The “Silent Enemy” Called Renal Artery Stenosis: A Mini-Review
Abstract
1. Introduction
2. Epidemiology
Gender, Age, and Race Influence on RAS Prevalence
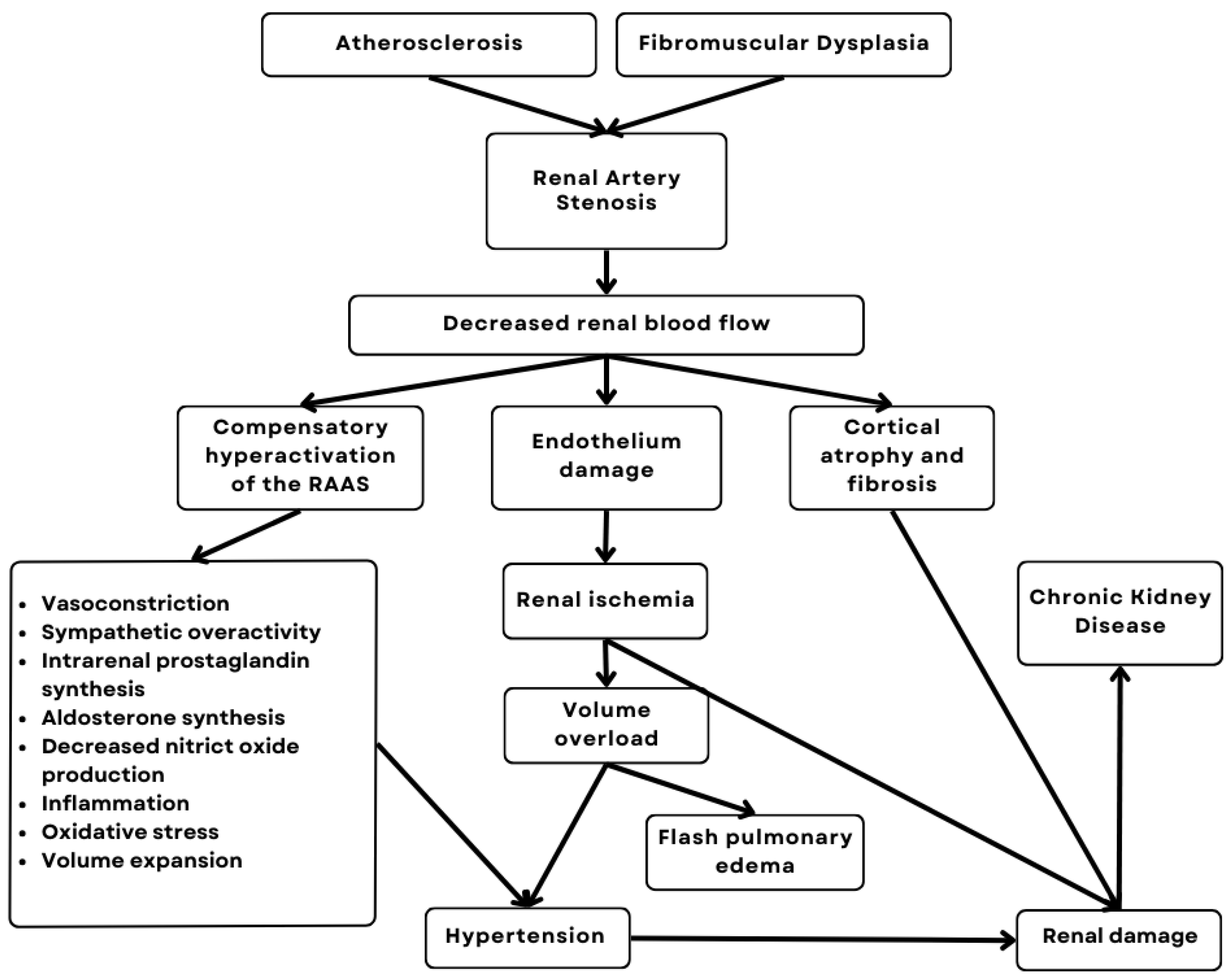
3. Physiopathology
- Relationship with the Activation of the Renin–Angiotensin–Aldosterone System (RAAS)
4. Diagnosis
4.1. Diagnostic Approach Using Imaging Modalities
4.2. Change in Laboratory Tests
4.3. The Role of Biomarkers in Early Diagnosis
5. Clinical Impacts
5.1. Renovascular Hypertension
- Early Diagnosis of Renovascular Hypertension: The Role of Laboratory Biomarkers
5.2. Ischemic Nephropathy
5.3. Chronic Kidney Disease (CKD)
- Early Detection of CKD: Role of Biomarkers and Advanced Imaging Techniques
5.4. Other Cardiovascular Events
6. Management
6.1. Distinguishing Between Approaches for Unilateral and Bilateral Stenosis
6.2. Drug Treatment
6.2.1. Renin–Angiotensin–Aldosterone System Inhibitors
6.2.2. Beta-Blockers and Combination Therapy
6.2.3. Recent Issues
6.3. Interventionist Treatment
6.3.1. Percutaneous Transluminal Angioplasty (PTA)
6.3.2. Stent Angioplasty
6.3.3. Renal Revascularization Surgery
6.4. Comparison of Efficacy Between Drug and Interventional Therapies
7. Emerging Therapies
8. Review and Knowledge Gaps
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RAS | Renal artery stenosis |
| RAAS | Renin–angiotensin–aldosterone system |
| SAH | Systemic arterial hypertension |
| FMD | Fibromuscular dysplasia |
| PAD | Peripheral arterial disease |
| CAD | Coronary arterial disease |
| CKD | Chronic kidney disease |
| CHF | Congestive heart failure |
| CHS | Cardiovascular Health Study |
| ACE | Angiotensin-converting enzyme |
| ARBs | Angiotensin II receptor blockers |
| CTA | Computed tomography angiography |
| MRA | Magnetic resonance angiography |
| PRA | Plasma renin activity |
| VEGF | Vascular endothelial growth factor |
| CRPqPCR | C-reactive proteinReal-time quantitative PCR |
| GFR | Glomerular filtration rate |
| AT1R | Angiotensin II type 1 receptor |
| AT2R | Angiotensin II type 2 receptors |
| ROS | Reactive oxygen species |
| ET-1 | Endothelin-1 |
| BNP | Brain natriuretic peptide |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| AKI | Acute kidney injury |
| ARVD | Atherosclerotic renovascular disease |
| Apo B | Apolipoprotein B |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor alpha |
| MCP-1 | Monocyte chemoattractant protein |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| TIMP-2 | Tissue inhibitors of metalloproteinases-2 |
| IGFBP7 | Insulin-like growth factor binding protein-7 |
| MRI | Magnetic resonance imaging |
| MT-MRI | Magnetization transfer-magnetic resonance imaging |
| BOLD-MRI | Blood oxygen level-dependent magnetic resonance imaging |
| ESC | European Society of Cardiology |
| ACEIs | Angiotensin-converting enzyme inhibitors |
| PTA | Percutaneous transluminal angioplasty |
| ISR | In-stent restenosis |
| CORAL | Cardiovascular outcomes in renal atherosclerotic lesions |
| PTRAS | Percutaneous transluminal renal angioplasty with stent |
References
- Herrmann, S.M.; Textor, S.C. Renovascular Hypertension. Endocrinol. Metab. Clin. N. Am. 2019, 48, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Schoepe, R.; McQuillan, S.; Valsan, D.; Teehan, G. Atherosclerotic Renal Artery Stenosis. In Hypertension: From Basic Research to Clinical Practice; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 956, pp. 209–213. ISBN 978-3-319-44250-1. [Google Scholar]
- Safian, R.D. Renal Artery Stenosis. Prog. Cardiovasc. Dis. 2021, 65, 60–70. [Google Scholar] [CrossRef]
- Lorenz, E.C.; Vrtiska, T.J.; Lieske, J.C.; Dillon, J.J.; Stegall, M.D.; Li, X.; Bergstralh, E.J.; Rule, A.D. Prevalence of Renal Artery and Kidney Abnormalities by Computed Tomography among Healthy Adults. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef] [PubMed]
- Rundback, J.H.; Sacks, D.; Kent, K.C.; Cooper, C.; Jones, D.; Murphy, T.; Rosenfield, K.; White, C.; Bettmann, M.; Cortell, S.; et al. Guidelines for the Reporting of Renal Artery Revascularization in Clinical Trials. American Heart Association. Circulation 2002, 106, 1572–1585. [Google Scholar] [CrossRef]
- Plouin, P.-F.; Bax, L. Diagnosis and Treatment of Renal Artery Stenosis. Nat. Rev. Nephrol. 2010, 6, 151–159. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Finocchiaro, P. Atherosclerotic Renal Artery Stenosis: Epidemiology, Cardiovascular Outcomes, and Clinical Prediction Rules. J. Am. Soc. Nephrol. 2002, 13, S179. [Google Scholar] [CrossRef]
