From Data to Decisions: AI in Varicose Veins—Predicting, Diagnosing, and Guiding Effective Management
Abstract
1. Introduction
2. Background
2.1. Pathogenesis and Risk Factors
2.1.1. Pathophysiology
- Venous hypertension can arise from valvular incompetence, flow obstruction, or dysfunction of the calf muscle pump [26]. Valvular incompetence, particularly, results from the tearing, thinning, deformation, or adhesion of valve leaflets, which ultimately leads to venous reflux and increased pressure.
- Structural changes in the vein wall further contribute to venous dilation and weakening. Research indicates that reduced vein wall elasticity is a critical factor in VV formation, with significant differences observed between normal and VV [27].
- Molecular changes also play a role in vein wall degradation. Overproduction of collagen type I and decreased synthesis of collagen type III, along with disorganized smooth muscle cells and elastin fibers, have been linked to structural weakening [28,29]. Elevated levels of transforming growth factor β1 (TGF-β1) and fibroblast growth factor β1 (FGF-β1) further contribute to extracellular matrix remodeling, leading to vein wall dysfunction.
- Prolonged exposure of venous valves to elevated pressures has been associated with structural remodeling, including reductions in leaflet length and thickness, which further impairs valve function [30].
2.1.2. Risk Factors
- Age is widely recognized as a key determinant in the development of VVs [40]. The incidence of VVs increases with age, mainly due to the weakening of lower leg muscles and impaired venous valve function [41]. A study by Sisto et al. reported the prevalence of VVs as 25% in women and 7% in men [42], with the Framingham study and others confirming that VVs are more common in women aged 40–79. However, some studies suggest no significant sex-based prevalence differences [3,43].
