Vascular and Neural Response to Focal Vibration, Sensory Feedback, and Piezo Ion Channel Signaling
Abstract
1. Introduction
2. Mechanosensitive Piezo Channels
2.1. Piezo1
2.1.1. Activation
2.1.2. Function
2.2. Piezo2
2.2.1. Activation
2.2.2. Function
2.3. TRPs and Other Mechanosensitive Channels
3. Sense of Touch
Touch, Proprioception, Pain, and Gate Control
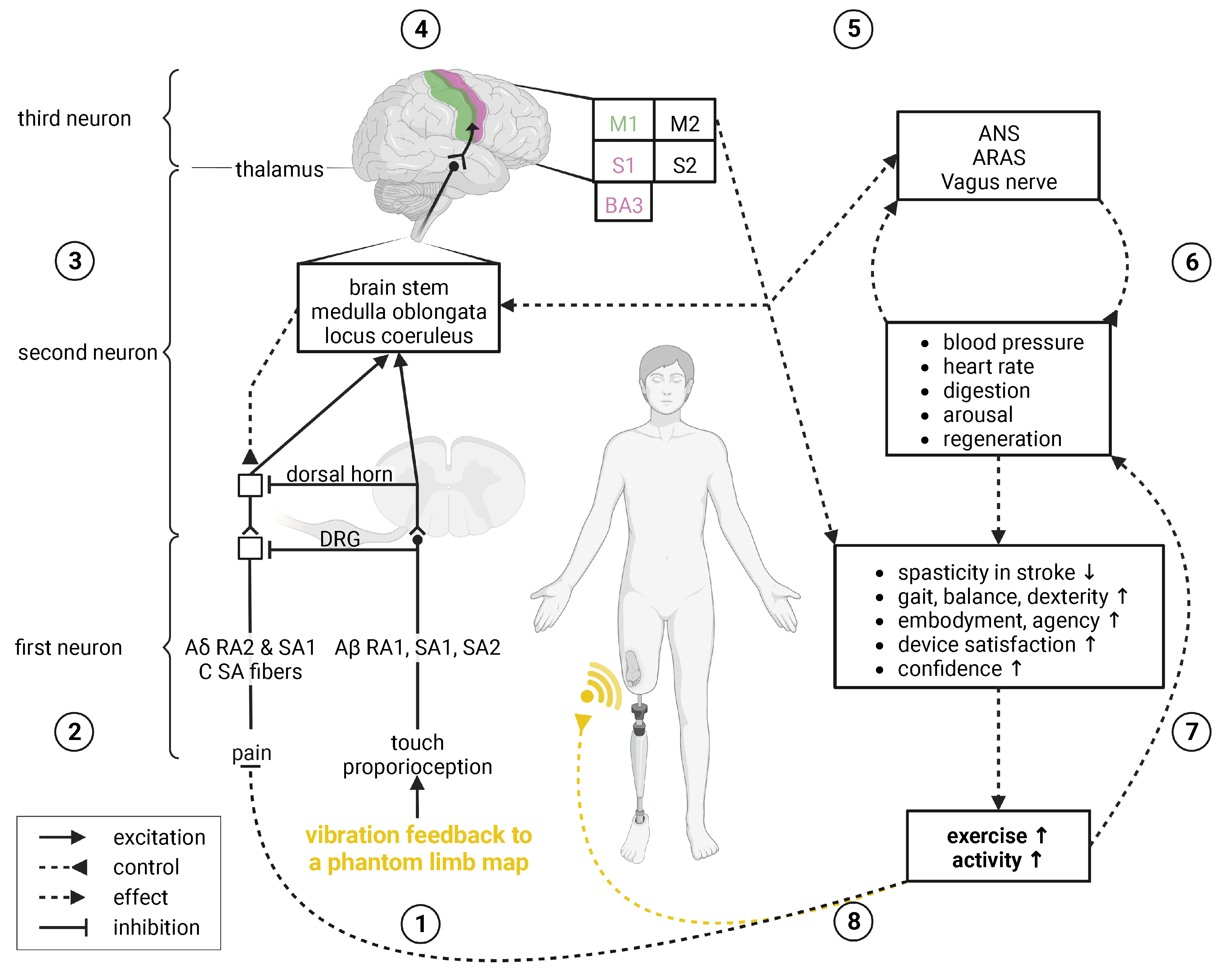
4. Focal Vibration Therapy
5. Peripheral Effects of Focal Vibration
5.1. Vascular Regeneration
- (a)
- (b)
- (c)
- High hydrostatic pressure in lung capillaries leads to endothelial barrier disruption (associated with lung edema) through Ca-induced calpain activation [367];
- (d)
- Laminar flow shear stress or S1P without mechanical stress lead to endothelial cell sprout formation through Ca-induced activation, and membrane translocation of membrane type-1 matrix metalloproteinase [40];
- (e)
- Laminar blood flow leads to atheroprotective vasodilatation in the periphery through phosphorylation of AKT and eNOS synthesizing endothelial [32,34,86,112,371,376,377,378,379,380,381,382,383,384] which is associated with factors, including VEGF [356], bFGF [368,385,386], microRNAs miR-126 [387,388], and miR-17–92 [369];
- (f)
- (g)
- Hypertension in smooth muscle cells of resistance arteries leads to arterial remodeling through stimulation of transglutaminase II (not depicted) [15].
5.2. Peripheral Nerve Repair
6. Neuromodulation Effects of Focal Vibration
“[...] appear to play a considerable role in reducing spasticity and improving gait, balance, and motor function in patients affected by stroke. In particular, focal muscle vibration [was] more useful if applied to nonspastic antagonist muscles with reciprocal inhibitory action on spastic muscles. Conversely, vibration therapy seems [unable] to reduce spasticity in multiple sclerosis and cerebral palsy. Concerning spinal cord injury, Parkinson’s disease, spinocerebellar ataxia, dystonia, and essential tremor, no evidence-based recommendation could be drawn from the literature to guide rehabilitation medicine clinicians to manage spasticity with vibration application” (Moggio et al. [242]).
6.1. Vibratory Sensory Feedback
6.2. Effects on the Autonomous Nervous System
6.2.1. Vagus Nerve
6.2.2. Ascending Reticular Activating System
“neurons wire together if they fire together” (Löwel and Singer [623]).