- Endo, M.; Kumakura, H.; Kanai, H.; Araki, Y.; Kasama, S.; Sumino, H.; Ichikawa, S.; Kurabayashi, M. Prevalence and Risk Factors for Renal Artery Stenosis and Chronic Kidney Disease in Japanese Patients with Peripheral Arterial Disease. Hypertens. Res. 2010, 33, 911–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conlon, P.J.; Little, M.A.; Pieper, K.; Mark, D.B. Severity of Renal Vascular Disease Predicts Mortality in Patients Undergoing Coronary Angiography. Kidney Int. 2001, 60, 1490–1497. [Google Scholar] [CrossRef]
- Cohen, M.G.; Andrés Pascua, J.; Garcia-Ben, M.; Rojas-Matas, C.A.; Gabay, J.M.; Berrocal, D.H.; Tan, W.A.; Stouffer, G.A.; Montoya, M.; Fernandez, A.D.; et al. A Simple Prediction Rule for Significant Renal Artery Stenosis in Patients Undergoing Cardiac Catheterization. Am. Heart J. 2005, 150, 1204–1211. [Google Scholar] [CrossRef]
- Alhaddad, I.A.; Blum, S.; Heller, E.N.; Beato, M.A.; Bhalodkar, N.C.; Keriaky, G.E.; Brown, E.J. Renal Artery Ste-nosis in Minority Patients Undergoing Diagnostic Cardiac Catheterization: Prevalence and Risk Factors. J. Cardiovasc. Pharmacol. Ther. 2001, 6, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Edwards, M.S.; Craven, T.E.; Cherr, G.S.; Jackson, S.A.; Appel, R.G.; Burke, G.L.; Dean, R.H. Prevalence of Renovascular Disease in the Elderly: A Population-Based Study. J. Vasc. Surg. 2002, 36, 443–451. [Google Scholar] [CrossRef]
- Novick, A.C.; Zaki, S.; Goldfarb, D.; Hodge, E.E. Epidemiologic and Clinical Comparison of Renal Artery Stenosis in Black Patients and White Patients. J. Vasc. Surg. 1994, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Deitch, J.S.; Hansen, K.J.; Craven, T.E.; Flack, J.M.; Appel, R.G.; Dean, R.H. Renal Artery Repair in African-Americans. J. Vasc. Surg. 1997, 26, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Debajyoti, R.; Sharma, S.; Bhatia, R.; Barbier, S.; Khoo, J.; Ng, J. Prevalence and Risk Factors of Renal Artery Stenosis in South Asian Patients with Type 2 Diabetes Using Renal Angiography. Indian J. Nephrol. 2014, 24, 68. [Google Scholar] [CrossRef]
- Svetkey, L.P.; Himmelstein, S.I.; Dunnick, N.R.; Wilkinson, R.H.; Bollinger, R.R.; McCann, R.L.; Beytas, E.M.; Klotman, P.E. Prospective Analysis of Strategies for Diagnosing Renovascular Hypertension. Hypertension 1989, 14, 247–257. [Google Scholar] [CrossRef]
- Svetkey, L.P.; Kadir, S.; Dunnick, N.R.; Smith, S.R.; Dunham, C.B.; Lambert, M.; Klotman, P.E. Similar Prevalence of Renovascular Hypertension in Selected Blacks and Whites. Hypertension 1991, 17, 678–683. [Google Scholar] [CrossRef]
- Olin, J.W.; Froehlich, J.; Gu, X.; Bacharach, J.M.; Eagle, K.; Gray, B.H.; Jaff, M.R.; Kim, E.S.H.; Mace, P.; Matsumoto, A.H.; et al. The United States Registry for Fibromuscular Dysplasia: Results in the First 447 Patients. Circulation 2012, 125, 3182–3190. [Google Scholar] [CrossRef]
- Olin, J.W.; Gornik, H.L.; Bacharach, J.M.; Biller, J.; Fine, L.J.; Gray, B.H.; Gray, W.A.; Gupta, R.; Hamburg, N.M.; Katzen, B.T.; et al. Fibromuscular Dysplasia: State of the Science and Critical Unanswered Questions: A Scientific Statement From the American Heart Association. Circulation 2014, 129, 1048–1078. [Google Scholar] [CrossRef] [PubMed]
- Slovut, D.P.; Olin, J.W. Fibromuscular Dysplasia. N. Engl. J. Med. 2004, 350, 1862–1871. [Google Scholar] [CrossRef]
- Dobrek, L. An Outline of Renal Artery Stenosis Pathophysiology—A Narrative Review. Life 2021, 11, 208. [Google Scholar] [CrossRef]
- Textor, S.C.; Lerman, L. State of the Art: Renovascular Hypertension and Ischemic Nephropathy. Am. J. Hypertens. 2010, 23, 1159–1169. [Google Scholar] [CrossRef]
- Messerli, F.; Bangalore, S.; Makani, H.; Rimoldi, S.; Allemann, Y.; White, C.; Textor, S.; Sleight, P. Flash Pulmonary Oedema and Bilateral Renal Artery Stenosis: The Pickering Syndrome. Eur. Heart J. 2011, 32, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Tafur, J.D.; White, C.J. Renal Artery Stenosis: When to Revascularize in 2017. Curr. Probl. Cardiol. 2017, 42, 110–135. [Google Scholar] [CrossRef]
- Chrysochou, C.; Kalra, P.A. Epidemiology and Natural History of Atherosclerotic Renovascular Disease. Prog. Cardiovasc. Dis. 2009, 52, 184–195. [Google Scholar] [CrossRef]
- Drieghe, B.; Van Loon, G.; Stuyvaert, S.; De Buyzere, M.L.; Bové, T.; De Backer, T. Renal Pressure-flow Relationship and Renin Activation in a Porcine Model Comparing Unilateral and Bilateral Renal Artery Stenosis. Physiol. Rep. 2024, 12, e70082. [Google Scholar] [CrossRef]
