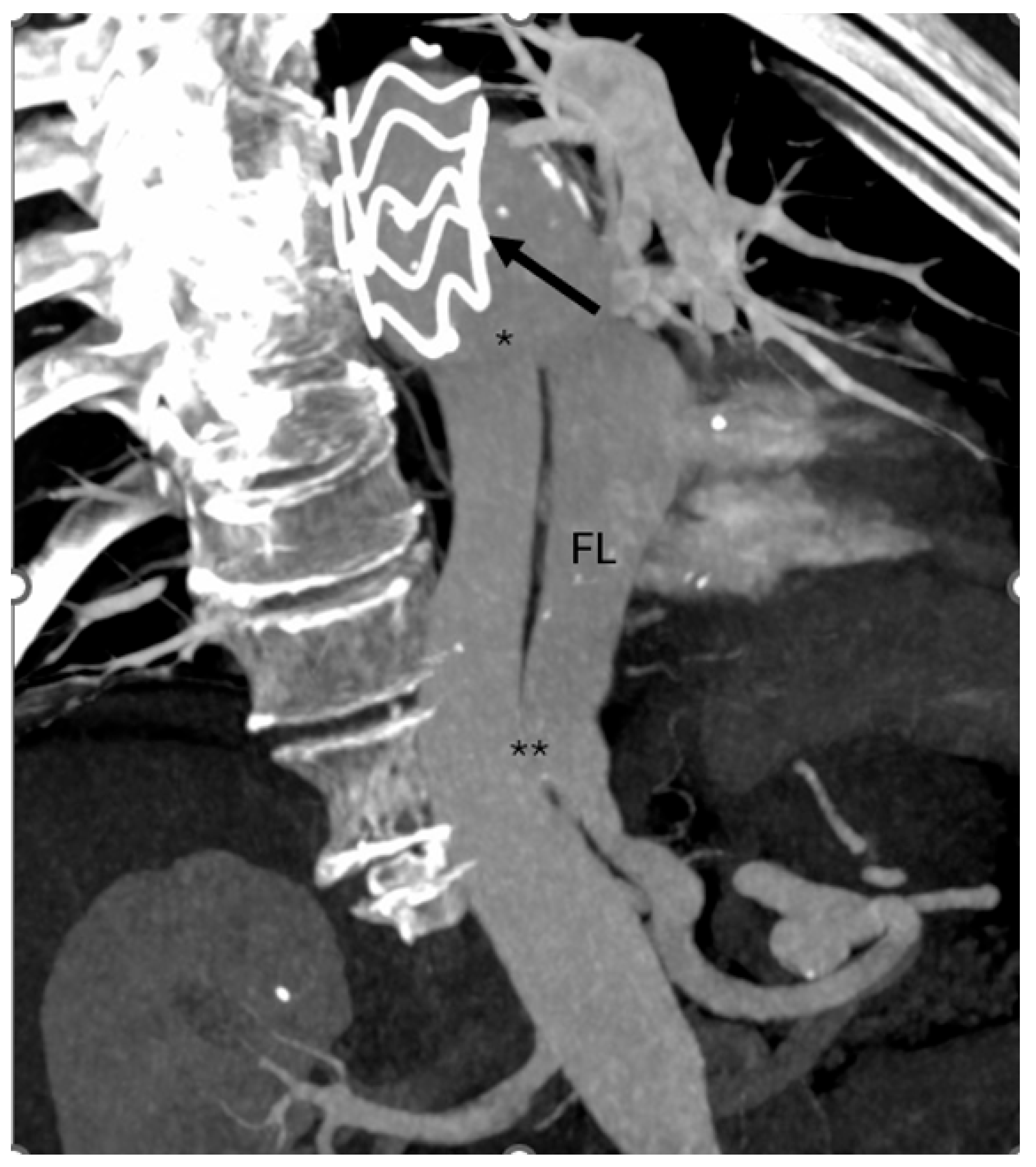
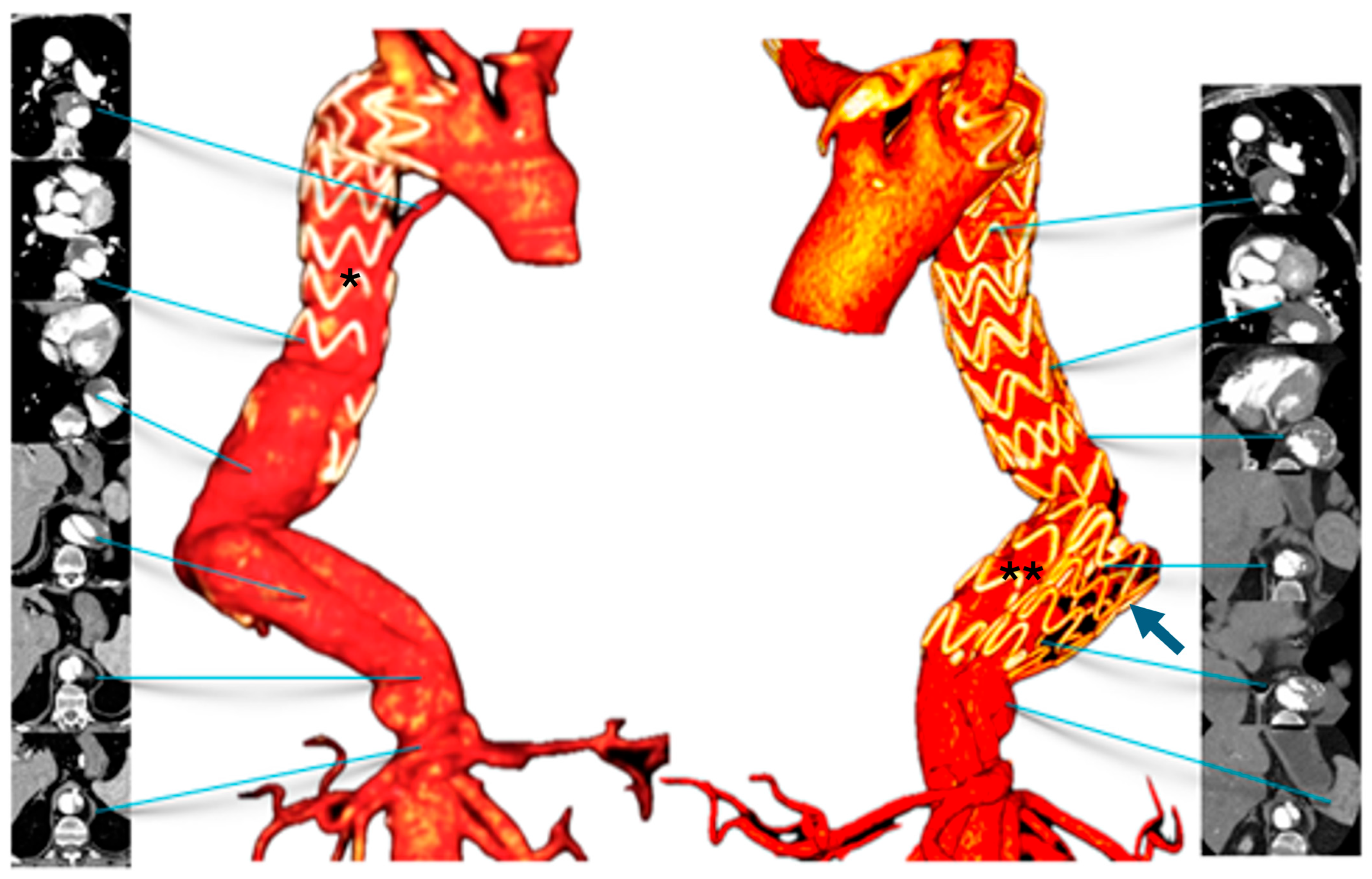
Follow-up computed tomography angiography (CTA) carried out in June 2025 revealed recanalization of the FL, extending from the distal aortic arch (zone 3) to the level of the superior mesenteric artery (zones 6–7). The descending thoracic aorta (DTA) demonstrated aneurysmal degeneration, with a maximum diameter of 58.5 mm (
Figure 1).
Considering the risk of progressive aortic dilatation and potential rupture, together with the high risk posed by open thoracoabdominal surgery (EURO II score: 5%), a multidisciplinary team—consisting of an interventional cardiologist, a clinical cardiologist, a cardiac surgeon, a cardiac anesthesiologist, and a vascular surgeon—recommended carrying out an endovascular reintervention. Under general anesthesia, with a spinal catheter employed to minimize the risk of spinal cord ischemia, both right and left common femoral arteries (RCFA and LCFA) were accessed using 6F introducers. Pre-closure was achieved with Perclose™ ProStyle™ suture-mediated closure systems (Abbott Vascular, Temecula, CA, USA). The RCFA was then upsized to a 24F dedicated introducer.