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ARAS | ascending reticular activating system |
| BDNF | brain-derived neurotrophic factor |
| bFGF | basic fibroblast growth factor |
| BA | Brodmann areas |
| DRG | dorsal root ganglion |
| EGF | epidermal growth factor |
| eNOS | endothelial nitric oxygen synthase |
| GDNF | glial-derived neurotrophic factor |
| HGF | hepatocyte growth factor |
| INHBA | inhibin subunit beta A |
| iNOS | inducible nitric oxygen synthase |
| LTMR | low-threshold mechanoreceptors |
| M1|2 | primary|secondary motor area |
| miR | micro RNA |
| NF-κB | nuclear factor κB |
| NGF | nerve growth factor |
| nNOS | neuronal nitric oxygen synthase |
| nitric oxygen | |
| NOS | nitric oxygen synthase |
| NRG1 | neuregulin 1 |
| NT | neurotrophin |
| RA | rapidly adapting |
| S1|2 | primary|secondary somatosensory area |
| S1P | sphingosine 1 phosphate |
| SA | slowly adapting |
| TAZ | transcriptional coactivator with PDZ-binding motif |
| TRP | transient receptor potential |
| VEGF | vascular endothelial growth factor |
| VEGFR | VEGF receptor |
| YAP | Yes-associated protein |
References
- Müller-Oerlinghausen, B.; Eggart, M.; Norholt, H.; Gerlach, M.; Kiebgis, G.M.; Arnold, M.M.; Moberg, K.U. Touch Medicine—a complementary therapeutic approach exemplified by the treatment of depression. Dtsch. Med. Wochenschr. 2022, 147, E32–E40. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J.; Kalli, A.C. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; MacKinnon, R. Structure-based membrane dome mechanism for piezo mechanosensitivity. eLife 2017, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the mechanically activated ion channel Piezo1. Nature 2018, 554, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.Q.; et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492. [Google Scholar] [CrossRef]
- Lin, Y.C.; Guo, Y.R.; Miyagi, A.; Levring, J.; Mackinnon, R.; Scheuring, S. Force-induced conformational changes in PIEZO1. Nature 2019, 573, 230–234. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229. [Google Scholar] [CrossRef]
- Geng, J.; Liu, W.; Zhou, H.; Zhang, T.; Wang, L.; Zhang, M.; Li, Y.; Shen, B.; Li, X.; Xiao, B. A Plug-and-Latch Mechanism for Gating the Mechanosensitive Piezo Channel. Neuron 2020, 106, 438–451.e6. [Google Scholar] [CrossRef]
- Szczot, M.; Nickolls, A.R.; Lam, R.M.; Chesler, A.T. The Form and Function of PIEZO2. Annu. Rev. Biochem. 2021, 90, 507–534. [Google Scholar] [CrossRef]
- Bagriantsev, S.N.; Gracheva, E.O.; Gallagher, P.G. Piezo Proteins: Regulators of Mechanosensation and Other Cellular Processes. J. Biol. Chem. 2014, 289, 31673–31681. [Google Scholar] [CrossRef]
- Murthy, S.E.; Dubin, A.E.; Patapoutian, A. Piezos thrive under pressure: Mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 771–783. [Google Scholar] [CrossRef]
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, Tension, and Transduction—The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci. 2017, 42, 57–71. [Google Scholar] [CrossRef]
- McCarter, G.C.; Reichling, D.B.; Levine, J.D. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci. Lett. 1999, 273, 179–182. [Google Scholar] [CrossRef]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef]
- Gaub, B.M.; Müller, D.J. Mechanical Stimulation of Piezo1 Receptors Depends on Extracellular Matrix Proteins and Directionality of Force. Nano Lett. 2017, 17, 2064–2072. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, J.; Kala, S.; Zhu, J.; Xian, Q.; Qiu, W.; Li, G.; Zhu, T.; Meng, L.; Zhang, R.; et al. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience 2019, 21, 448–457. [Google Scholar] [CrossRef]
- Ellefsen, K.L.; Holt, J.R.; Chang, A.C.; Nourse, J.L.; Arulmoli, J.; Mekhdjian, A.H.; Abuwarda, H.; Tombola, F.; Flanagan, L.A.; Dunn, A.R.; et al. Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca2+ flickers. Commun. Biol. 2019, 2, 1–13. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015, 4, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 2018, 9, 1300. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeon, W.Y.; Ji, Y.; Park, E.J.; Yoon, D.S.; Lee, N.H.; Park, S.M.; Mandakhbayar, N.; Lee, J.H.; Lee, H.H.; et al. Electricity auto-generating skin patch promotes wound healing process by activation of mechanosensitive ion channels. Biomaterials 2021, 275, 120948. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zhang, H.; Guo, X.W.; Lu, Y.; Wang, S.N.; Cheng, B.; Dong, S.H.; Lyu, X.L.; Li, F.S.; Li, Y.W. Mechanically Activated Calcium Channel PIEZO1 Modulates Radiation-Induced Epithelial-Mesenchymal Transition by Forming a Positive Feedback With TGF-β1. Front. Mol. Biosci. 2021, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Li, L.; Wang, P.; Wang, Z.; Shi, X.; Guo, M.; Zhang, P. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale 2019, 11, 23423–23437. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Bae, C.; Gottlieb, P.A.; Sachs, F. Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS ONE 2015, 10, e0125503. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Xiao, B.; Santos, J.S.; Syeda, R.; Grandl, J.; Spencer, K.S.; Kim, S.E.; Schmidt, M.; Mathur, J.; Dubin, A.E.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Qin, L.; He, T.; Chen, S.; Yang, D.; Yi, W.; Cao, H.; Xiao, G. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Shah, V.; Patel, S.; Shah, J. Emerging role of Piezo ion channels in cardiovascular development. Dev. Dyn. 2022, 251, 276–286. [Google Scholar] [CrossRef]
- Douguet, D.; Patel, A.; Xu, A.; Vanhoutte, P.M.; Honoré, E. Piezo Ion Channels in Cardiovascular Mechanobiology. Trends Pharmacol. Sci. 2019, 40, 956–970. [Google Scholar] [CrossRef]
- Gilchrist, C.L.; Leddy, H.A.; Kaye, L.; Case, N.D.; Rothenberg, K.E.; Little, D.; Liedtke, W.; Hoffman, B.D.; Guilak, F. TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc. Natl. Acad. Sci. 2019, 116, 1992–1997. [Google Scholar] [CrossRef]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kühnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 2018, 10, eaat9897. [Google Scholar] [CrossRef]
- Szczot, M.; Liljencrantz, J.; Ghitani, N.; Barik, A.; Lam, R.; Thompson, J.H.; Bharucha-Goebel, D.; Saade, D.; Necaise, A.; Donkervoort, S.; et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 2018, 10, eaat9892. [Google Scholar] [CrossRef]
- Ranade, S.S.; Qiu, Z.; Woo, S.H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef]
- Zeng, W.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef]
- Wang, S.P.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef]
- Rode, B.; Shi, J.; Endesh, N.; Drinkhill, M.J.; Webster, P.J.; Lotteau, S.J.; Bailey, M.A.; Yuldasheva, N.Y.; Ludlow, M.J.; Cubbon, R.M.; et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat. Commun. 2017, 8, 350. [Google Scholar] [CrossRef]
- Nonomura, K.; Lukacs, V.; Sweet, D.T.; Goddard, L.M.; Kanie, A.; Whitwam, T.; Ranade, S.S.; Fujimori, T.; Kahn, M.L.; Patapoutian, A. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc. Natl. Acad. Sci. USA 2018, 115, 12817–12822. [Google Scholar] [CrossRef]
- Choi, D.; Park, E.; Jung, E.; Cha, B.; Lee, S.; Yu, J.; Kim, P.M.; Lee, S.; Hong, Y.J.; Koh, C.J.; et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Faucherre, A.; Moha Ou Maati, H.; Nasr, N.; Pinard, A.; Theron, A.; Odelin, G.; Desvignes, J.P.; Salgado, D.; Collod-Béroud, G.; Avierinos, J.F.; et al. Piezo1 is required for outflow tract and aortic valve development. J. Mol. Cell. Cardiol. 2020, 143, 51–62. [Google Scholar] [CrossRef]