- Occupational factors are also significant. A case–control study by Elamrawy et al. found that frequent heavy lifting, standing for more than four hours per day, and insufficient sleep increased the risk of VVs [46]. Professions involving prolonged standing, such as teaching, retail, manual labor, and healthcare (especially nursing), are linked to a higher prevalence of VVs [3].
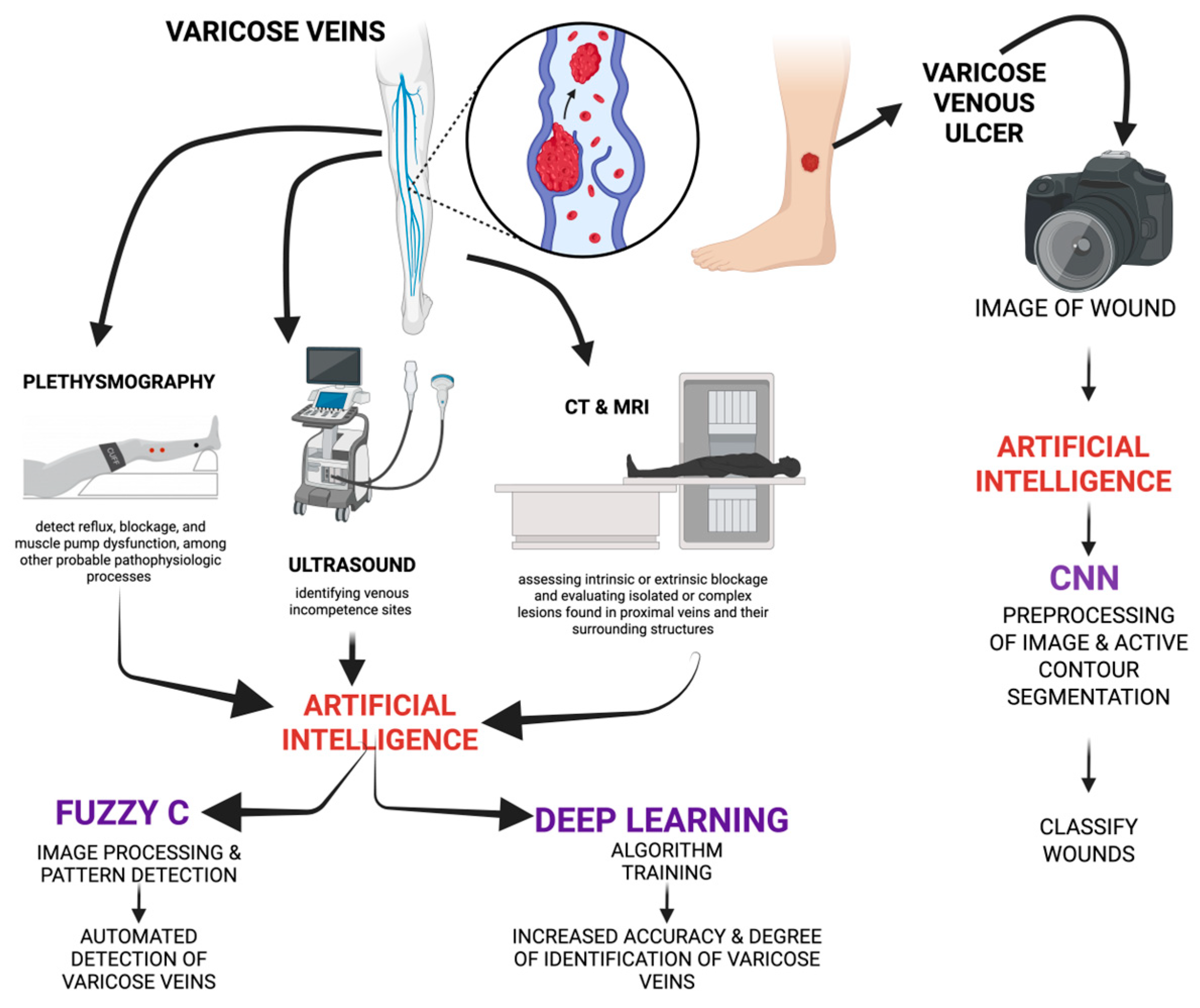
2.2. Current Technologies in Diagnosing VVs
2.2.1. Venous Duplex Ultrasonography
2.2.2. Plethysmography
2.2.3. Light Reflection Rheography
2.2.4. CT and MRI
2.3. Current Treatment and AI in Enhancing the Management of VVs
2.3.1. Conservative Management
2.3.2. Invasive Treatment Option
3. Primary Studies on AI in Varicose Veins
3.1. Role of AI in Risk Factor Identification and Recurrence Estimation
3.2. Role of AI in Risk Factor Identification and Recurrence Estimation
3.2.1. Machine Learning in Risk Factor Identification
- WNT1-inducible-signaling pathway protein 2 (WISP2);
- Cysteine Rich Protein 1 (CRIP1);
- Odd-Skipped Related Transcription Factor 1 (OSR1).
- Fukaya E. et al. conducted a community-based study on approximately 500,000 individuals aged 40 to 69 from the United Kingdom (UK) Biobank [83]. The application of machine learning facilitated the confirmation of established risk factors such as age, sex, obesity, pregnancy, and history of deep vein thrombosis for VVs. Additionally, the utilization of this approach uncovered novel risk factors, including height. The application of machine learning techniques in genome-wide association studies (GWAS) has facilitated the identification of 30 novel genetic loci that are associated with the occurrence of VVs;
- Blood pressure regulation;
- Vascular mechanosensing channels;
- Vascular maturation and development;
- Structural integrity of veins;
- Genes located near the hemochromatosis gene [84].
3.2.2. AI in Recurrence Estimation
- Multiple factors are also involved in the recurrence of VVs, but they are difficult to identify.
- Age and gender;
- Obesity;
- Genetic predisposition;
- Inadequate preoperative assessment;
- Presence of double short or long saphenous veins;
- Neovascularization;
- Surgical incompetence;
- Length of the recurrence period [86].