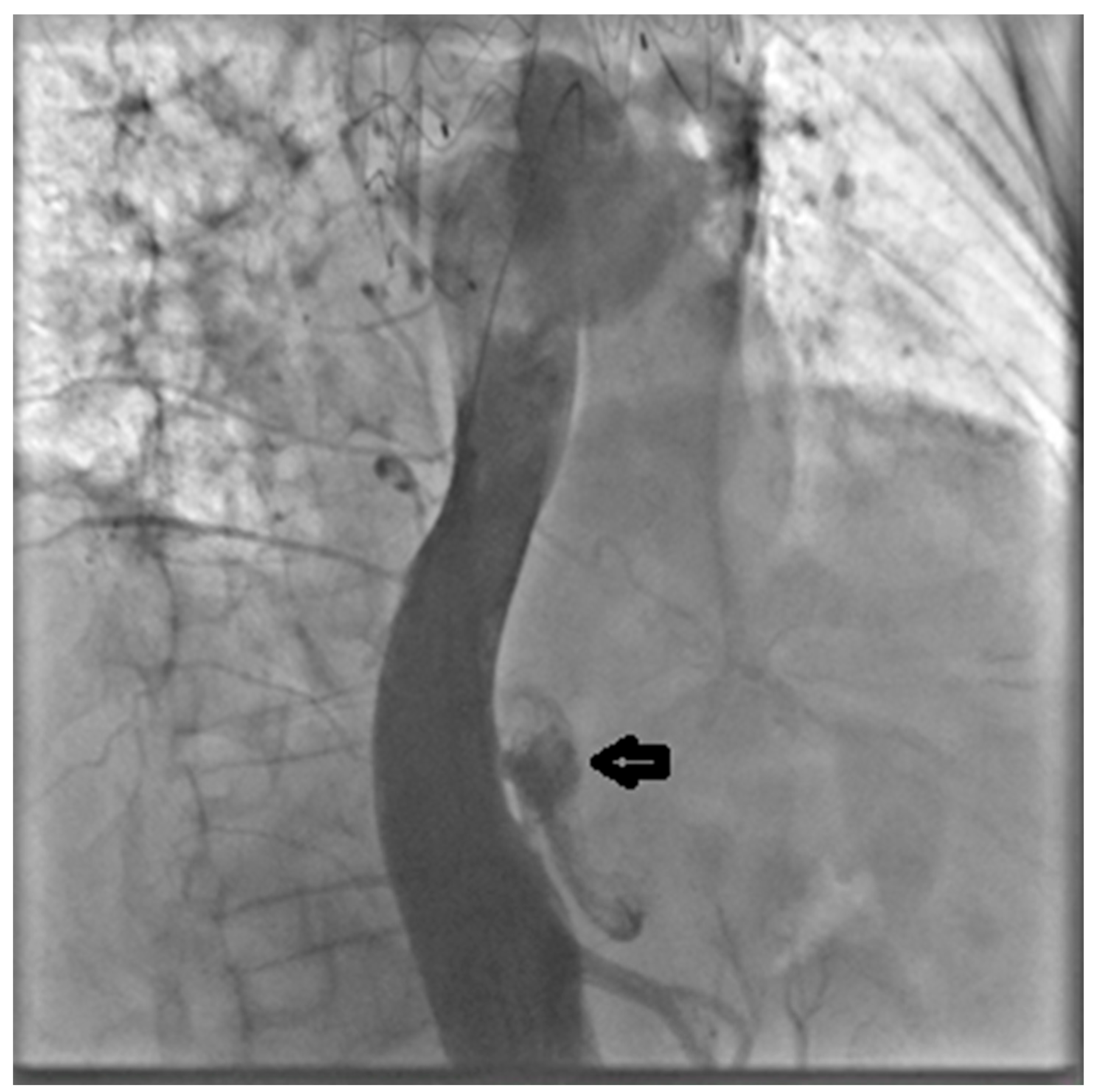
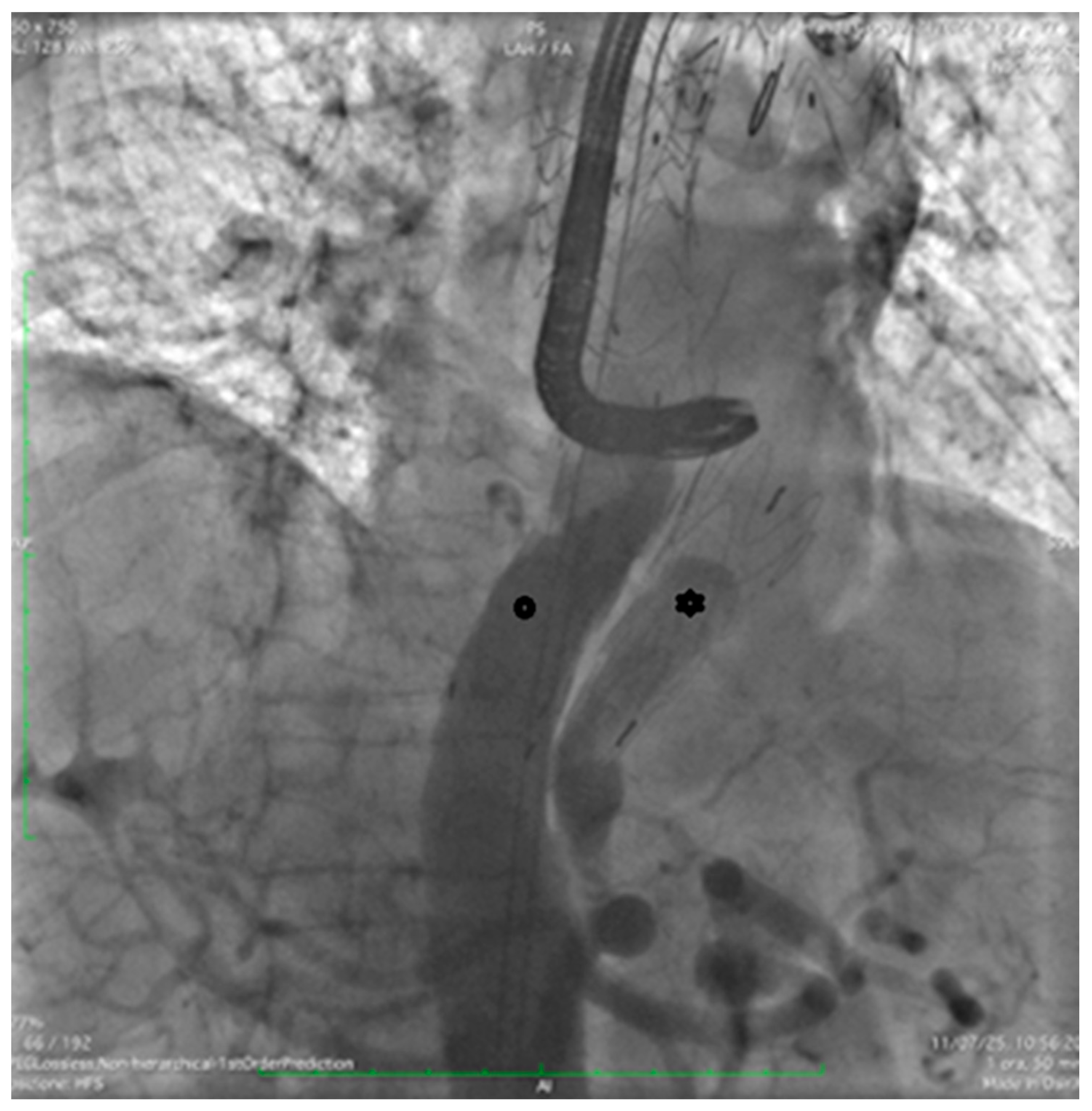
Through the LCFA, a 6F pigtail catheter was advanced into the thoracoabdominal aorta. Intraoperative angiography confirmed substantial FL dilation and two distal re-entry tears beyond the prior TEVAR, with clear retrograde backflow (
Figure 2).
Two extra-stiff guidewires were positioned: one via the RCFA into the true lumen (TL), and the other via the LCFA into the FL. A Terumo Aortic