- Duchemin, A.L.; Vignes, H.; Vermot, J. Mechanically activated Piezo channels modulate outflow tract valve development through the yap1 and KLF2-notch signaling axis. eLife 2019, 8, 1–27. [Google Scholar] [CrossRef]
- Kang, H.; Hong, Z.; Zhong, M.; Klomp, J.; Bayless, K.J.; Mehta, D.; Karginov, A.V.; Hu, G.; Malik, A.B. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am. J. Physiol.-Cell Physiol. 2019, 316, C92–C103. [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Si, G.; Huang, J.; Samuel, A.D.; Perrimon, N. Mechanical regulation of stem-cell differentiation by the stretch-activated Piezo channel. Nature 2018, 555, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of piezo1 by mechanical signals promotes bone anabolism. eLife 2019, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, D.; Farrelly, O.; Miles, L.; Li, F.; Kim, S.E.; Lo, T.Y.; Wang, F.; Li, T.; Thompson-Peer, K.L.; et al. The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 2019, 102, 373–389.e6. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.; Yun, S.; De Zoete, M.R.; Warnock, J.N.; To, S.D.; York, A.G.; et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef]
- Ma, S.; Cahalan, S.; Lamonte, G.; Grubaugh, N.D.; Zeng, W.; Murthy, S.E.; Paytas, E.; Gamini, R.; Lukacs, V.; Whitwam, T.; et al. Common PIEZO1 Allele in African Populations Causes RBC Dehydration and Attenuates Plasmodium Infection. Cell 2018, 173, 443–455.e12. [Google Scholar] [CrossRef]
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.B.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549. [Google Scholar] [CrossRef]
- Reeh, P.W.; Fischer, M.J.M. Nobel somatosensations and pain. Pflügers Arch.-Eur. J. Physiol. 2022, 474, 405–420. [Google Scholar] [CrossRef]
- Lewis, A.H.; Cui, A.F.; McDonald, M.F.; Grandl, J. Transduction of Repetitive Mechanical Stimuli by Piezo1 and Piezo2 Ion Channels. Cell Rep. 2017, 19, 2572–2585. [Google Scholar] [CrossRef]
- Wang, J.; La, J.H.; Hamill, O.P. PIEZO1 is selectively expressed in small diameter mouse DRG neurons distinct from neurons strongly expressing TRPV1. Front. Mol. Neurosci. 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Roh, J.; Hwang, S.M.; Lee, S.H.; Lee, K.; Kim, Y.H.; Park, C.K. Functional expression of piezo1 in dorsal root ganglion (DRG) neurons. Int. J. Mol. Sci. 2020, 21, 3834. [Google Scholar] [CrossRef]
- Nagel, M.; Chesler, A.T. PIEZO2 ion channels in proprioception. Curr. Opin. Neurobiol. 2022, 75, 102572. [Google Scholar] [CrossRef]
- Ranade, S.S.; Woo, S.H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Bégay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef]
- Liao, D.; Hsiao, M.Y.; Xiang, G.; Zhong, P. Optimal pulse length of insonification for Piezo1 activation and intracellular calcium response. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Fallon, J.B.; Macefield, V.G. Vibration sensitivity of human muscle spindles and golgi tendon organs. Muscle Nerve 2007, 36, 21–29. [Google Scholar] [CrossRef]
- Handler, A.; Ginty, D.D. The mechanosensory neurons of touch and their mechanisms of activation. Nat. Rev. Neurosci. 2021, 22, 521–537. [Google Scholar] [CrossRef]
- Roll, J.P.; Vedel, J.P. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp. Brain Res. 1982, 47, 177–190. [Google Scholar] [CrossRef]
- Roll, J.P.; Vedel, J.P.; Ribot, E. Alteration of proprioceptive messages induced by tendon vibration in man: A microneurographic study. Exp. Brain Res. 1989, 76, 213–222. [Google Scholar] [CrossRef]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L.; Wallin, B.G. The responses of human muscle spindle endings to vibration during isometric contraction. J. Physiol. 1976, 261, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.J.; Park, H.S. Analysis of the tonic vibration reflex: Influence of vibration variables on motor unit synchronization and fatigue. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.G.; Oh, B.M.; Leigh, J.H.; Chun, C.; Park, C.; Kim, C.H. Effect of Focal Muscle Vibration on Calf Muscle Spasticity: A Proof-of-Concept Study. PM R 2016, 8, 1083–1089. [Google Scholar] [CrossRef]
- Maloney-Hinds, C.; Petrofsky, J.S.; Zimmerman, G.; Hessinger, D.A. The Role of Nitric Oxide in Skin Blood Flow Increases Due to Vibration in Healthy Adults and Adults with Type 2 Diabetes. Diabetes Technol. Ther. 2009, 11, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Pu, F.; Luan, H.; Duan, Y.; Su, H.; Fan, Y.; Jan, Y.K. Effects of Local Vibration With Different Intermittent Durations on Skin Blood Flow Responses in Diabetic People. Front. Bioeng. Biotechnol. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Liao, F.; Zhang, K.; Zhou, L.; Chen, Y.; Elliott, J.; Jan, Y. Effect of Different Local Vibration Frequencies on the Multiscale Regularity of Plantar Skin Blood Flow. Entropy 2020, 22, 1288. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, F.; Zhu, T.; Liao, F.; Ren, Y.; Jan, Y. Effects of Preconditioning Local Vibrations on Subsequent Plantar Skin Blood Flow Response to Walking. Int. J. Low. Extrem. Wounds 2021, 20, 143–149. [Google Scholar] [CrossRef]
- Marasco, P.D.; Hebert, J.S.; Sensinger, J.W.; Shell, C.E.; Schofield, J.S.; Thumser, Z.C.; Nataraj, R.; Beckler, D.T.; Dawson, M.R.; Blustein, D.H.; et al. Illusory movement perception improves motor control for prosthetic hands. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Goodwin, G.M.; McCloskey, D.I.; Matthews, P.B.C. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 1972, 95, 705–748. [Google Scholar] [CrossRef]
- Collins, D.F.; Refshauge, K.M.; Todd, G.; Gandevia, S.C. Cutaneous Receptors Contribute to Kinesthesia at the Index Finger, Elbow, and Knee. J. Neurophysiol. 2005, 94, 1699–1706. [Google Scholar] [CrossRef]
- Gay, A.; Parratte, S.; Salazard, B.; Guinard, D.; Pham, T.; Legré, R.; Roll, J.P. Proprioceptive feedback enhancement induced by vibratory stimulation in complex regional pain syndrome type I: An open comparative pilot study in 11 patients. Jt. Bone Spine 2007, 74, 461–466. [Google Scholar] [CrossRef]
- Hamaue, Y.; Nakano, J.; Sekino, Y.; Chuganji, S.; Sakamoto, J.; Yoshimura, T.; Okita, M.; Origuchi, T. Effects of Vibration Therapy on Immobilization-Induced Hypersensitivity in Rats. Phys. Ther. 2015, 95, 1015–1026. [Google Scholar] [CrossRef]
- Rippetoe, J.; Wang, H.; James, S.A.; Dionne, C.; Block, B.; Beckner, M. Improvement of gait after 4 weeks of wearable focal muscle vibration therapy for individuals with diabetic peripheral neuropathy. J. Clin. Med. 2020, 9, 3767. [Google Scholar] [CrossRef]
- Chesler, A.T.; Szczot, M.; Bharucha-Goebel, D.; Čeko, M.; Donkervoort, S.; Laubacher, C.; Hayes, L.H.; Alter, K.; Zampieri, C.; Stanley, C.; et al. The Role of PIEZO2 in Human Mechanosensation. N. Engl. J. Med. 2016, 375, 1355–1364. [Google Scholar] [CrossRef]
- Coste, B.; Crest, M.; Delmas, P. Pharmacological Dissection and Distribution of NaN/Nav1.9, T-type Ca2+ Currents, and Mechanically Activated Cation Currents in Different Populations of DRG Neurons. J. Gen. Physiol. 2007, 129, 57–77. [Google Scholar] [CrossRef]