3.3. Role of AI in Diagnosing VVs
4. Discussion
4.1. CN—Convolutional Neural Network
4.2. Current Challenges
4.3. Limitations
4.4. Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piazza, G. Varicose veins. Circulation 2014, 130, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Melander, S. Varicose veins: Diagnosis, management, and treatment. J. Nurse Pract. 2014, 10, 417–424. [Google Scholar] [CrossRef]
- Aslam, M.R.; Muhammad, A.H.; Ahmad, K.; Jabbar, S.; Hayee, A.; Sagheer, M.S.; Rehman, J.U.; Khalid, S.; Hashmi, A.S.; Rajpoot, S.R.; et al. Global impact and contributing factors in varicose vein disease development. SAGE Open Med. 2022, 10, 20503121221118992. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Pfeifer, J.R.; Engle, J.S.; Schottenfeld, D. The epidemiology of chronic venous insufficiency and varicose veins. Ann. Epidemiol. 2005, 15, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, R.T.; Raffetto, J.D. Chronic venous insufficiency. Circulation 2005, 111, 2398–2409. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Al Mamun, A.; Majumder, A. Clinical Presentation of Varicose Veins. Indian J. Surg. 2023, 85, 7–14. [Google Scholar] [CrossRef]
- Jones, R.H.; Carek, P.J. Management of varicose veins. Am. Fam. Physician 2008, 78, 1289–1294. [Google Scholar]
- Nicholls, S.C. Sequelae of untreated venous insufficiency. Semin. Interv. Radiol. 2005, 22, 162–168. [Google Scholar] [CrossRef]
- Raetz, J.; Wilson, M.; Collins, K. Varicose veins: Diagnosis and treatment. Am. Fam. Physician 2019, 99, 682–688. [Google Scholar]
- National Clinical Guideline Centre (UK). Assessment prior to treatment. In Varicose Veins in the Legs: The Diagnosis and Management of Varicose Veins; National Institute for Health and Care Excellence (NICE): London, UK, 2013. [Google Scholar]
- Cavezzi, A.; Labropoulos, N.; Partsch, H.; Ricci, S.; Caggiati, A.; Myers, K.; Nicolaides, A.; Smith, P.C. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs—UIP consensus document. Part II. Anatomy. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 288–299. [Google Scholar] [CrossRef]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2S–48S. [Google Scholar] [CrossRef]
- Secinaro, S.; Calandra, D.; Secinaro, A.; Muthurangu, V.; Biancone, P. The role of artificial intelligence in healthcare: A structured literature review. BMC Med. Inform. Decis. Mak. 2021, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Crema, C.; Attardi, G.; Sartiano, D.; Redolfi, A. Natural language processing in clinical neuroscience and psychiatry: A review. Front. Psychiatry 2022, 13, 946387. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94. [Google Scholar] [CrossRef]
- Butova, X.; Shayakhmetov, S.; Fedin, M.; Zolotukhin, I.; Gianesini, S. Artificial Intelligence Evidence-Based Current Status and Potential for Lower Limb Vascular Management. J. Pers. Med. 2021, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.P. Flow through collapsible tubes and through in situ veins. IEEE Trans. Biomed. Eng. 1969, 16, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.H. Lower extremity venous anatomy. Semin. Interv. Radiol. 2005, 22, 147–156. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Duong, H. Anatomy, Shoulder and Upper Limb, Veins; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hill, B.G.; van Rij, A.M. The lower limb perforator veins in normal subjects. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 669–675.el. [Google Scholar] [CrossRef] [PubMed]
- Young, T.W. Anomalous Pulmonary Venous Return. In Clinical Management of Congenital Heart Disease from Infancy to Adulthood; Cardiotext Publishing: Hopkins, MN, USA, 2013. [Google Scholar]