- Mansoor, S.; Shah, A.; Scoble, J.E. ‘Flash Pulmonary Oedema’—A Diagnosis for Both the Cardiologist and the Nephrologist? Nephrol. Dial. Transplant. 2001, 16, 1311–1313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pickering, T.; Devereux, R.; James, G.; Silane, M.; Herman, L.; Sotelo, J.; Sos, T.; Laragh, J. Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: Treatment by angioplasty or surgical revascularisation. Lancet 1988, 332, 551–552. [Google Scholar] [CrossRef]
- Rimoldi, S.F.; Yuzefpolskaya, M.; Allemann, Y.; Messerli, F. Flash Pulmonary Edema. Prog. Cardiovasc. Dis. 2009, 52, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Missouris, C.G. “Apparent” Heart Failure: A Syndrome Caused by Renal Artery Stenoses. Heart 2000, 83, 152–155. [Google Scholar] [CrossRef]
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology 2017, 32, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Straume Bah, I.; Will, M.; Kwok, C.S.; Mascherbauer, J.; Kumric, M.; Bozic, J.; Borovac, J.A. Prevalence and Risk Factors of Renal Artery Stenosis in Patients Undergoing Simultaneous Coronary and Renal Artery Angiography: A Systematic Review and Meta-Analysis of 31,689 Patients from 31 Studies. Diseases 2024, 12, 208. [Google Scholar] [CrossRef]
- Payami, B.; Jafarizade, M.; Beladi Mousavi, S.S.; Sattari, S.-A.; Nokhostin, F. Prevalence and Predictors of Atherosclerotic Renal Artery Stenosis in Hypertensive Patients Undergoing Simultaneous Coronary and Renal Artery Angiography; a Cross-Sectional Study. J. Ren. Inj. Prev. 2016, 5, 34–38. [Google Scholar] [CrossRef][Green Version]
- Kiando, S.R.; Tucker, N.R.; Castro-Vega, L.-J.; Katz, A.; D’Escamard, V.; Tréard, C.; Fraher, D.; Albuisson, J.; Kadian-Dodov, D.; Ye, Z.; et al. PHACTR1 Is a Genetic Susceptibility Locus for Fibromuscular Dysplasia Supporting Its Complex Genetic Pattern of Inheritance. PLoS Genet. 2016, 12, e1006367. [Google Scholar] [CrossRef] [PubMed]
- Savard, S.; Azarine, A.; Jeunemaitre, X.; Azizi, M.; Plouin, P.-F.; Steichen, O. Association of Smoking With Phenotype at Diagnosis and Vascular Interventions in Patients With Renal Artery Fibromuscular Dysplasia. Hypertension 2013, 61, 1227–1232. [Google Scholar] [CrossRef]
- Persu, A.; Van Der Niepen, P.; Touzé, E.; Gevaert, S.; Berra, E.; Mace, P.; Plouin, P.-F.; Jeunemaitre, X. Revisiting Fibromuscular Dysplasia: Rationale of the European Fibromuscular Dysplasia Initiative. Hypertension 2016, 68, 832–839. [Google Scholar] [CrossRef]
- O’Connor, S.; Kim, E.S.; Brinza, E.; Moran, R.; Fendrikova-Mahlay, N.; Wolski, K.; Gornik, H.L. Systemic Connective Tissue Features in Women with Fibromuscular Dysplasia. Vasc. Med. 2015, 20, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Yorgun, H.; Kabakçı, G.; Canpolat, U.; Aytemir, K.; Fatihoğlu, G.; Karakulak, U.N.; Kaya, E.B.; Şahiner, L.; Tokgözoğlu, L.; Oto, A. Frequency and Predictors of Renal Artery Stenosis in Hypertensive Patients Undergoing Coronary Angiography. Angiology 2013, 64, 385–390. [Google Scholar] [CrossRef]
- Khatami, M.; Edalati-Fard, M.; Sadeghian, S.; Salari-Far, M.; Bs, M. Renal Artery Stenosis in Patients with Established Coronary Artery Disease: Prevalence and Predicting Factors. Saudi J. Kidney Dis. Transplant. 2014, 25, 986. [Google Scholar] [CrossRef]
- Colbert, G.B.; Abra, G.; Lerma, E.V. Update and Review of Renal Artery Stenosis. Dis. Mon. 2021, 67, 101118. [Google Scholar] [CrossRef]
- Odudu, A.; Vassallo, D.; Kalra, P.A. From Anatomy to Function: Diagnosis of Atherosclerotic Renal Artery Stenosis. Expert Rev. Cardiovasc. Ther. 2015, 13, 1357–1375. [Google Scholar] [CrossRef]
- AbuRahma, A.F.; Srivastava, M.; Mousa, A.Y.; Dearing, D.D.; Hass, S.M.; Campbell, J.R.; Dean, L.S.; Stone, P.A.; Keiffer, T. Critical Analysis of Renal Duplex Ultrasound Parameters in Detecting Significant Renal Artery Stenosis. J. Vasc. Surg. 2012, 56, 1052–1060.e1. [Google Scholar] [CrossRef]
- Petrovic, D.; Sreckovic, M.; Nikolic, T.; Zivkovic-Radojevic, M.; Miloradovic, V. Renovascular Hypertension: Clinical Features, Differential Diagnoses and Basic Principles of Treatment. Exp. Appl. Biomed. Res. EABR 2016, 17, 251–256. [Google Scholar] [CrossRef][Green Version]
- Arab, S.F.; Alhumaid, A.A.; Alnasr, M.T.A.; Altuwaijri, T.A.; Al-Ghofili, H.; Al-Salman, M.M.; Altoijry, A. Review of Renal Artery Stenosis and Hypertension: Diagnosis, Management, and Recent Randomized Control Trials. Saudi J. Kidney Dis. Transplant. 2022, 33, 147–159. [Google Scholar]