- Drew, L.J.; Wood, J.N.; Cesare, P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 1–5. [Google Scholar] [CrossRef]
- Hu, J.; Lewin, G.R. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J. Physiol. 2006, 577, 815–828. [Google Scholar] [CrossRef]
- Drew, L.J.; Rohrer, D.K.; Price, M.P.; Blaver, K.E.; Cockayne, D.A.; Cesare, P.; Wood, J.N. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J. Physiol. 2004, 556, 691–710. [Google Scholar] [CrossRef]
- Drew, L.J.; Rugiero, F.; Cesare, P.; Gale, J.E.; Abrahamsen, B.; Bowden, S.; Heinzmann, S.; Robinson, M.; Brust, A.; Colless, B.; et al. High-Threshold Mechanosensitive Ion Channels Blocked by a Novel Conopeptide Mediate Pressure-Evoked Pain. PLoS ONE 2007, 2, e515. [Google Scholar] [CrossRef]
- Nakamichi, R.; Ma, S.; Nonoyama, T.; Chiba, T.; Kurimoto, R.; Ohzono, H.; Olmer, M.; Shukunami, C.; Fuku, N.; Wang, G.; et al. The mechanosensitive ion channel PIEZO1 is expressed in tendons and regulates physical performance. Sci. Transl. Med. 2022, 14, eabj5557. [Google Scholar] [CrossRef]
- Wetzel, C.; Hu, J.; Riethmacher, D.; Benckendorff, A.; Harder, L.; Eilers, A.; Moshourab, R.; Kozlenkov, A.; Labuz, D.; Caspani, O.; et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature 2007, 445, 206–209. [Google Scholar] [CrossRef]
- Moehring, F.; Halder, P.; Seal, R.P.; Stucky, C.L. Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 2018, 100, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Stucky, C.L.; Mikesell, A.R. When soft touch hurts: How hugs become painful after spinal cord injury. In Spinal Cord Injury Pain; Elsevier: Amsterdam, The Netherlands, 2022; pp. 341–351. [Google Scholar] [CrossRef]
- Fuchs, E. Keratins and the Skin. Annu. Rev. Cell Dev. Biol. 1995, 11, 123–154. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; Mckeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- Poole, K. The Diverse Physiological Functions of Mechanically Activated Ion Channels in Mammals. Annu. Rev. Physiol. 2022, 84, 307–329. [Google Scholar] [CrossRef]
- Shen, X.; Song, Z.; Xu, E.; Zhou, J.; Yan, F. Sensitization of nerve cells to ultrasound stimulation through Piezo1-targeted microbubbles. Ultrason. Sonochemistry 2021, 73, 105494. [Google Scholar] [CrossRef]
- Lewis, A.H.; Grandl, J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 2015, 4, e12088. [Google Scholar] [CrossRef]
- Poole, K.; Herget, R.; Lapatsina, L.; Ngo, H.D.; Lewin, G.R. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 2014, 5, 3520. [Google Scholar] [CrossRef]
- Gibbons, C.H. Basics of autonomic nervous system function. In Clinical Neurophysiology: Basis and Technical Aspect, 160th ed.; Levin, K.H., Chauvel, P., Eds.; Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 27; pp. 407–418. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Miyamoto, T.; Mochizuki, T.; Nakagomi, H.; Kira, S.; Watanabe, M.; Takayama, Y.; Suzuki, Y.; Koizumi, S.; Takeda, M.; Tominaga, M. Functional Role for Piezo1 in Stretch-evoked Ca2+ Influx and ATP Release in Urothelial Cell Cultures. J. Biol. Chem. 2014, 289, 16565–16575. [Google Scholar] [CrossRef]
- Ridone, P.; Vassalli, M.; Martinac, B. Piezo1 mechanosensitive channels: What are they and why are they important. Biophys. Rev. 2019, 11, 795–805. [Google Scholar] [CrossRef]
- Hung, W.C.; Yang, J.R.; Yankaskas, C.L.; Wong, B.S.; Wu, P.H.; Pardo-Pastor, C.; Serra, S.A.; Chiang, M.J.; Gu, Z.; Wirtz, D.; et al. Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways. Cell Rep. 2016, 15, 1430–1441. [Google Scholar] [CrossRef]
- Bartel, L.; Mosabbir, A. Possible Mechanisms for the Effects of Sound Vibration on Human Health. Healthcare 2021, 9, 597. [Google Scholar] [CrossRef]
- Shibasaki, M.; Secher, N.H.; Johnson, J.M.; Crandall, C.G. Central command and the cutaneous vascular response to isometric exercise in heated humans. J. Physiol. 2005, 565, 667–673. [Google Scholar] [CrossRef]
- McHugh, B.J.; Murdoch, A.; Haslett, C.; Sethi, T. Loss of the Integrin-Activating Transmembrane Protein Fam38A (Piezo1) Promotes a Switch to a Reduced Integrin-Dependent Mode of Cell Migration. PLoS ONE 2012, 7, e40346. [Google Scholar] [CrossRef]
- Orsini, E.M.; Perelas, A.; Southern, B.D.; Grove, L.M.; Olman, M.A.; Scheraga, R.G. Stretching the Function of Innate Immune Cells. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Liang, J.; Huang, B.; Yuan, G.; Chen, Y.; Liang, F.; Zeng, H.; Zheng, S.; Cao, L.; Geng, D.; Zhou, S. Stretch-activated channel Piezo1 is up-regulated in failure heart and cardiomyocyte stimulated by AngII. Am. J. Transl. Res. 2017, 9, 2945–2955. [Google Scholar]
- Rocio Servin-Vences, M.; Moroni, M.; Lewin, G.R.; Poole, K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. eLife 2017, 6. [Google Scholar] [CrossRef]
- Ma, N.; Chen, D.; Lee, J.; Kuri, P.; Hernandez, E.B.; Kocan, J.; Mahmood, H.; Tichy, E.D.; Rompolas, P.; Mourkioti, F. Piezo1 regulates the regenerative capacity of skeletal muscles via orchestration of stem cell morphological states. Sci. Adv. 2022, 8, eabn0485. [Google Scholar] [CrossRef]
- Liu, H.; Hu, J.; Zheng, Q.; Feng, X.; Zhan, F.; Wang, X.; Xu, G.; Hua, F. Piezo1 Channels as Force Sensors in Mechanical Force-Related Chronic Inflammation. Front. Immunol. 2022, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 2017, 7, 17696. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, E.; Martin-Almedina, S.; Simpson, M.A.; Lin, S.; Gordon, K.; Brice, G.; Atton, G.; Jeffery, I.; Rees, D.C.; Mignot, C.; et al. Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rezania, S.; Kammerer, S.; Sokolowski, A.; Devaney, T.; Gorischek, A.; Jahn, S.; Hackl, H.; Groschner, K.; Windpassinger, C.; et al. Piezo1 forms mechanosensitive ion channels in the human MCF-7 breast cancer cell line. Sci. Rep. 2015, 5, 8364. [Google Scholar] [CrossRef] [PubMed]
- Albarrán-Juárez, J.; Iring, A.; Wang, S.P.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef]
- Cahalan, S.M.; Lukacs, V.; Ranade, S.S.; Chien, S.; Bandell, M.; Patapoutian, A. Piezo1 links mechanical forces to red blood cell volume. eLife 2015, 4. [Google Scholar] [CrossRef]
- Dampney, R.A.L.; Coleman, M.J.; Fontes, M.A.P.; Hirooka, Y.; Horiuchi, J.; Li, Y.W.; Polson, J.W.; Potts, P.D.; Tagawa, T. Central Mechanisms Underlying Short- And Long-Term Regulation Of The Cardiovascular System. Clin. Exp. Pharmacol. Physiol. 2002, 29, 261–268. [Google Scholar] [CrossRef]
- Nourse, J.L.; Pathak, M.M. How cells channel their stress: Interplay between Piezo1 and the cytoskeleton. Semin. Cell Dev. Biol. 2017, 71, 3–12. [Google Scholar] [CrossRef]
- Evans, E.L.; Cuthbertson, K.; Endesh, N.; Rode, B.; Blythe, N.M.; Hyman, A.J.; Hall, S.J.; Gaunt, H.J.; Ludlow, M.J.; Foster, R.; et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br. J. Pharmacol. 2018, 175, 1744–1759. [Google Scholar] [CrossRef]
- Wetzel, C.; Pifferi, S.; Picci, C.; Gök, C.; Hoffmann, D.; Bali, K.K.; Lampe, A.; Lapatsina, L.; Fleischer, R.; Smith, E.S.J.; et al. Small-molecule inhibition of STOML3 oligomerization reverses pathological mechanical hypersensitivity. Nat. Neurosci. 2017, 20, 209–218. [Google Scholar] [CrossRef]
- Shutova, M.S.; Svitkina, T.M. Common and Specific Functions of Nonmuscle Myosin II Paralogs in Cells. Biochemistry 2018, 83, 1459–1468. [Google Scholar] [CrossRef]