- Bergan, J.J.; Schmid-Schönbein, G.W.; Smith, P.D.; Nicolaides, A.N.; Boisseau, M.R.; Eklof, B. Chronic venous disease. N. Engl. J. Med. 2006, 355, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J. New Treatment of Varicose Veins through Muscle Regeneration of Lower Leg Muscles (Especially Calf Muscle) Without Removal of Varicose Veins. Ann. Phlebol. 2022, 20, 68–77. [Google Scholar] [CrossRef]
- Surendran, S.; Ramegowda, K.S.; Suresh, A.; Binil Raj, S.S.; Lakkappa, R.K.; Kamalapurkar, G.; Radhakrishnan, N.; Kartha, C.C. Arterialization and anomalous vein wall remodeling in varicose veins is associated with upregulated FoxC2-Dll4 pathway. Lab. Investig. 2016, 96, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.N.; Andraska, E.A.; Obi, A.T.; Wakefield, T.W. Pathophysiology of varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, M.; Corduneanu, D. Chronic venous insufficiency: A frequently underdiagnosed and undertreated pathology. Mædica 2017, 12, 59. [Google Scholar]
- Clarke, H.; Smith, S.R.; Vasdekis, S.N.; Hobbs, J.T.; Nicolaides, A.N. Role of venous elasticity in the development of varicose veins. Br. J. Surg. 1989, 76, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Sansilvestri-Morel, P.; Rupin, A.; Badier-Commander, C.; Kern, P.; Fabiani, J.N.; Verbeuren, T.J.; Vanhoutte, P.M. Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J. Vasc. Res. 2001, 38, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Serralheiro, P.; Soares, A.; Costa Almeida, C.M.; Verde, I. TGF-β1 in vascular wall pathology: Unraveling chronic venous insufficiency pathophysiology. Int. J. Mol. Sci. 2017, 18, 2534. [Google Scholar] [CrossRef] [PubMed]
- Takase, S.; Pascarella, L.; Bergan, J.J.; Schmid-Schönbein, G.W. Hypertension-induced venous valve remodeling. J. Vasc. Surg. 2004, 39, 1329–1334. [Google Scholar] [CrossRef]
- Ascher, E.; Jacob, T.; Hingorani, A.; Gunduz, Y.; Mazzariol, F.; Kallakuri, S. Programmed cell death (Apoptosis) and its role in the pathogenesis of lower extremity varicose veins. Ann. Vasc. Surg. 2000, 14, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, M.J.; Baker, D.M.; Turmaine, M.; Burnstock, G. Alterations in purinoceptor expression in human long saphenous vein during varicose disease. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Kiriakidis, S.; Paleolog, E.M.; Davies, A.H. Increased activation of the hypoxia-inducible factor pathway in varicose veins. J. Vasc. Surg. 2012, 55, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Davies, A.H. Pathogenesis of primary varicose veins. Br. J. Surg. 2009, 96, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Asuwa, N.; Ishii, T.; Ito, K.; Akasaka, Y.; Masuda, T.; Zhang, L.; Kiguchi, H. Collagen alteration in vascular remodeling by hemodynamic factors. Virchows Arch. 2000, 437, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Ross, R.L.; Khalil, R.A. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: Relevance to varicose vein formation. J. Vasc. Surg. 2007, 45, 373–380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tisi, P.V. Varicose veins. BMJ Clin. Evid. 2007, 2007, 0212. [Google Scholar]
- Lee, A.J.; Evans, C.J.; Allan, P.L.; Ruckley, C.V.; Fowkes, F.G. Lifestyle factors and the risk of varicose veins: Edinburgh Vein Study. J. Clin. Epidemiol. 2003, 56, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.H.; Maricq, H.R.; Biro, C.; Ponçot-Makinen, C.O.; Franco, A. Prevalence, risk factors, and clinical patterns of chronic venous disorders of lower limbs: A population-based study in France. J. Vasc. Surg. 2004, 40, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Naoum, J.J.; Hunter, G.C. Pathogenesis of varicose veins and implications for clinical management. Vascular 2007, 15, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Naoum, J.J.; Hunter, G.C.; Woodside, K.J.; Chen, C. Current advances in the pathogenesis of varicose veins. J. Surg. Res. 2007, 141, 311–316. [Google Scholar] [CrossRef]