- Cardia, P.P.; Penachim, T.J.; Prando, A.; Torres, U.S.; D’Ippólito, G. Non-Contrast MR Angiography Using Three-Dimensional Balanced Steady-State Free-Precession Imaging for Evaluation of Stenosis in the Celiac Trunk and Superior Mesenteric Artery: A Preliminary Comparative Study with Computed Tomography Angiography. Br. J. Radiol. 2017, 90, 20170011. [Google Scholar] [CrossRef]
- Rajgopal, R.; Khan, A.; Syed, A.A. Renal Artery Stenosis Presenting as Hypertension with Hypokalemia. CMAJ Can. Med. Assoc. J. 2022, 194, E1248–E1249. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Georgianos, P.I.; Germanidis, G.; Giavroglou, C.; Nikolaidis, P.; Lasaridis, A.N.; Madias, N.E. Hypertension and Symptomatic Hypokalemia in a Patient With Simultaneous Unilateral Stenoses of Intrarenal Arteries and Mesangioproliferative Glomerulonephritis. Am. J. Kidney Dis. 2012, 59, 434–438. [Google Scholar] [CrossRef]
- Saeed, A.; Herlitz, H.; Nowakowska-Fortuna, E.; Nilsson, U.; Alhadad, A.; Jensen, G.; Mattiasson, I.; Lindblad, B.; Gottsäter, A.; Guron, G. Oxidative Stress and Endothelin-1 in Atherosclerotic Renal Artery Stenosis and Effects of Renal Angioplasty. Kidney Blood Press. Res. 2011, 34, 396–403. [Google Scholar] [CrossRef]
- Wang, W.; Saad, A.; Herrmann, S.M.; Eirin Massat, A.; McKusick, M.A.; Misra, S.; Lerman, L.O.; Textor, S.C. Changes in Inflammatory Biomarkers after Renal Revascularization in Atherosclerotic Renal Artery Stenosis. Nephrol. Dial. Transplant. 2016, 31, 1437–1443. [Google Scholar] [CrossRef]
- Park, M.Y.; Herrmann, S.M.; Saad, A.; Widmer, R.J.; Tang, H.; Zhu, X.-Y.; Lerman, A.; Textor, S.C.; Lerman, L.O. Circulating and Renal Vein Levels of microRNAs in Patients with Renal Artery Stenosis. Nephrol. Dial. Transplant. 2015, 30, 480–490. [Google Scholar] [CrossRef]
- Herrmann, S.M.S.; Saad, A.; Textor, S.C. Management of Atherosclerotic Renovascular Disease after Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL). Nephrol. Dial. Transplant. 2015, 30, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, H.; Lynch, J.; Hanzal, R.F.; Summerville, W.W. Studies on experimental hypertension. J. Exp. Med. 1934, 59, 347–379. [Google Scholar] [CrossRef]
- Brooks, D.P. SYMPOSIUM Experimental Biology 1995 Endothelin Receptors: Role in Renal Function and Dysfunction Role of Endothelin in Renal Function and Dysfunction. Clin. Exp. Pharmacol. Physiol. 1996, 23, 345–348. [Google Scholar] [CrossRef]
- Kurata, H.; Takaoka, M.; Kubo, Y.; Katayama, T.; Tsutsui, H.; Takayama, J.; Ohkita, M.; Matsumura, Y. Protective Effect of Nitric Oxide on Ischemia/Reperfusion-Induced Renal Injury and Endothelin-1 Overproduction. Eur. J. Pharmacol. 2005, 517, 232–239. [Google Scholar] [CrossRef]
- da Silveira, K.D.; Pompermayer Bosco, K.S.; Diniz, L.R.L.; Carmona, A.K.; Cassali, G.D.; Bruna-Romero, O.; de Sousa, L.P.; Teixeira, M.M.; Santos, R.A.S.; Simões e Silva, A.C.; et al. ACE2–Angiotensin-(1–7)–Mas Axis in Renal Ischaemia/Reperfusion Injury in Rats. Clin. Sci. 2010, 119, 385–394. [Google Scholar] [CrossRef]
- Dieter, R.S.; Schmidt, W.S.; Pacanowski Jr, J.P.; Jaff, M.R. Renovascular Hypertension. Expert Rev. Cardiovasc. Ther. 2005, 3, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Dalman, J.; Coleman, D.M. Nonatherosclerotic Renovascular Hypertension. Surg. Clin. N. Am. 2023, 103, 733–743. [Google Scholar] [CrossRef]
- Marchesi, C.; Paradis, P.; Schiffrin, E.L. Role of the Renin-Angiotensin System in Vascular Inflammation. Trends Pharmacol. Sci. 2008, 29, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Baker, A.Q.; Gallacher, B.; Lodwick, D. Angiotensin II Increases Vascular Permeability Factor Gene Expression by Human Vascular Smooth Muscle Cells. Hypertension 1995, 25, 913–917. [Google Scholar] [CrossRef]
- Álvarez, Á.; Cerdá-Nicolás, M.; Naim Abu Nabah, Y.; Mata, M.; Issekutz, A.C.; Panés, J.; Lobb, R.R.; Sanz, M.-J. Direct Evidence of Leukocyte Adhesion in Arterioles by Angiotensin II. Blood 2004, 104, 402–408. [Google Scholar] [CrossRef]
- Nobuhiko, A.; Suganuma, E.; Babaev, V.R.; Fogo, A.; Swift, L.L.; Linton, M.F.; Fazio, S.; Ichikawa, I.; Kon, V. Angiotensin II Amplifies Macrophage-Driven Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, J.; Liu, X.; Li, M. Angiotensin II Induces C-Reactive Protein Expression through ERK1/2 and JNK Signaling in Human Aortic Endothelial Cells. Atherosclerosis 2010, 212, 206–212. [Google Scholar] [CrossRef]
- Wang, C.-H.; Li, S.-H.; Weisel, R.D.; Fedak, P.W.M.; Dumont, A.S.; Szmitko, P.; Li, R.-K.; Mickle, D.A.G.; Verma, S. C-Reactive Protein Upregulates Angiotensin Type 1 Receptors in Vascular Smooth Muscle. Circulation 2003, 107, 1783–1790. [Google Scholar] [CrossRef]