- Hyman, A.J.; Tumova, S.; Beech, D.J. Piezo1 Channels in Vascular Development and the Sensing of Shear Stress. Curr. Top. Membr. 2017, 79, 37–57. [Google Scholar] [CrossRef]
- McHugh, B.J.; Buttery, R.; Lad, Y.; Banks, S.; Haslett, C.; Sethi, T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 2010, 123, 51–61. [Google Scholar] [CrossRef]
- Velasco-Estevez, M.; Rolle, S.O.; Mampay, M.; Dev, K.K.; Sheridan, G.K. Piezo1 regulates calcium oscillations and cytokine release from astrocytes. Glia 2020, 68, 145–160. [Google Scholar] [CrossRef]
- Perko, M.J.; Nielsen, H.B.; Skak, C.; Clemmesen, J.O.; Schroeder, T.V.; Secher, N.H. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J. Physiol. 1998, 513, 907–913. [Google Scholar] [CrossRef]
- Qamar, M.I.; Read, A.E. Effects of exercise on mesenteric blood flow in man. Gut 1987, 28, 583–587. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Dawson, E.A.; Black, M.A.; Hopman, M.T.E.; Cable, N.T.; Green, D.J. Brachial artery blood flow responses to different modalities of lower limb exercise. Med. Sci. Sport. Exerc. 2009, 41, 1072–1079. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (Hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Clifford, P.S. Skeletal muscle vasodilatation at the onset of exercise. J. Physiol. 2007, 583, 825–833. [Google Scholar] [CrossRef]
- Holwerda, S.W.; Restaino, R.M.; Fadel, P.J. Adrenergic and non-adrenergic control of active skeletal muscle blood flow: Implications for blood pressure regulation during exercise. Auton. Neurosci. Basic Clin. 2015, 188, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Hudlicka, O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: Involvement of VEGF and metalloproteinases. Angiogenesis 2003, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- García-Mesa, Y.; García-Piqueras, J.; García, B.; Feito, J.; Cabo, R.; Cobo, J.; Vega, J.A.; García-Suárez, O. Merkel cells and Meissner’s corpuscles in human digital skin display Piezo2 immunoreactivity. J. Anat. 2017, 231, 978–989. [Google Scholar] [CrossRef] [PubMed]
- García-Piqueras, J.; García-Mesa, Y.; Cárcaba, L.; Feito, J.; Torres-Parejo, I.; Martín-Biedma, B.; Cobo, J.; García-Suárez, O.; Vega, J.A. Ageing of the somatosensory system at the periphery: Age-related changes in cutaneous mechanoreceptors. J. Anat. 2019, 234, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Ranade, S.; Weyer, A.D.; Dubin, A.E.; Baba, Y.; Qiu, Z.; Petrus, M.; Miyamoto, T.; Reddy, K.; Lumpkin, E.A.; et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014, 509, 622–626. [Google Scholar] [CrossRef]
- Ikeda, R.; Cha, M.; Ling, J.; Jia, Z.; Coyle, D.; Gu, J.G. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 2014, 157, 664–675. [Google Scholar] [CrossRef]
- Maksimovic, S.; Nakatani, M.; Baba, Y.; Nelson, A.M.; Marshall, K.L.; Wellnitz, S.A.; Firozi, P.; Woo, S.H.; Ranade, S.; Patapoutian, A.; et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014, 509, 617–621. [Google Scholar] [CrossRef]
- Woo, S.H.; Lukacs, V.; De Nooij, J.C.; Zaytseva, D.; Criddle, C.R.; Francisco, A.G.; Jessell, T.M.; Wilkinson, K.A.; Patapoutian, A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015, 18, 1756–1762. [Google Scholar] [CrossRef]
- Ernfors, P.; Manira, A.E.; Svenningson, P. Discoveries of Receptors for Temperature and Touch; The Noble Assembly at Karolinska Institutet: Stockholm, Sweden, 2021; pp. 2–5. [Google Scholar]
- Von Buchholtz, L.J.; Ghitani, N.; Lam, R.M.; Licholai, J.A.; Chesler, A.T.; Ryba, N.J. Decoding Cellular Mechanisms for Mechanosensory Discrimination. Neuron 2021, 109, 285–298.e5. [Google Scholar] [CrossRef]
- Florez-Paz, D.; Bali, K.K.; Kuner, R.; Gomis, A. A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Zhou, R.M.; Yao, J.; Li, X.M.; Shen, Y.; Cheng, H.; Yuan, J.; Yan, B.; Jiang, Q. Piezo2 protein: A novel regulator of tumor angiogenesis and hyperpermeability. Oncotarget 2016, 7, 44630–44643. [Google Scholar] [CrossRef]
- Nonomura, K.; Woo, S.H.; Chang, R.B.; Gillich, A.; Qiu, Z.; Francisco, A.G.; Ranade, S.S.; Liberles, S.D.; Patapoutian, A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017, 541, 176–181. [Google Scholar] [CrossRef]
- Ferrari, L.F.; Bogen, O.; Green, P.; Levine, J.D. Contribution of Piezo2 to endothelium-dependent pain. Mol. Pain 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Case, L.K.; Liljencrantz, J.; Madian, N.; Necaise, A.; Tubbs, J.; McCall, M.; Bradson, M.L.; Szczot, M.; Pitcher, M.H.; Ghitani, N.; et al. Innocuous pressure sensation requires A-type afferents but not functional PIEZO2 channels in humans. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Nagi, S.S.; Marshall, A.G.; Makdani, A.; Jarocka, E.; Liljencrantz, J.; Ridderström, M.; Shaikh, S.; O’Neill, F.; Saade, D.; Donkervoort, S.; et al. An ultrafast system for signaling mechanical pain in human skin. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Bevan, S.; Quallo, T.; Andersson, D.A. TRPV1. In Handbook of Experimental Pharmacology, 222th ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 207–245. [Google Scholar] [CrossRef]
- Moriello, A.S.; De Petrocellis, L. Assay of TRPV1 Receptor Signaling. In Methods in Molecular Biology, 1412th ed.; Springer: New York, NY, USA, 2016; pp. 65–76. [Google Scholar] [CrossRef]
- Storozhuk, M.V.; Moroz, O.F.; Zholos, A.V. Multifunctional TRPV1 Ion Channels in Physiology and Pathology with Focus on the Brain, Vasculature, and Some Visceral Systems. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Ben-Shahar, Y. Sensory Functions for Degenerin/Epithelial Sodium Channels (DEG/ENaC). In Advances in Genetics, 76th ed.; Academic Press: Cambridge, MA, USA, 2011; Chapter 1; pp. 1–26. [Google Scholar] [CrossRef]
- Lin, S.H.; Cheng, Y.R.; Banks, R.W.; Min, M.Y.; Bewick, G.S.; Chen, C.C. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat. Commun. 2016, 7, 11460. [Google Scholar] [CrossRef]
- Perozo, E.; Kloda, A.; Cortes, D.M.; Martinac, B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002, 9, 696–703. [Google Scholar] [CrossRef]
- Nomura, T.; Cranfield, C.G.; Deplazes, E.; Owen, D.M.; Macmillan, A.; Battle, A.R.; Constantine, M.; Sokabe, M.; Martinac, B. Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc. Natl. Acad. Sci. USA 2012, 109, 8770–8775. [Google Scholar] [CrossRef]
- Brohawn, S.G.; Su, Z.; Mackinnon, R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. USA 2014, 111, 3614–3619. [Google Scholar] [CrossRef]
- Murthy, S.E.; Dubin, A.E.; Whitwam, T.; Jojoa-Cruz, S.; Cahalan, S.M.; Mousavi, S.A.R.; Ward, A.B.; Patapoutian, A. OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. eLife 2018, 7, e41844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Yin, Y.W.; Li, B.H.; Liu, Y.; Liao, S.Q.; Gao, C.Y.; Li, J.C.; Zhang, L.L. The role of TRPV1 in improving VSMC function and attenuating hypertension. Prog. Biophys. Mol. Biol. 2015, 117, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Baylie, R.L.; Brayden, J.E. TRPV channels and vascular function. Acta Physiol. 2011, 203, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by Dietary Capsaicin Improves Endothelium-Dependent Vasorelaxation and Prevents Hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lim, J.Y.; Yoo, S.; Kim, H.; Hwang, S.W. Emerging Role of Spinal Cord TRPV1 in Pain Exacerbation. Neural Plast. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Yang, S.; Yang, F.; Wei, N.; Hong, J.; Li, B.; Luo, L.; Rong, M.; Yarov-Yarovoy, V.; Zheng, J.; Wang, K.; et al. A pain-inducing centipede toxin targets the heat activation machinery of nociceptor TRPV1. Nat. Commun. 2015, 6, 8297. [Google Scholar] [CrossRef]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef]