- Sisto, T.; Reunanen, A.; Laurikka, J.; Impivaara, O.; Heliövaara, M.; Knekt, P.; Aromaa, A. Prevalence and risk factors of varicose veins in lower extremities: Mini-Finland health survey. Eur. J. Surg. = Acta Chir. 1995, 161, 405–414. [Google Scholar]
- Ahti, T. Risk Factors of Varicose Veins; Tampere University Press: Tampere, Finland, 2010. [Google Scholar]
- Bernstein, I.M.; Ziegler, W.; Badger, G.J. Plasma volume expansion in early pregnancy. Obstet. Gynecol. 2001, 97, 669–672. [Google Scholar]
- Thornberg, K.L.; Jacobson, S.L.; Giraud, G.D.; Morton, M.J. Hemodynamic changes in pregnancy. Semin. Perinatol. 2000, 24, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Elamrawy, S.; Darwish, I.; Moustafa, S.; Elshaer, N.; Ahmed, N. Epidemiological, life style, and occupational factors associated with lower limb varicose veins: A case control study. J. Egypt. Public Health Assoc. 2021, 96, 1–11. [Google Scholar] [CrossRef]
- Ahti, T.M.; Mäkivaara, L.A.; Luukkaala, T.; Hakama, M.; Laurikka, J.O. Effect of family history on the incidence of varicose veins: A population-based follow-up study in Finland. Angiology 2009, 60, 487–491. [Google Scholar] [CrossRef]
- Cleave, T.L. Varicose veins: Nature′s error or man′s? Lancet 1959, 2, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P. A deficiency in dietary fiber may be one cause of certain colonic and venous disorders. Am. J. Dig. Dis. 1976, 21, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P.; Walker, A.R.P.; Painter, N.S. Dietary fiber and disease. JAMA 1972, 229, 1068–1074. [Google Scholar] [CrossRef]
- Labropoulos, N.; Giannoukas, A.D.; Delis, K.; Mansour, M.A.; Kang, S.S.; Nicolaides, A.N.; Lumley, J.; Baker, W.H. Where does venous reflux start? J. Vasc. Surg. 1997, 26, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Pedrycz, A.; Budzyńska, B. Diagnosis of varicose veins of the lower limbs—Functional tests. Arch. Physiother. Glob. Res. 2016, 20, 29–32. [Google Scholar] [CrossRef]
- Ramelet, A.A.; Monti, M.; Bounameaux, H.; Buchheim, G.; Capasso, P. Flebologia: Przewodnik; Wydawnictwo Medyczne “Via Medica”: Warszawa, Poland, 2003. [Google Scholar]
- Zegarra, T.I.; Tadi, P. CEAP Classification of Venous Disorders. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pannier, F.; Noppeney, T.; Alm, J.; Breu, F.X.; Bruning, G.; Flessenkämper, I.; Gerlach, H.; Hartmann, K.; Kahle, B.; Kluess, H.; et al. S2k guidelines: Diagnosis and treatment of varicose veins. Der Hautarzt 2022, 73 (Suppl. S1), 1–44. [Google Scholar] [CrossRef]
- Campbell, B. Varicose veins and their management. BMJ 2006, 333, 287–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whiteley, M.S. Current best practice in the management of varicose veins. Clin. Cosmet. Investig. Dermatol. 2022, 6, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Nasra, K.; Negussie, E. Sonography Vascular Peripheral Vein Assessment, Protocols, and Interpretation; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chan, K.S.; Liang, S.; Cho, Y.T.; Chan, Y.M.; Tan, A.H.M.; Muthuveerappa, S.; Lai, T.P.; Goh, C.C.; Joseph, A.; Hong, Q.; et al. Clinical validation of a machine-learning-based handheld 3-dimensional infrared wound imaging device in venous leg ulcers. Int. Wound J. 2022, 19, 436–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plethysmographic Techniques in the Diagnosis of Venous Disease | SpringerLink. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-030-49616-6_39-1#Abs1 (accessed on 1 January 1970).