- Kim, M.P.; Zhou, M.; Wahl, L.M. Angiotensin II Increases Human Monocyte Matrix Metalloproteinase-1 through the AT2 Receptor and Prostaglandin E2: Implications for Atherosclerotic Plaque Rupture. J. Leukoc. Biol. 2005, 78, 195–201. [Google Scholar] [CrossRef]
- Kim, S.; Iwao, H. Molecular and Cellular Mechanisms of Angiotensin II-Mediated Cardiovascular and Renal Diseases. Pharmacol. Rev. 2000, 52, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-Induced Production of Mitochondrial Reactive Oxygen Species: Potential Mechanisms and Relevance for Cardiovascular Disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Romero, J.C. Role of Oxidative Stress in Angiotensin-Induced Hypertension. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R893–R912. [Google Scholar] [CrossRef]
- Diekmann, F.; Zart, R.; Thöne-Reineke, C.; Bauer, C.; Neumayer, H.-H.; Hocher, B. Regulation of the Renal Endothelin System in the Two-Kidney, One Clip Renal Hypertensive Rat. J. Cardiovasc. Pharmacol. 2000, 36, S191–S194. [Google Scholar] [CrossRef]
- Deng, L.-Y.; Schiffrin, E.L. Endothelin-1 Gene Expression in Blood Vessels and Kidney of Spontaneously Hypertensive Rats (SHR), L-NAME-Treated SHR, and Renovascular Hypertensive Rats. J. Cardiovasc. Pharmacol. 1998, 31, S380–S383. [Google Scholar] [CrossRef]
- Cecioni, I.; Modesti, P.A.; Poggesi, L.; Rocchi, F.; Rega, L.; Serneri, G.G.N. Endothelin-1 Urinary Excretion, but Not Endothelin-1 Plasma Concentration, Is Increased in Renovascular Hypertension. J. Lab. Clin. Med. 1999, 134, 386–391. [Google Scholar] [CrossRef]
- De Jong, W. Release of Renin by Rat Kidney Slices; Relationship to Plasma Renin after Desoxycorticosterone and Renal Hypertension. Exp. Biol. Med. 1969, 130, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Eide, I.; Myers, M.R.; DeQuattro, V.; Kolloch, R.; Eide, K.; Whigham, H. Increased Hypothalamic Noradrenergic Activity in One-Kidney, One-Clip Renovascular Hypertensive Rats. J. Cardiovasc. Pharmacol. 1980, 2, 833–842. [Google Scholar] [CrossRef]
- Klassen, P.S.; Svetkey, L.P. Diagnosis and Management of Renovascular Hypertension. Cardiol. Rev. 2000, 8, 17–29. [Google Scholar] [CrossRef]
- Park, M.Y.; Herrmann, S.M.; Saad, A.; Eirin, A.; Tang, H.; Lerman, A.; Textor, S.C.; Lerman, L.O. Biomarkers of Kidney Injury and Klotho in Patients with Atherosclerotic Renovascular Disease. Clin. J. Am. Soc. Nephrol. 2015, 10, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S. Cardiovascular and Renal Surrogate Markers in the Clinical Management of Hypertension. Cardiovasc. Drugs Ther. 2009, 23, 317–326. [Google Scholar] [CrossRef]
- Eirin, A.; Gloviczki, M.L.; Tang, H.; Rule, A.D.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Lerman, L.O. Chronic Renovascular Hypertension Is Associated with Elevated Levels of Neutrophil Gelatinase-Associated Lipocalin. Nephrol. Dial. Transplant. 2012, 27, 4153–4161. [Google Scholar] [CrossRef]
- Schoenberg, S.O.; Rieger, J.R.; Michaely, H.J.; Rupprecht, H.; Samtleben, W.; Reiser, M.F. Functional Magnetic Resonance Imaging in Renal Artery Stenosis. Abdom. Imaging 2006, 31, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Kelsen, S. Renal Microvascular Disease Determines the Responses to Revascularization in Experimental Renovascular Disease. Circ. Cardiovasc. Interv. 2010, 3, 376–383. [Google Scholar] [CrossRef]
- Jacobson, H.R. Ischemic Renal Disease: An Overlooked Clinical Entity? Kidney Int. 1988, 34, 729–743. [Google Scholar] [CrossRef]
- Breyer, J.A.; Jacobson, H.R. Ischemic Nephropathy. Curr. Opin. Nephrol. Hypertens. 1993, 2, 216–224. [Google Scholar] [CrossRef]
- Adamczak, M.; Wiecek, A. Ischemic Nephropathy–Pathogenesis and Treatment. Nefrología 2012, 32, 432–438. [Google Scholar] [PubMed]
- Greco, B.A.; Breyer, J.A. Atherosclerotic Ischemic Renal Disease. Am. J. Kidney Dis. 1997, 29, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.; Wright, J.R.; Shurrab, A.E.; Mamtora, H.; Foley, R.N.; O’Donoghue, D.J.; Waldek, S.; Kalra, P.A. Epidemiology of Renal Dysfunction and Patient Outcome in Atherosclerotic Renal Artery Occlusion. J. Am. Soc. Nephrol. 2002, 13, 149–157. [Google Scholar] [CrossRef]
- Kwon, O.; Hong, S.-M.; Ramesh, G. Diminished NO Generation by Injured Endothelium and Loss of Macula Densa nNOS May Contribute to Sustained Acute Kidney Injury after Ischemia-Reperfusion. Am. J. Physiol.-Ren. Physiol. 2009, 296, F25–F33. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Zuk, A. Ischemic Acute Renal Failure: An Inflammatory Disease? Kidney Int. 2004, 66, 480–485. [Google Scholar] [CrossRef]