- Woolums, B.M.; Mccray, B.A.; Sung, H.; Tabuchi, M.; Sullivan, J.M.; Ruppell, K.T.; Yang, Y.; Mamah, C.; Aisenberg, W.H.; Saavedra-Rivera, P.C.; et al. TRPV4 disrupts mitochondrial transport and causes axonal degeneration via a CaMKII-dependent elevation of intracellular Ca2+. Nat. Commun. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Phan, T.X.; Ton, H.T.; Gulyás, H.; Pórszász, R.; Tóth, A.; Russo, R.; Kay, M.W.; Sahibzada, N.; Ahern, G.P. TRPV1 expressed throughout the arterial circulation regulates vasoconstriction and blood pressure. J. Physiol. 2020, 598, 5639–5659. [Google Scholar] [CrossRef]
- Christie, S.; Wittert, G.A.; Li, H.; Page, A.J. Involvement of TRPV1 Channels in Energy Homeostasis. Front. Endocrinol. 2018, 9, 420. [Google Scholar] [CrossRef]
- Amantini, C.; Farfariello, V.; Cardinali, C.; Morelli, M.B.; Marinelli, O.; Nabissi, M.; Santoni, M.; Bonfili, L.; Cecarini, V.; Eleuteri, A.M.; et al. The TRPV1 ion channel regulates thymocyte differentiation by modulating autophagy and proteasome activity. Oncotarget 2017, 8, 90766–90780. [Google Scholar] [CrossRef]
- Li, Y.; Gupta, P. Immune aspects of the bi-directional neuroimmune facilitator TRPV1. Mol. Biol. Rep. 2019, 46, 1499–1510. [Google Scholar] [CrossRef]
- Lee, E.; Jung, D.Y.; Kim, J.H.; Patel, P.R.; Hu, X.; Lee, Y.; Azuma, Y.; Wang, H.; Tsitsilianos, N.; Shafiq, U.; et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015, 29, 3182–3192. [Google Scholar] [CrossRef]
- Mistretta, F.; Buffi, N.M.; Lughezzani, G.; Lista, G.; Larcher, A.; Fossati, N.; Abrate, A.; Dell’Oglio, P.; Montorsi, F.; Guazzoni, G.; et al. Bladder Cancer and Urothelial Impairment: The Role of TRPV1 as Potential Drug Target. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Sterle, I.; Zupančič, D.; Romih, R. Correlation between Urothelial Differentiation and Sensory Proteins P2X3, P2X5, TRPV1, and TRPV4 in Normal Urothelium and Papillary Carcinoma of Human Bladder. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- McGarvey, L.P.; Butler, C.A.; Stokesberry, S.; Polley, L.; Mcquaid, S.; Abdullah, H.; Ashraf, S.; Mcgahon, M.K.; Curtis, T.M.; Arron, J.; et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J. Allergy Clin. Immunol. 2014, 133, 704–712.e4. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z.; et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Bai, L.; Lehnert, B.P.; Liu, J.; Neubarth, N.L.; Dickendesher, T.L.; Nwe, P.H.; Cassidy, C.; Woodbury, C.J.; Ginty, D.D. Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking. Cell 2015, 163, 1783–1795. [Google Scholar] [CrossRef]
- Neubarth, N.L.; Emanuel, A.J.; Liu, Y.; Springel, M.W.; Handler, A.; Zhang, Q.; Lehnert, B.P.; Guo, C.; Orefice, L.L.; Abdelaziz, A.; et al. Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 2020, 368, eabb2751. [Google Scholar] [CrossRef]
- Lewin, G.R.; McMahon, S.B. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skin in the adult rat. J. Neurophysiol. 1991, 66, 1205–1217. [Google Scholar] [CrossRef]
- Koltzenburg, M.; Stucky, C.L.; Lewin, G.R. Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 1997, 78, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Vallbo, Å.B.; Westling, G. Thresholds of mechanosensitive afferents in the human hand as measured with von Frey hairs. Brain Res. 1980, 184, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.M.; Khasabov, S.G.; Simone, D.A. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: An in vivo study. J. Neurophysiol. 2001, 85, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rutlin, M.; Abraira, V.E.; Cassidy, C.; Kus, L.; Gong, S.; Jankowski, M.P.; Luo, W.; Heintz, N.; Koerber, H.R.; et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 2011, 147, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Mauguière, F.; Merlet, I.; Forss, N.; Vanni, S.; Jousmäki, V.; Adeleine, P.; Hari, R. Activation of a distributed somatosensory cortical network in the human brain: A dipole modelling study of magnetic fields evoked by median nerve stimulation. Part II: Effects of stimulus rate, attention and stimulus detection. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1997, 104, 290–295. [Google Scholar] [CrossRef]
- Mauguière, F.; Merlet, I.; Forss, N.; Vanni, S.; Jousmäki, V.; Adeleine, P.; Hari, R. Activation of a distributed somatosensory cortical network in the human brain. A dipole modelling study of magnetic fields evoked by median nerve stimulation. Part I: Location and activation timing of SEF sources. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1997, 104, 281–289. [Google Scholar] [CrossRef]
- Ackerley, R.; Kavounoudias, A. The role of tactile afference in shaping motor behaviour and implications for prosthetic innovation. Neuropsychologia 2015, 79, 192–205. [Google Scholar] [CrossRef]
- Perl, E.R. Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J. Physiol. 1968, 197, 593–615. [Google Scholar] [CrossRef]
- Burgess, P.R.; Petit, D.; Warren, R.M. Receptor types in cat hairy skin supplied by myelinated fibers. J. Neurophysiol. 1968, 31, 833–848. [Google Scholar] [CrossRef]
- Brown, A.G.; Iggo, A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J. Physiol. 1967, 193, 707–733. [Google Scholar] [CrossRef]
- Knibestöl, M. Stimulus–response functions of rapidly adapting mechanoreceptors in the human glabrous skin area. J. Physiol. 1973, 232, 427–452. [Google Scholar] [CrossRef]
- Leem, J.W.; Willis, W.D.; Chung, J.M. Cutaneous sensory receptors in the rat foot. J. Neurophysiol. 1993, 69, 1684–1699. [Google Scholar] [CrossRef]
- Zimmerman, A.; Bai, L.; Ginty, D.D. The gentle touch receptors of mammalian skin. Science 2014, 346, 950–954. [Google Scholar] [CrossRef]
- Bardouille, T.; Picton, T.W.; Ross, B. Attention modulates beta oscillations during prolonged tactile stimulation. Eur. J. Neurosci. 2010, 31, 761–769. [Google Scholar] [CrossRef]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Danilov, Y.P. Translingual Neurostimulation (TLNS): A novel approach to neurorehabilitation. J. Neurol. Neurophysiol. 2017, 08. [Google Scholar] [CrossRef]
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2018, 11. [Google Scholar] [CrossRef]
- Gillespie, P.G.; Walker, R.G. Molecular basis of mechanosensory transduction. Nature 2001, 413, 194–202. [Google Scholar] [CrossRef]
- McGlone, F.; Reilly, D. The cutaneous sensory system. Neurosci. Biobehav. Rev. 2010, 34, 148–159. [Google Scholar] [CrossRef]
- Rice, F.L.; Albrecht, P.J. Cutaneous Mechanisms of Tactile Perception: Morphological and Chemical Organization of the Innervation to the Skin. Senses: A Compr. Ref. 2008, 6, 1–31. [Google Scholar] [CrossRef]
- Cobo, R.; García-Piqueras, J.; Cobo, J.; Vega, J.A. The human cutaneous sensory corpuscles: An update. J. Clin. Med. 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Munger, L.B.; Ide, C. The Structure and Function of Cutaneous Sensory Receptors. Arch. Histol. Cytol. 1988, 51, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Zelená, J. Nerves and Mechanoreceptors: The Role of Innervation in the Development and Maintenance of Mammalian Mechanoreceptors; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Gardner, E.P.; Martin, J.H.; Jessell, T.M. Coding of sensory information. In Principles of Neural Science; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds.; Oxford University Press: New York, NY, USA, 2000; Chapter 21; pp. 430–449. [Google Scholar]