- Singh, A.; Zahra, F. Chronic Venous Insufficiency; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Youn, Y.J.; Lee, J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J. Intern. Med. 2019, 34, 269–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dezotti, N.R.A.; Dalio, M.B.; Ribeiro, M.S.; Piccinato, C.E.; Joviliano, E.E. The clinical importance of air plethysmography in the assessment of chronic venous disease. J. Vasc. Bras. 2016, 15, 287–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, P.M.; Barrie, W.W.; Donnelly, P.K. Light reflection rheography: A simple method of assessing lower limb venous filling. J. R. Coll. Surg. Edinb. 1994, 39, 89–92. [Google Scholar] [PubMed]
- Jin, K.N.; Lee, W.; Jae, H.J.; Yin, Y.H.; Chung, J.W.; Park, J.H. Venous reflux from the pelvis and vulvoperineal region as a possible cause of lower extremity varicose veins: Diagnosis with computed tomographic and ultrasonographic findings. J. Comput. Assist. Tomogr. 2009, 33, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Kim, S.Y.; Park, Y.J.; Lee, W.; Jung, I.M.; Lee, T.; Ha, J.; Kim, S.J. Role of three-dimensional computed tomography venography as a powerful navigator for varicose vein surgery. J. Vasc. Surg. 2010, 51, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Peng, J.; Zheng, L.; Lu, H.; Yu, W.; Jiang, X.; Zhang, L.; Song, H.; Zhao, Z. Application of computed tomography venography in the diagnosis and severity assessment of iliac vein compression syndrome: A retrospective study. Medicine 2018, 97, e12002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leopardi, D.; Hoggan, B.L.; Fitridge, R.A.; Woodruff, P.W.; Maddern, G.J. Systematic review of treatments for varicose veins. Ann. Vasc. Surg. 2009, 23, 264–276. [Google Scholar] [CrossRef]
- Palacios, F.S.; Rathbun, S.W. Medical treatment for Postthrombotic syndrome. Semin. Interv. Radiol. 2017, 34, 61–67. [Google Scholar]
- Knight, S.L.; Robertson, L.; Stewart, M. Graduated compression stockings for the initial treatment of varicose veins in people without venous ulceration. Cochrane Database Syst. Rev. 2021. [Google Scholar] [CrossRef]
- Pittler, M.H.; Ernst, E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Dudek-Makuch, M.; Studzińska-Sroka, E. Horse chestnut-efficacy and safety in chronic venous insufficiency: An overview. Rev. Bras. De Farmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef]
- Hamdan, A. Management of varicose veins and venous insufficiency. JAMA 2012, 308, 2612–2621. [Google Scholar] [CrossRef]
- Stirling, M.; Shortell, C.K. Endovascular treatment of varicose veins. Semin. Vasc. Surg. 2006, 19, 109–115. [Google Scholar] [CrossRef]
- Brasic, N.; Lopresti, D.; McSwain, H. Endovenous laser ablation and sclerotherapy for treatment of varicose veins. Semin. Cutan. Med. Surg. 2008, 27, 264–275. [Google Scholar] [CrossRef]
- Myers, T.T. Results and technique of stripping operation for varicose veins. J. Am. Med. Assoc. 1957, 163, 87–92. [Google Scholar] [CrossRef]
- Andrews, R.H.; Dixon, R.G. Ambulatory Phlebectomy and Sclerotherapy as Tools for the Treatment of Varicose Veins and Telangiectasias. Semin. Interv. Radiol. 2021, 38, 160–166. [Google Scholar] [CrossRef]
- Manga, S.; Muthavarapu, N.; Redij, R.; Baraskar, B.; Kaur, A.; Gaddam, S.; Gopalakrishnan, K.; Shinde, R.; Rajagopal, A.; Samaddar, P.; et al. Estimation of Physiologic Pressures: Invasive and Non-Invasive Techniques, AI Models, and Future Perspectives. Sensors 2023, 23, 5744. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Artificial intelligence in surgery: Promises and perils. Ann. Surg. 2018, 268, 70. [Google Scholar] [CrossRef]