endograft (36–32–199 mm) was deployed over the RCFA guidewire with partial overlap of the existing stent graft, successfully sealing the distal re-entry tears. (
Figure 3).
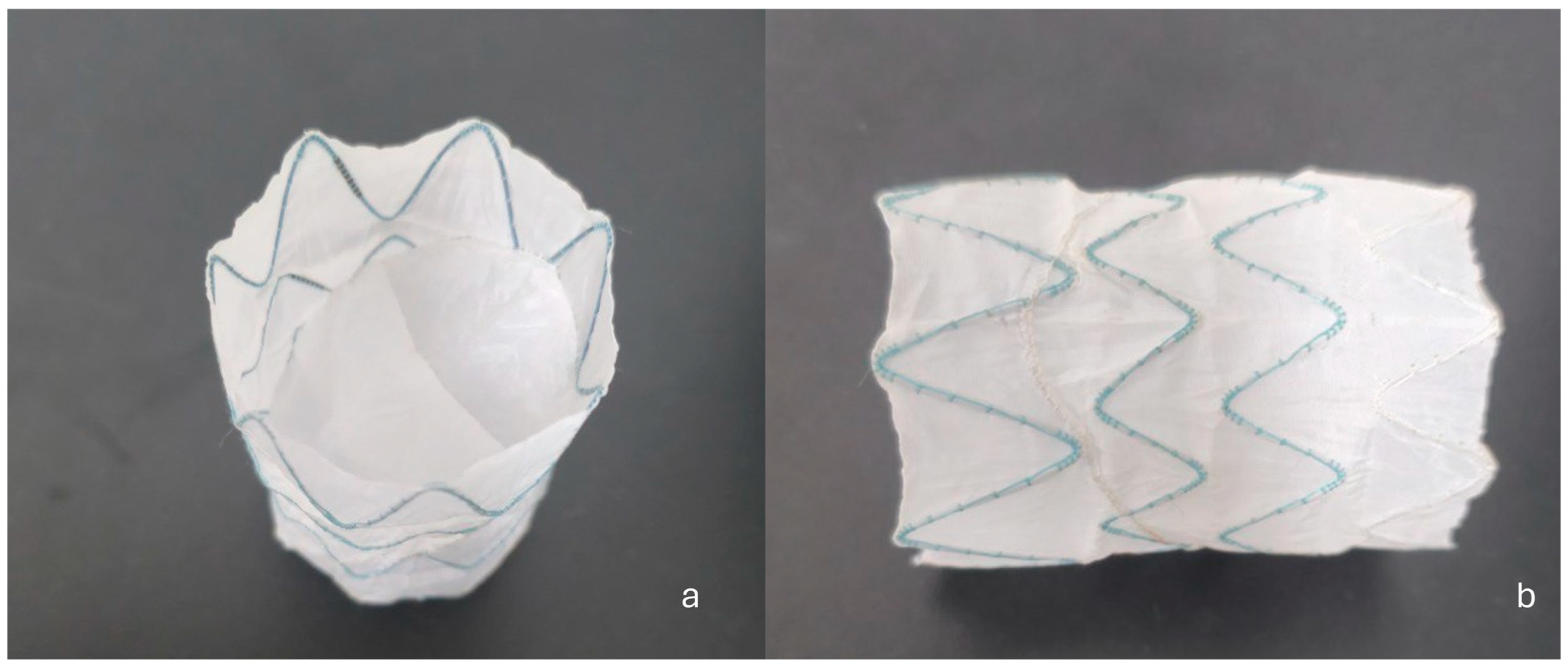
Subsequently, a custom-made FLOD (Braile Biomedica, São José do Rio Preto, Brazil) (
Figure 4a,b) was deployed via the LCFA into the FL. The FLOD consists of a tubular nitinol stent structure internally lined with polyester fabric secured by suture