- Strzalkowski, N.D.J.; Peters, R.M.; Inglis, J.T.; Bent, L.R. Cutaneous afferent innervation of the human foot sole: What can we learn from single-unit recordings? J. Neurophysiol. 2018, 120, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Vallbo, Å.B.; Olausson, H.; Wessberg, J.; Norrsell, U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993, 628, 301–304. [Google Scholar] [CrossRef]
- Vallbo, Å.B.; Olausson, H.; Wessberg, J.; Kakuda, N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J. Physiol. 1995, 483, 783–795. [Google Scholar] [CrossRef]
- Vallbo, Å.B.; Olausson, H.; Wessberg, J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999, 81, 2753–2763. [Google Scholar] [CrossRef]
- Seal, R.P.; Wang, X.; Guan, Y.; Raja, S.N.; Woodbury, C.J.; Basbaum, A.I.; Edwards, R.H. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 2009, 462, 651–655. [Google Scholar] [CrossRef]
- Abdo, H.; Calvo-Enrique, L.; Lopez, J.M.; Song, J.; Zhang, M.D.; Usoskin, D.; Manira, A.E.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized cutaneous schwann cells initiate pain sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef]
- Hoffman, B.U.; Baba, Y.; Griffith, T.N.; Mosharov, E.V.; Woo, S.H.; Roybal, D.D.; Karsenty, G.; Patapoutian, A.; Sulzer, D.; Lumpkin, E.A. Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses. Neuron 2018, 100, 1401–1413.e6. [Google Scholar] [CrossRef]
- Schwaller, F.; Bégay, V.; García-García, G.; Taberner, F.J.; Moshourab, R.; Mcdonald, B.; Docter, T.; Kühnemund, J.; Ojeda-Alonso, J.; Paricio-Montesinos, R.; et al. USH2A is a Meissner’s corpuscle protein necessary for normal vibration sensing in mice and humans. Nat. Neurosci. 2021, 24, 74–81. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Talagas, M.; Lebonvallet, N.; Berthod, F.; Misery, L. Lifting the veil on the keratinocyte contribution to cutaneous nociception. Protein Cell 2020, 11, 239–250. [Google Scholar] [CrossRef]
- Sadler, K.E.; Moehring, F.; Stucky, C.L. Keratinocytes contribute to normal cold and heat sensation. eLife 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Chung, M.K.; Lee, H.; Mizuno, A.; Suzuki, M.; Caterina, M.J. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J. Biol. Chem. 2004, 279, 21569–21575. [Google Scholar] [CrossRef]
- Moehring, F.; Cowie, A.M.; Menzel, A.D.; Weyer, A.D.; Grzybowski, M.; Arzua, T.; Geurts, A.M.; Palygin, O.; Stucky, C.L. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. eLife 2018, 7, 1–35. [Google Scholar] [CrossRef]
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef]
- Marshall, K.; Patapoutian, A. Getting a grip on touch receptors. Science 2020, 368, 1311–1312. [Google Scholar] [CrossRef]
- Delmas, P.; Hao, J.; Rodat-Despoix, L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 2011, 12, 139–153. [Google Scholar] [CrossRef]
- Fleming, M.S.; Luo, W. The anatomy, function, and development of mammalian Aβ low-threshold mechanoreceptors. Front. Biol. 2013, 8, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Olson, W.; Dong, P.; Fleming, M.; Luo, W. The specification and wiring of mammalian cutaneous low-threshold mechanoreceptors. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.T.; Bahramali, H.; Zhang, H.Q.; Rowe, M.J. Characterization of Tactile Afferent Fibers in the Hand of the Marmoset Monkey. J. Neurophysiol. 2001, 85, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Tzen, Y.T.; Weinheimer-Haus, E.M.; Corbiere, T.F.; Koh, T.J. Increased skin blood flow during low intensity vibration in human participants: Analysis of control mechanisms using short-time fourier transform. PLoS ONE 2018, 13, e0200247. [Google Scholar] [CrossRef] [PubMed]
- Krajnak, K.; Dong, R.G.; Flavahan, S.; Welcome, D.; Flavahan, N.A. Acute vibration increases α2C-adrenergic smooth muscle constriction and alters thermosensitivity of cutaneous arteries. J. Appl. Physiol. 2006, 100, 1230–1237. [Google Scholar] [CrossRef]
- Krajnak, K.; Miller, G.R.; Waugh, S.; Johnson, C.; Kashon, M.L. Characterization of frequency-dependent responses of the vascular system to repetitive vibration. J. Occup. Environ. Med. 2012, 54, 1010–1016. [Google Scholar] [CrossRef]
- Bovenzi, M.; Lindsell, C.J.; Griffin, M.J. Magnitude of acute exposures to vibration and finger circulation. Scand. J. Work. Environ. Health 1999, 25, 278–284. [Google Scholar] [CrossRef]
- Souron, R.; Besson, T.; Millet, G.Y.; Lapole, T. Acute and chronic neuromuscular adaptations to local vibration training. Eur. J. Appl. Physiol. 2017, 117, 1939–1964. [Google Scholar] [CrossRef]
- Salter, M.W.; Henry, J.L. Evidence that adenosine mediates the depression of spinal dorsal horn neurons induced by peripheral vibration in the cat. Neuroscience 1987, 22, 631–650. [Google Scholar] [CrossRef]
- Naghdi, L.; Ahonen, H.; Macario, P.; Bartel, L. The effect of low-frequency sound stimulation on patients with fibromyalgia: A clinical study. Pain Res. Manag. 2015, 20, e21–e27. [Google Scholar] [CrossRef]
- Serritella, E.; Scialanca, G.; Di Giacomo, P.; Di Paolo, C. Local Vibratory Stimulation for Temporomandibular Disorder Myofascial Pain Treatment: A Randomised, Double-Blind, Placebo-Controlled Preliminary Study. Pain Res. Manag. 2020, 2020. [Google Scholar] [CrossRef]
- Chen, L.; Feng, Y.; Chen, B.; Wang, Q.; Wei, K. Improving postural stability among people with lower-limb amputations by tactile sensory substitution. J. NeuroEngineering Rehabil. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Steyvers, M.; Levin, O.; Verschueren, S.M.; Swinnen, S.P. Frequency-dependent effects of muscle tendon vibration on corticospinal excitability: A TMS study. Exp. Brain Res. 2003, 151, 9–14. [Google Scholar] [CrossRef]
- Kawahira, K.; Higashihara, K.; Matsumoto, S.; Shimodozono, M.; Etoh, S.; Tanaka, N.; Sueyoshi, Y. New functional vibratory stimulation device for extremities in patients with stroke. Int. J. Rehabil. Res. 2004, 27, 335–337. [Google Scholar] [CrossRef]
- Chandrashekhar, R.; Wang, H.; Dionne, C.; James, S.; Burzycki, J. Wearable focal muscle vibration on pain, balance, mobility, and sensation in individuals with diabetic peripheral neuropathy: A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 2415. [Google Scholar] [CrossRef]
- Koike, Y.; Iwamoto, S.; Kimata, Y.; Nohno, T.; Hiragami, F.; Kawamura, K.; Numata, K.; Murai, H.; Okisima, K.; Iwata, M.; et al. Low-Frequency Vibratory Sound Induces Neurite Outgrowth In PC12M3 Cells In Which Nerve Growth Factor-Induced Neurite Outgrowth Is Impaired. Tissue Cult. Res. Commun. 2004, 23, 81–90. [Google Scholar] [CrossRef]
- Koike, Y.; Tutida, R.; Hayashi, Y.; Yamanishi, Y.; Kano, Y. Low-Frequency, Whole Body Vibration Induced Neurite Outgrowth by Pc12m3 Cells with Impaired Nerve Growth Factor-Induced Neurite Outgrowth. J. Nov. Physiother. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Barralon, P.; Dumont, G.; Schwarz, S.K.; Ansermino, J.M. Autonomic nervous system response to vibrating and electrical stimuli on the forearm and wrist. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 931–934. [Google Scholar] [CrossRef]