- Totoń-Żurańska, J.; Mikolajczyk, T.P.; Saju, B.; Guzik, T.J. Vascular Remodelling in Cardiovascular Diseases: Hypertension, Oxidation, and Inflammation. Clin. Sci. 2024, 138, 817–850. [Google Scholar] [CrossRef]
- Tabas, I.; Williams, K.J.; Borén, J. Subendothelial Lipoprotein Retention as the Initiating Process in Atherosclerosis: Update and Therapeutic Implications. Circulation 2007, 116, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Pentikäinen, M.O.; Öörni, K.; Ala-Korpela, M.; Kovanen, P.T. Modified LDL–Trigger of Atherosclerosis and Inflammation in the Arterial Intima. J. Intern. Med. 2000, 247, 359–370. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current Pathogenesis and Therapeutic Options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Urbieta-Caceres, V.H.; Grande, J.P.; Lerman, A.; Textor, S.C.; Lerman, L.O. Persistent Kidney Dysfunction in Swine Renal Artery Stenosis Correlates with Outer Cortical Microvascular Remodeling. Am. J. Physiol.-Ren. Physiol. 2011, 300, F1394–F1401. [Google Scholar] [CrossRef]
- Chade, A.R.; Zhu, X.; Mushin, O.P.; Napoli, C.; Lerman, A.; Lerman, L.O.; Chade, A.R.; Zhu, X.; Mushin, O.P.; Napoli, C.; et al. Simvastatin Promotes Angiogenesis and Prevents Microvascular Remodeling in Chronic Renal Ischemia. FASEB J. 2006, 20, 1706–1708. [Google Scholar] [CrossRef] [PubMed]
- Kotliar, C.; Juncos, L.; Inserra, F.; De Cavanagh, E.M.V.; Chuluyan, E.; Aquino, J.B.; Hita, A.; Navari, C.; Sánchez, R. Local and Systemic Cellular Immunity in Early Renal Artery Atherosclerosis. Clin. J. Am. Soc. Nephrol. 2012, 7, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.-S.; Ruhrberg, C.; Cantley, L.G. Distinct Macrophage Phenotypes Contribute to Kidney Injury and Repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef]
- Duffield, J.S. Macrophages in Kidney Repair and Regeneration. J. Am. Soc. Nephrol. 2011, 22, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, M.L.; Keddis, M.T.; Garovic, V.D.; Friedman, H.; Herrmann, S.; McKusick, M.A.; Misra, S.; Grande, J.P.; Lerman, L.O.; Textor, S.C. TGF Expression and Macrophage Accumulation in Atherosclerotic Renal Artery Stenosis. Clin. J. Am. Soc. Nephrol. 2013, 8, 546–553. [Google Scholar] [CrossRef]
- Abumoawad, A.; Saad, A.; Ferguson, C.M.; Eirin, A.; Woollard, J.R.; Herrmann, S.M.; Hickson, L.J.; Bendel, E.C.; Misra, S.; Glockner, J.; et al. Tissue Hypoxia, Inflammation, and Loss of Glomerular Filtration Rate in Human Atherosclerotic Renovascular Disease. Kidney Int. 2019, 95, 948–957. [Google Scholar] [CrossRef]
- Puranik, A.S.; Leaf, I.A.; Jensen, M.A.; Hedayat, A.F.; Saad, A.; Kim, K.-W.; Saadalla, A.M.; Woollard, J.R.; Kashyap, S.; Textor, S.C.; et al. Kidney-Resident Macrophages Promote a Proangiogenic Environment in the Normal and Chronically Ischemic Mouse Kidney. Sci. Rep. 2018, 8, 13948. [Google Scholar] [CrossRef]
- Perez-Gomez, M.V.; Bartsch, L.-A.; Castillo-Rodriguez, E.; Fernandez-Prado, R.; Fernandez-Fernandez, B.; Martin-Cleary, C.; Gracia-Iguacel, C.; Ortiz, A. Clarifying the Concept of Chronic Kidney Disease for Non-Nephrologists. Clin. Kidney J. 2019, 12, 258–261. [Google Scholar] [CrossRef]
- Hu, L.; Napoletano, A.; Provenzano, M.; Garofalo, C.; Bini, C.; Comai, G.; La Manna, G. Mineral Bone Disorders in Kidney Disease Patients: The Ever-Current Topic. Int. J. Mol. Sci. 2022, 23, 12223. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39, S1–S266. [Google Scholar]
- Ebrahimi, B.; Eirin, A.; Li, Z.; Zhu, X.-Y.; Zhang, X.; Lerman, A.; Textor, S.C.; Lerman, L.O. Mesenchymal Stem Cells Improve Medullary Inflammation and Fibrosis after Revascularization of Swine Atherosclerotic Renal Artery Stenosis. PLoS ONE 2013, 8, e67474. [Google Scholar] [CrossRef] [PubMed]
- Parienty, I.; Rostoker, G.; Jouniaux, F.; Piotin, M.; Admiraal-Behloul, F.; Miyazaki, M. Renal Artery Stenosis Evaluation in Chronic Kidney Disease Patients: Nonenhanced Time-Spatial Labeling Inversion-Pulse Three-Dimensional MR Angiography with Regulated Breathing versus DSA. Radiology 2011, 259, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kozawa, E.; Okada, H.; Inukai, K.; Watanabe, S.; Kikuta, T.; Watanabe, Y.; Takenaka, T.; Katayama, S.; Tanaka, J.; et al. Noninvasive Evaluation of Kidney Hypoxia and Fibrosis Using Magnetic Resonance Imaging. J. Am. Soc. Nephrol. 2011, 22, 1429–1434. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Coca, A.; Kahan, T.; Boriani, G.; Manolis, A.S.; Olsen, M.H.; Oto, A.; Potpara, T.S.; Steffel, J.; Marín, F.; et al. Hypertension and Cardiac Arrhythmias: Executive Summary of a Consensus Document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, Endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Eur. Heart J.-Cardiovasc. Pharmacother. 2017, 3, 235–250. [Google Scholar] [CrossRef]