thread, incorporating two semi-leaflets that prevent retrograde backflow once deployed. Its low radial force allows excellent adaptability within the FL without interfering with the TL endoprosthesis. Anchoring is achieved owing to the conformability of the stent structure to the FL walls and the stabilizing effect of the internal lining and semi-leaflets, ensuring secure apposition without exerting excessive radial stress. These features make the FLOD particularly suitable for chronic dissections where the intimal flap is fibrotic and hypomobile, and in the presence of a large FL not amenable to closure with conventional plugs.
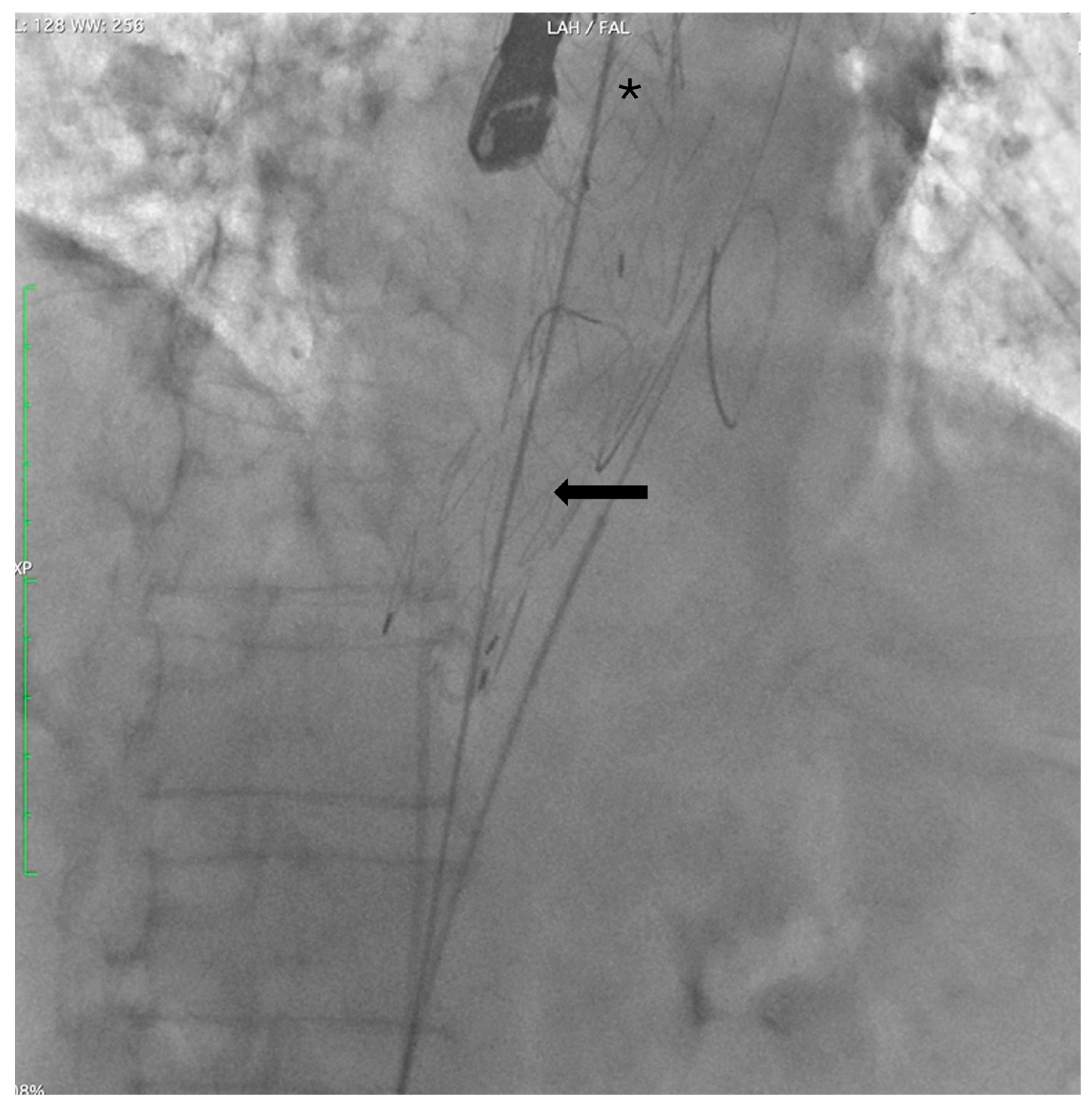
Finally, angiography confirmed proper device positioning, preserved visceral artery patency, and complete FL exclusion (
Figure 5 and
Figure 6).
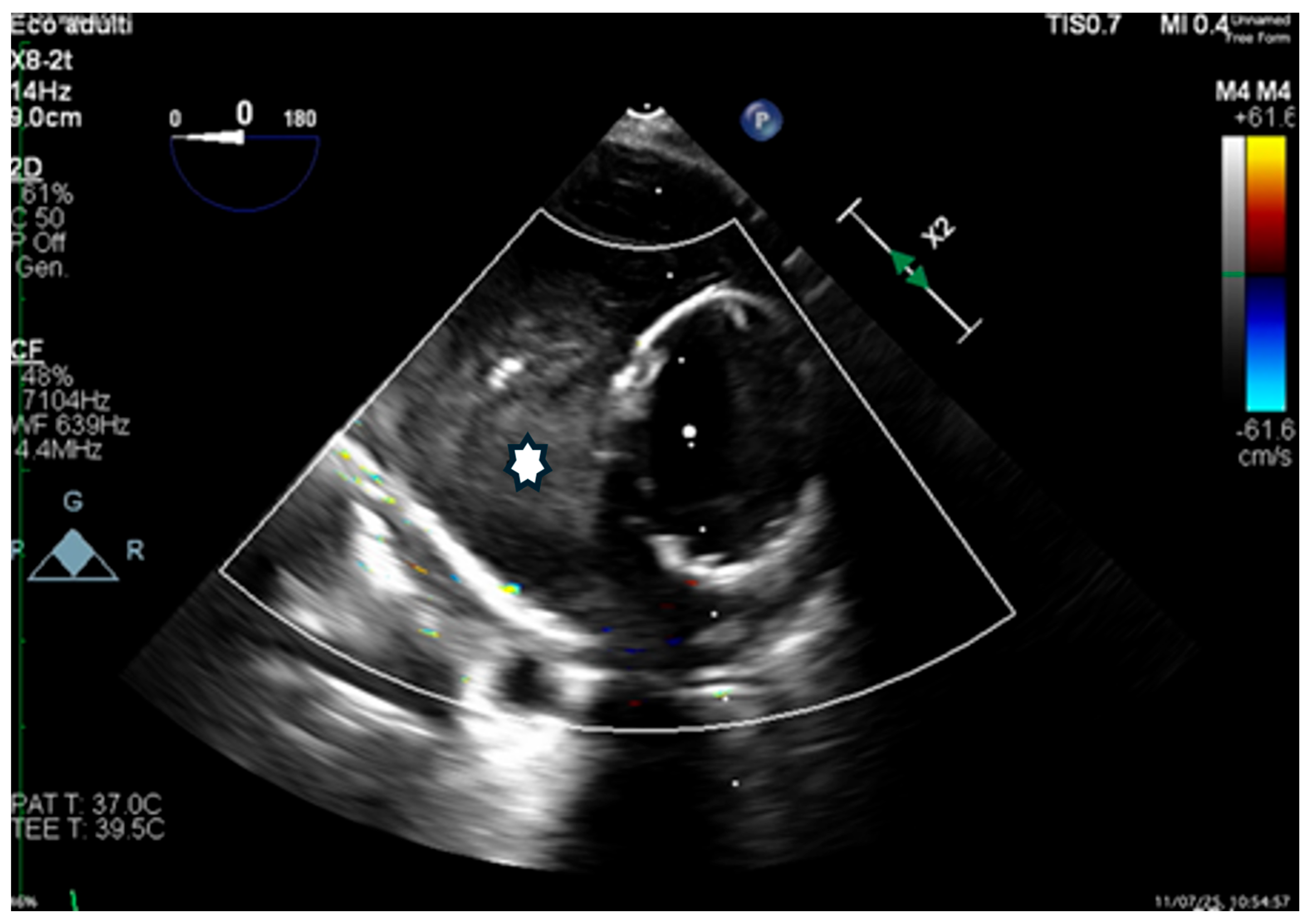
Transesophageal echocardiography (TEE) confirmed rapid thrombosis of the FL (
Figure 7).