- Reiner, B.I.; Siegel, E.L.; Hooper, F.; Pomerantz, S.M.; Protopapas, Z.; Pickar, E.; Killewich, L. Picture archiving and communication systems and vascular surgery: Clinical impressions and suggestions for improvement. J. Digit. Imaging 1996, 9, 167–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fischer, U.M.; Shireman, P.K.; Lin, J.C. Current applications of artificial intelligence in vascular surgery. Semin. Vasc. Surg. 2021, 34, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Liu, M.; Wang, L.; Li, X. Comprehensive bioinformatics analysis reveals biomarkers of DNA methylation-related genes in varicose veins. Front. Genet. 2022, 13, 1013803. [Google Scholar] [CrossRef]
- Fukaya, E.; Flores, A.M.; Lindholm, D.; Gustafsson, S.; Zanetti, D.; Ingelsson, E.; Leeper, N.J. Clinical and Genetic Determinants of Varicose Veins. Circulation 2018, 138, 2869–2880. [Google Scholar] [CrossRef]
- Seddik, E.H.; Abelhalim, K.; Abdelaziz, B. Intelligent Analysis of Some Factors Characterizing Patients Operated for Varicose Veins at Setif University Hospital-Algeria. ARC J. Cardiol. 2017, 3, 21–26. [Google Scholar]
- Bouharati, I.; El-Hachmi, S.; Babouche, F.; Khenchouche, A.; Bouharati, K.; Bouharati, S. Radiology and management of recurrent varicose veins: Risk factors analysis using artificial neural networks. J. Med. Radiol. Pathol. Surg. 2018, 5, 1–5. [Google Scholar]
- Liu, L.; Qin, F.; Zhao, X.; Li, M.; Yan, Q. Deep learning in medical ultrasound analysis: A review. Engineering 2019, 5, 261–275. [Google Scholar] [CrossRef]
- Mirunalini, S.; Jeyalakshmi, C.; Muralikrishnan, P. Fuzzy C means-based approach for analysis of Varicose Veins. J. Pharm. Negat. Results 2022, 13, 2288–2295. [Google Scholar] [CrossRef]
- Rajathi, V.; Bhavani, R.R.; Wiselin Jiji, G. Varicose ulcer (C6) wound image tissue classification using multidimensional convolutional neural networks. Imaging Sci. J. 2019, 67, 374–384. [Google Scholar] [CrossRef]

| Therapy | Mechanism | Indication | Overall Results | Comparative Results |
|---|---|---|---|---|
| Conservative treatment: Stockings/lifestyle modifications/medications | Relief from pain, edema and pressure | Temporary | Surgery vs. conservative Management: Surgery enhanced quality-adjusted life-years and symptoms | |
| Sclerotherapy | A foreign substance is injected into a vascular lumen, causing thrombosis and fibrosis. Endofibrosis then ensues, which finally causes the vein to be ablated [74]. | Spider veins and small VV | 60–70% Cosmetic improvement | Foam sclerotherapy vs. endovenous Ablation: Foam sclerotherapy can cause neurologic or retinal complications. |
| Thermocoagulation/Laser | An extremely small needle put into the target vessel transmits heat from a radio frequency pulse resulting in endothelial damage and hence occluded [76]. | Spider veins and small VV | 60–70% Cosmetic improvement | |
| Endovenous ablation: Radiofrequency ablation | Heats the vein wall, denaturing the collagen fibers, and causing constriction of the artery wall rather than thrombosis. The collagen in the venous wall contracts as a result, causing vein shrinkage or blockage [75]. | Ablation of GSV or SSV | 70–90% Durable GSV occlusion | Surgery vs. radiofrequency ablation: Radiofrequency ablation reduces pain, speeds up recovery, and improves short-term quality of life. Radiofrequency ablation vs. endovenous laser therapy: Radiofrequency ablation reduces bruising and tenderness. |
| Endovenous ablation: Endovenous laser therapy | ELA causes localized tissue injury and transfers thermal energy into the blood and venous wall, encouraging vein collapse [75]. | Ablation of GSV or SSV | 70–90% Durable GSV occlusion | Surgery vs. endovenous laser therapy: Endovenous laser therapy showed faster recovery and shorter postoperative impairment. |