- Shenasa, M.; Shenasa, H. Hypertension, Left Ventricular Hypertrophy, and Sudden Cardiac Death. Int. J. Cardiol. 2017, 237, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K. Pathogenesis and Therapeutic Implications of Cardiorenal Syndrome. Clin. Exp. Nephrol. 2011, 15, 187–194. [Google Scholar] [CrossRef]
- Subbiah, A.K.; Chhabra, Y.K.; Mahajan, S. Cardiovascular Disease in Patients with Chronic Kidney Disease: A Neglected Subgroup. Heart Asia 2016, 8, 56–61. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Balk, E.; Raman, G.; Chung, M.; Ip, S.; Tatsioni, A.; Alonso, A.; Chew, P.; Gilbert, S.J.; Lau, J. Effectiveness of Management Strategies for Renal Artery Stenosis: A Systematic Review. Ann. Intern. Med. 2006, 145, 901–912. [Google Scholar] [CrossRef]
- Cooper, C.J.; Murphy, T.P.; Cutlip, D.E.; Jamerson, K.; Henrich, W.; Reid, D.M.; Cohen, D.J.; Matsumoto, A.H.; Steffes, M.; Jaff, M.R.; et al. Stenting and Medical Therapy for Atherosclerotic Renal-Artery Stenosis. N. Engl. J. Med. 2014, 370, 13–22. [Google Scholar] [CrossRef]
- Maideen, N.M.P.; Balasubramanian, R.; Muthusamy, S.; Nallasamy, V. An Overview of Clinically Imperative and Pharmacodynamically Significant Drug Interactions of Renin-Angiotensin-Aldosterone System (RAAS) Blockers. Curr. Cardiol. Rev. 2022, 18, e110522204611. [Google Scholar] [CrossRef]
- Prince, M.; Tafur, J.D.; White, C.J. When and How Should We Revascularize Patients With Atherosclerotic Renal Artery Stenosis? JACC Cardiovasc. Interv. 2019, 12, 505–517. [Google Scholar] [CrossRef]
- Kihm, M.; Vogel, B.; Zeier, M.; Kihm, L. Renal Artery Stenosis–Are There Patients Who Benefit from Intervention? Exp. Clin. Endocrinol. Diabetes 2016, 124, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.J.; Murphy, T.P.; Matsumoto, A.; Steffes, M.; Cohen, D.J.; Jaff, M.; Kuntz, R.; Jamerson, K.; Reid, D.; Rosenfield, K.; et al. Stent Revascularization for the Prevention of Cardiovascular and Renal Events among Patients with Renal Artery Stenosis and Systolic Hypertension: Rationale and Design of the CORAL Trial. Am. Heart J. 2006, 152, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Dubel, G.J.; Murphy, T.P. The Role of Percutaneous Revascularization for Renal Artery Stenosis. Vasc. Med. 2008, 13, 141–156. [Google Scholar] [CrossRef]
- Zeller, T.; Macharzina, R.; Rastan, A.; Beschorner, U.; Noory, E. Renal Artery Stenosis: Up-Date on Diagnosis and Treatment. Vasa 2014, 43, 27–38. [Google Scholar] [CrossRef]
- Sattur, S.; Prasad, H.; Bedi, U.; Kaluski, E.; Stapleton, D.D. Renal Artery Stenosis-An Update. Postgrad. Med. 2013, 125, 43–50. [Google Scholar] [CrossRef]
- Edgar, B.; Pearson, R.; Kasthuri, R.; Gillis, K.; Geddes, C.; Rostron, M.; Brady, A.; Hussey, K.; Roditi, G.; Delles, C.; et al. The Impact of Renal Artery Stenting on Therapeutic Aims. J. Hum. Hypertens. 2022, 37, 265–272. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Dworkin, L.D.; Henrich, W.; Greco, B.A.; Steffes, M.; Tobe, S.; Shapiro, J.I.; Jamerson, K.; Lyass, A.; Pencina, K.; et al. Effects of Stenting for Atherosclerotic Renal Artery Stenosis on eGFR and Predictors of Clinical Events in the CORAL Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 1180–1188. [Google Scholar] [CrossRef]
- Kądziela, J.; Jóźwik-Plebanek, K.; Pappaccogli, M.; Van Der Niepen, P.; Prejbisz, A.; Dobrowolski, P.; Michałowska, I.; Talarowska, P.; Warchoł-Celińska, E.; Stryczyński, Ł.; et al. Risks and Benefits of Renal Artery Stenting in Fibromuscular Dysplasia: Lessons from the ARCADIA-POL Study. Vasc. Med. 2024, 29, 50–57. [Google Scholar] [CrossRef]
- Alfonso, F.; Byrne, R.A.; Rivero, F.; Kastrati, A. Current Treatment of In-Stent Restenosis. J. Am. Coll. Cardiol. 2014, 63, 2659–2673. [Google Scholar] [CrossRef]
- Lao, D.; Parasher, P.S.; Cho, K.C.; Yeghiazarians, Y. Atherosclerotic Renal Artery Stenosis—Diagnosis and Treatment. Mayo Clin. Proc. 2011, 86, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Boateng, F.K.; Greco, B.A. Renal Artery Stenosis: Prevalence of, Risk Factors for, and Management of In-Stent Stenosis. Am. J. Kidney Dis. 2013, 61, 147–160. [Google Scholar] [CrossRef]