On post-operative day (POD) 3, CTA confirmed correct positioning of both endografts, extending from the posterior aortic arch to just above the celiac trunk. The FLOD remained stable in the mid-distal DTA, with near-complete thrombosis of the proximal FL. The patient was started on lifelong antiplatelet therapy with 100 mg/day of acetylsalicylic acid and discharged on POD 5 in good condition and without complications.
At the three-month follow-up, the patient was asymptomatic, and the control CTA confirmed the previous result.
3. Discussion
Chronic type B aortic dissection (cTBAD) remains a therapeutic challenge. Up to 40% of patients with initially uncomplicated TBAD may later require intervention due to aneurysmal degeneration driven by persistent FL perfusion [
1]. Although open surgical repair is considered the gold-standard treatment, it carries high risks, including spinal cord ischemia and significant postoperative mortality.
Over the past two decades, endovascular techniques have become the preferred strategy in many centers. The PETTICOAT (Provisional Extension to Induce Complete Attachment) technique, in which a distal bare-metal stent is used to expand the TL and stabilize the intimal flap, has shown promise—particularly for malperfusion—but is less effective at preventing late aneurysmal degeneration when distal FL perfusion persists [
2].
The STABILISE (Stent-Assisted Balloon-Induced Intimal Disruption and Relamination in Aortic Dissection Repair) technique builds upon PETTICOAT by incorporating balloon-induced disruption of the intimal flap, creating a single-channel aorta and promoting FL thrombosis [
3]. The DEEVAR (Distal Extended EndoVascular Aortic Repair) approach extends coverage into the abdominal aorta, offering more comprehensive remodeling but with increased technical complexity [
1].
Therapeutic focus has progressively shifted from treating only the TL to directly addressing the FL, recognizing that persistent FL perfusion is a major driver of long-term complications in cTBAD, even after technically successful TEVAR.
Several targeted FL occlusion strategies have been developed. In the candy-plug technique, first described by Kölbel, a customized endograft is employed to seal the FL, though it often requires extensive intraoperative modification [
4]. In the Knickerbocker technique, oversized stent grafts are used to intentionally fenestrate the dissection flap and collapse the FL, thereby promoting remodeling [
5]. In cases where the intimal flap is markedly thickened and fibrotic, adequate stent graft expansion may be hindered, perpetuating FL perfusion. To overcome this issue, the guidewire fenestration technique was introduced, which entails creating or enlarging a septal fenestration to reestablish a single aortic channel and redirect flow into the TL, followed by balloon dilation to optimize endograft expansion [
3].
Another approach, described by Loubert et al., is the “cork-in-the-bottleneck” technique, which involves the deployment of devices such as vena cava filters, detachable balloons, thrombin, or occluders into the FL to achieve exclusion [
6]. Other studies have reported success with coils, cyanoacrylate glue, and iliac occluders, with high technical success and complete FL thrombosis [
7].
In cases with large FL diameters, embolization becomes technically challenging because of the limitations of available materials. In our patient, combining proximal endograft extension with a custom-made FLOD excluded the FL and enabled early thrombosis, as confirmed by TEE and CTA. FLOD typically promotes rapid and often complete thrombosis of the FL, underscoring the importance of careful pre-procedural planning to avoid ischemic complications. Preoperative CTA is systematically performed to assess whether visceral or spinal branches originate from the FL. In addition, protective strategies such as cerebrospinal fluid drainage and strict hemodynamic management are adopted to minimize the risk of spinal cord ischemia. In our case, the sizing strategy was based on precise preoperative CTA measurements of the FL. The thoracic endograft was first positioned from the level of the LSA down to the level of the celiac tripod, where the FL typically re-enters. At this point, the maximum FL diameter was measured just above the tripod (30 × 14 mm), and again 7 cm proximally, where the diameter was 42 mm. Based on these measurements, an FLOD with a nominal diameter of 42 mm was selected. The device’s low radial force allows it to adapt to variations in diameter, ranging from 42 mm down to 30 mm in this case. Although completion angiography confirmed correct device positioning, a mild antegrade residual perfusion of the FL was observed near the celiac trunk, in a dissected but non-dilated aortic segment. Given the lower propensity of distal aortic tracts to dilate—owing to their smaller diameter, reduced wall stress, and structural characteristics—this finding may be considered relatively reassuring [
8]. Nevertheless, structured CTA follow-up remains mandatory to ensure long-term stability and early detection of potential enlargement, as imaging surveillance is an integral part of cTBAD management. Our combined strategy provides a minimally invasive and reproducible therapeutic option for patients with chronic dissection and persistent FL perfusion. Importantly, the use of a custom-made device allows the treatment of complex anatomies and broadens applicability to a wider patient population, while maintaining costs broadly comparable to those of other available alternatives. Our findings are consistent with the insights of Fukuhara et al. [
3], who emphasized the need for innovative, anatomy-specific endovascular strategies in the treatment of cTBAD.