| Stripping and excision | A stripper can be used to remove a non-tortuous part of the VV from proximal to distal incisions or vice versa [77]. | Removal of GSV or SSV (axial reflux) and excision of branch VV | 80% Intermediate term | |
| Microphlebectomy | Specialized hooks and clamps remove superficial VV by many tiny “stab” incisions [78]. | Removal of branch VV alone or after endovenous ablation | 90% Intermediate term |
| Risk Factor Identification and Recurrence Estimation of Varicose Veins | ||
|---|---|---|
| AI Method | Process/Method | Results |
Machine Learning [83]
| Explore biomarkers associated with VVs
| Identified genetic biomarkers of VVs, namely WNT1-inducible-signaling pathway protein 2 (WISP2), Cysteine Rich Protein 1 (CRIP1), and Odd-Skipped Related Transcription Factor 1 (OSR1). |
| Fuzzy modeling [85] | Assessing risk factors and the anticipated output on the veins. | Identified risk factors such as age, sex, obesity, pregnancy, history of deep vein thrombosis and height were associated with VVs. |
| Artificial Neural Network (ANN) systems [86] | Input is variables like probable causes of recurrence and output is recurrence rate. | Does not have published results yet. |
| Diagnosis/Detection of Varicose Veins | ||
|---|---|---|
| AI Method | Process/Method | Results |
| Deep Learning Techniques [87] | Algorithm was trained using a substantial collection of ultrasound images to diagnose VVs more effectively. | Sensitivity rate of 94% and a specificity rate of 93% in its automated detection of VVs. |
| Fuzzy C [88] | Fuzzy C means images from MRI for image processing, extraction and pattern recognition | Increased accuracy and degree of identification of VVs. |
| Multidimensional convolutional neural network (CNN) [88] | Pre-processing of images (by removing the flashlight), active contour segmentation, and multidimensional CNN. Their dataset used images and has two parts—training set (65%) and the validation set (35%) images for tissue classification. | Accuracy of 99.55, a specificity of 98.06, and a sensitivity of 95.66. Helps in classifying the wound and plan management accordingly. |
| Management of VVs | ||
|---|---|---|
| AI Method | Process/Method | Results |
| Artificial Neural Network [86] | Identify risk factors like previous venous leg ulcers, high BMI, and male sex that would help in predicting healing time. | Accurately predicted healing time in 68% of patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugalenthi, L.S.; Garapati, C.; Maddukuri, S.; Kanwal, F.; Kumar, J.; Asadimanesh, N.; Dadwal, S.; Ahluwalia, V.; Senapati, S.G.; Arunachalam, S.P. From Data to Decisions: AI in Varicose Veins—Predicting, Diagnosing, and Guiding Effective Management. J. Vasc. Dis. 2025, 4, 19. https://doi.org/10.3390/jvd4020019
Pugalenthi LS, Garapati C, Maddukuri S, Kanwal F, Kumar J, Asadimanesh N, Dadwal S, Ahluwalia V, Senapati SG, Arunachalam SP. From Data to Decisions: AI in Varicose Veins—Predicting, Diagnosing, and Guiding Effective Management. Journal of Vascular Diseases. 2025; 4(2):19. https://doi.org/10.3390/jvd4020019
Chicago/Turabian StylePugalenthi, Lakshmi Sree, Chris Garapati, Srivarshini Maddukuri, Fnu Kanwal, Jaspreet Kumar, Naghmeh Asadimanesh, Surbhi Dadwal, Vibhor Ahluwalia, Sidhartha Gautam Senapati, and Shivaram P. Arunachalam. 2025. "From Data to Decisions: AI in Varicose Veins—Predicting, Diagnosing, and Guiding Effective Management" Journal of Vascular Diseases 4, no. 2: 19. https://doi.org/10.3390/jvd4020019
APA StylePugalenthi, L. S., Garapati, C., Maddukuri, S., Kanwal, F., Kumar, J., Asadimanesh, N., Dadwal, S., Ahluwalia, V., Senapati, S. G., & Arunachalam, S. P. (2025). From Data to Decisions: AI in Varicose Veins—Predicting, Diagnosing, and Guiding Effective Management. Journal of Vascular Diseases, 4(2), 19. https://doi.org/10.3390/jvd4020019