- Patel, P.M.; Eisenberg, J.; Islam, M.A.; Maree, A.O.; Rosenfield, K.A. Percutaneous Revascularization of Persistent Renal Artery In-Stent Restenosis. Vasc. Med. 2009, 14, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, B.C.; Krijnen, P.; Pieterman, H.; Derkx, F.H.M.; Deinum, J.; Postma, C.T.; Dees, A.; Woittiez, A.J.J.; Bartelink, A.K.M.; Man In `T Veld, A.J.; et al. The Effect of Balloon Angioplasty on Hypertension in Atherosclerotic Renal-Artery Stenosis. N. Engl. J. Med. 2000, 342, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Plouin, P.-F.; Chatellier, G.; Darné, B.; Raynaud, A. Blood Pressure Outcome of Angioplasty in Atherosclerotic Renal Artery Stenosis: A Randomized Trial. Hypertension 1998, 31, 823–829. [Google Scholar] [CrossRef]
- Webster, J.; Marshall, F.; Abdalla, M.; Dominiczak, A.; Edwards, R.; Isles, C.; Loose, H.; Main, J.; Padfield, P.; Russell, I.; et al. Randomised Comparison of Percutaneous Angioplasty vs Continued Medical Therapy for Hypertensive Patients with Atheromatous Renal Artery Stenosis. J. Hum. Hypertens. 1998, 12, 329–335. [Google Scholar] [CrossRef]
- Bax, L. Stent Placement in Patients With Atherosclerotic Renal Artery Stenosis and Impaired Renal Function: A Randomized Trial. Ann. Intern. Med. 2009, 150, 840. [Google Scholar] [CrossRef]
- ASTRAL Investigators; Wheatley, K.; Ives, N.; Gray, R.; Kalra, P.A.; Moss, J.G.; Baigent, C.; Carr, S.; Chalmers, N.; Eadington, D.; et al. Revascularization versus Medical Therapy for Renal-Artery Stenosis. N. Engl. J. Med. 2009, 361, 1953–1962. [Google Scholar] [CrossRef]
- Fournier, T.; Sens, F.; Rouvière, O.; Millon, A.; Juillard, L. Prise en charge de la sténose athéromateuse d’artère rénale en 2016. Néphrologie Thérapeutique 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Balk, E.M.; Raman, G.; Adam, G.P.; Halladay, C.W.; Langberg, V.N.; Azodo, I.A.; Trikalinos, T.A. Renal Artery Stenosis Management Strategies: An Updated Comparative Effectiveness Review; AHRQ Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2016. [Google Scholar]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the Management of Patients With Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): A Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery,* Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Halperin, J.L.; Albert, N.M.; Bozkurt, B.; Brindis, R.G.; Curtis, L.H.; DeMets, D.; Guyton, R.A.; Hochman, J.S.; Kovacs, R.J.; et al. Management of Patients With Peripheral Artery Disease (Compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 1425–1443. [Google Scholar] [CrossRef] [PubMed]
- Ikee, R.; Kobayashi, S.; Hemmi, N.; Imakiire, T.; Kikuchi, Y.; Moriya, H.; Suzuki, S.; Miura, S. Correlation Between the Resistive Index by Doppler Ultrasound and Kidney Function and Histology. Am. J. Kidney Dis. 2005, 46, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, J.; Chavan, A.; Bleck, J.; Vitzthum, A.; Stoess, B.; Gebel, M.J.; Galanski, M.; Koch, K.M.; Haller, H. Use of Doppler Ultrasonography to Predict the Outcome of Therapy for Renal-Artery Stenosis. N. Engl. J. Med. 2001, 344, 410–417. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Wang, H.; Yin, Y.; Cao, C.; Luo, J.; Wang, Y. Percutaneous Renal Artery Stent Implantation in the Treatment of Atherosclerotic Renal Artery Stenosis. Exp. Ther. Med. 2018, 16, 2331–2336. [Google Scholar] [CrossRef]
- Di Palma, G.; Cortese, B. Intravascular Lithotripsy and Dual Drug-Coated Balloons for the Treatment of Severely Calcific Renal In-Stent Restenosis: All Is Fair in War. Cardiovasc. Revasc. Med. 2021, 28, 193–196. [Google Scholar] [CrossRef]
- Zhang, H.; McCarty, N. CRISPR-Cas9 Technology and Its Application in Haematological Disorders. Br. J. Haematol. 2016, 175, 208–225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; Tonheiro, J.; Rodrigues, F. The “Silent Enemy” Called Renal Artery Stenosis: A Mini-Review. J. Vasc. Dis. 2025, 4, 10. https://doi.org/10.3390/jvd4010010
Silva J, Tonheiro J, Rodrigues F. The “Silent Enemy” Called Renal Artery Stenosis: A Mini-Review. Journal of Vascular Diseases. 2025; 4(1):10. https://doi.org/10.3390/jvd4010010
Chicago/Turabian StyleSilva, José, Juan Tonheiro, and Fernanda Rodrigues. 2025. "The “Silent Enemy” Called Renal Artery Stenosis: A Mini-Review" Journal of Vascular Diseases 4, no. 1: 10. https://doi.org/10.3390/jvd4010010
APA StyleSilva, J., Tonheiro, J., & Rodrigues, F. (2025). The “Silent Enemy” Called Renal Artery Stenosis: A Mini-Review. Journal of Vascular Diseases, 4(1), 10. https://doi.org/10.3390/jvd4010010