4. Conclusions
This case highlights that combining endograft extension with a dedicated FLOD is a safe, effective, and technically feasible approach for selected patients with cTBAD and aneurysmal degeneration. Early and complete FL thrombosis was achieved without complications, supporting the growing role of customized endovascular solutions in treating complex aortic pathologies.
Author Contributions
Conceptualization, A.R. and M.C. (Marta Casula); data curation, M.C. (Marta Casula); writing—original draft preparation, M.C. (Marta Casula); writing—review and editing, A.R.; visualization, A.R.; supervision, A.R., S.B. and M.C. (Michele Collareta). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures complied with the Declaration of Helsinki. As it is a retrospective case report without any intervention, the Institutional Ethical Board does not need to give approval.
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available from the corresponding author on request because of privacy concerns.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to have influenced the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| cTBAD | Chronic Type B Aortic Dissection |
| FLOD | False Lumen Occlusion Device |
| CTA | Computed Tomography Angiography |
| DTA | Descending Thoracic Aorta |
| FL | False Lumen |
| LCFA | Left Common Femoral Artery |
| POD | Postoperative Day |
| RCFA | Right Common Femoral Artery |
| STABILISE | Stent-Assisted Balloon-Induced Intimal Disruption and Relamination in Aortic Dissection |
| TBAD | Type B Aortic Dissection |
| TEVAR | Thoracic Endovascular Aortic Repair |
| TL | True lumen |
| TEE | Transesophageal Echocardiography |
| PETTICOAT | Provisional Extension to Induce Complete Attachment |
| DEEVAR | Distal Extended EndoVascular Aortic Repair |
| LSA | Left Subclavian Artery |
References
- Jubouri, M.; Patel, R.; Tan, S.Z.; Al-Tawil, M.; Bashir, M.; Bailey, D.M.; Williams, I.M. Fate and Consequences of the False Lumen After Thoracic Endovascular Aortic Repair in Type B Aortic Dissection. Ann. Vasc. Surg. 2023, 94, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, L.; Rinaldi, E.; Melissano, G.; Chiesa, R. The PETTICOAT concept for endovascular treatment of type B aortic dissection. J. Cardiovasc. Surg. 2019, 60, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Tchouta, L.; Yokoyama, Y.; Pampati, R.; Khaja, M.S. Recent developments in thoracic endovascular aortic repair for chronic type B dissection. J. Vis. Surg. 2021, 7, 40. [Google Scholar] [CrossRef]
- Kölbel, T.; Lohrenz, C.; Kieback, A.; Diener, H.; Debus, E.S.; Larena-Avellaneda, A. Distal False Lumen Occlusion in Aortic Dissection With a Homemade Extra-Large Vascular Plug: The Candy-Plug Technique. J. Endovasc. Ther. 2013, 20, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Rohlffs, F.; Tsilimparis, N.; Panuccio, G.; Heidemann, F.; Behrendt, C.-A.; Kölbel, T. The Knickerbocker Technique: Technical Aspects and Single-Center Results of a New Endovascular Method for False Lumen Occlusion in Chronic Aortic Dissection. J. Endovasc. Ther. 2023, 30, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Loubert, M.C.; van der Hulst, V.P.M.; De Vries, C.; Bloemendaal, K.; Vahl, A.C. How to Exclude the Dilated False Lumen in Patients after a Type B Aortic Dissection? The Cork in the Bottleneck. J. Endovasc. Ther. 2003, 10, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, K.; Katsargyris, A.; Kouvelos, G.; Renner, H.; Verhoeven, E.L.G. Treatment algorithms for patients with (sub)acute type B aortic dissections. J. Cardiovasc. Surg. 2016, 57, 212–220. [Google Scholar]
- Goldfinger, J.Z.; Halperin, J.L.; Marin, M.L.; Stewart, A.S.; Eagle, K.A.; Fuster, V. Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2014, 64, 1725–1739. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).