- Chambers, M.R.; Andres, K.H.; Duering, M.V.; Iggo, A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q. J. Exp. Physiol. Cogn. Med. Sci. 1972, 57, 417–445. [Google Scholar] [CrossRef]
- Johansson, R.S.; Vallbo, A.B. Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979, 286, 283–300. [Google Scholar] [CrossRef]
- Paré, M.; Smith, A.M.; Rice, F.L. Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. J. Comp. Neurol. 2002, 445, 347–359. [Google Scholar] [CrossRef]
- Torebjörk, H.E.; Hallin, R.G. Perceptual changes accompanying controlled preferential blocking of A and C fibre responses in intact human skin nerves. Exp. Brain Res. 1973, 16, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.A.; Burke, D.; Skuse, N.F.; Lethlean, A.K. Fibre function and perception during cutaneous nerve block. J. Neurol. Neurosurg. Psychiatry 1975, 38, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.; Torebjörk, E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J. Physiol. 1989, 415, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Bromm, B.; Treede, R.D. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum. Neurobiol. 1984, 3, 33–40. [Google Scholar]
- Löken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547–548. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Marasco, P.D.; Lock, B.A.; Harden, R.N.; Dewald, J.P.A. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc. Natl. Acad. Sci. USA 2007, 104, 20061–20066. [Google Scholar] [CrossRef]
- Melzack, R. Pain and the Neuromatrix in the Brain. J. Dent. Educ. 2001, 65, 1378–1382. [Google Scholar] [CrossRef]
- Moayedi, M.; Davis, K.D. Theories of pain: From specificity to gate control. J. Neurophysiol. 2013, 109, 5–12. [Google Scholar] [CrossRef]
- Guyenet, P.G. Regulation of breathing and autonomic outflows by chemoreceptors. Compr. Physiol. 2014, 4, 1511–1562. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache J. Head Face Pain 2016, 56, 71–78. [Google Scholar] [CrossRef]
- Darrow, M.J.; Mian, T.M.; Torres, M.; Haider, Z.; Danaphongse, T.; Seyedahmadi, A.; Rennaker, R.L.; Hays, S.A.; Kilgard, M.P. The tactile experience paired with vagus nerve stimulation determines the degree of sensory recovery after chronic nerve damage. Behav. Brain Res. 2021, 396, 112910. [Google Scholar] [CrossRef]
- Baig, S.S.; Kamarova, M.; Ali, A.; Su, L.; Dawson, J.; Redgrave, J.N.; Majid, A. Transcutaneous vagus nerve stimulation (tVNS) in stroke: The evidence, challenges and future directions. Auton. Neurosci. Basic Clin. 2022, 237. [Google Scholar] [CrossRef]
- Moggio, L.; De Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Vibration therapy role in neurological diseases rehabilitation: An umbrella review of systematic reviews. Disabil. Rehabil. 2021, 1–9. [Google Scholar] [CrossRef]
- Flor, H.; Denke, C.; Schaefer, M.; Grüsser, S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet 2001, 357, 1763–1764. [Google Scholar] [CrossRef]
- Flor, H.; Diers, M.; Andoh, J. The neural basis of phantom limb pain. Trends Cogn. Sci. 2013, 17, 307–308. [Google Scholar] [CrossRef]
- Flor, H.; Nikolajsen, L.; Jensen, T.S. Phantom limb pain: A case of maladaptive CNS plasticity? Nat. Rev. Neurosci. 2006, 7, 873–881. [Google Scholar] [CrossRef]
- Baron, R.; Hans, G.; Dickenson, A.H. Peripheral input and its importance for central sensitization. Ann. Neurol. 2013, 74, 630–636. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef] [PubMed]
- Gay, A.; Aimonetti, J.M.; Roll, J.P.; Ribot-Ciscar, E. Kinesthetic illusions attenuate experimental muscle pain, as do muscle and cutaneous stimulation. Brain Res. 2015, 1615, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wall, P.D. Pain, itch, and vibration. Arch. Neurol. 1960, 2, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D.; Weisz, A.Z. Masking and Metacontrast Phenomena in Skin Sensory System. Exp. Neurol. 1963, 8, 35–46. [Google Scholar] [CrossRef]
- Melzack, R.; Schecter, B. Itch and vibration. Science 1965, 147, 1047–1048. [Google Scholar] [CrossRef]
- Bank, P.J.M.; Peper, C.E.; Marinus, J.; Beek, P.J.; Van Hilten, J.J. Motor dysfunction of complex regional pain syndrome is related to impaired central processing of proprioceptive information. J. Pain 2013, 14, 1460–1474. [Google Scholar] [CrossRef]
- Russell, W.R.; Spalding, J.M.K. Treatment of Painful Amputation Stumps. Br. Med. J. 1950, 2, 68. [Google Scholar] [CrossRef]
- Livingston, W.K. Pain Mechanisms; Macmillan: New York, NY, USA, 1943. [Google Scholar]
- Gordon, I.; Voos, A.C.; Bennett, R.H.; Bolling, D.Z.; Pelphrey, K.A.; Kaiser, M.D. Brain mechanisms for processing affective touch. Hum. Brain Mapp. 2013, 34, 914–922. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Handlin, L.; Petersson, M. Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Wu, J.Z.; Welcome, D.E.; Krajnak, K.; Dong, R.G. Finite element analysis of the penetrations of shear and normal vibrations into the soft tissues in a fingertip. Med. Eng. Phys. 2007, 29, 718–727. [Google Scholar] [CrossRef]
- Welcome, D.E.; Krajnak, K.; Kashon, M.L.; Dong, R.G. An investigation on the biodynamic foundation of a rat tail vibration model. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 1127–1141. [Google Scholar] [CrossRef]
- Curry, B.D.; Bain, J.L.W.; Yan, J.G.; Zhang, L.L.; Yamaguchi, M.; Matloub, H.S.; Riley, D.A. Vibration injury damages arterial endothelial cells. Muscle Nerve 2002, 25, 527–534. [Google Scholar] [CrossRef]
- Lindblad, L.E.; Ekenvall, L. Alpha2-Adrenoceptor Inhibition in Patients with Vibration White Fingers. Kurume Med. J. 1990, 37, S95–S99. [Google Scholar] [CrossRef]
- Eklund, G.; Hagbarth, K.E. Normal variability of tonic vibration reflexes in man. Exp. Neurol. 1966, 16, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Izumizaki, M.; Sekihara, C.; Atsumi, T.; Homma, I. Combined effects of preceding muscle vibration and contraction on the tonic vibration reflex. Exp. Brain Res. 2009, 192, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Gregory, J.E.; Proske, U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J. Physiol. 1996, 497, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hultborn, H.; Illert, M.; Nielsen, J.; Paul, A.; Ballegaard, M.; Wiese, H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp. Brain Res. 1996, 108, 450–462. [Google Scholar] [CrossRef]
- Hagbarth, K.E.; Eklund, G. Motor effects of muscle vibration in spasticity, rigidity and cerebellar disorders. Electroencephalogr. Clin. Neurophysiol. 1968, 25, 407. [Google Scholar] [PubMed]
- Paoloni, M.; Mangone, M.; Scettri, P.; Procaccianti, R.; Cometa, A.; Santilli, V. Segmental muscle vibration improves walking in chronic stroke patients with foot drop: A randomized controlled trial. Neurorehabilit. Neural Repair 2010, 24, 254–262. [Google Scholar] [CrossRef]
- Paoloni, M.; Tavernese, E.; Fini, M.; Sale, P.; Franceschini, M.; Santilli, V.; Mangone, M. Segmental muscle vibration modifies muscle activation during reaching in chronic stroke: A pilot study. NeuroRehabilitation 2014, 35, 405–414. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Romagnoli, C.; Annino, G. Effectiveness of focal muscle vibration on hemiplegic upper extremity spasticity in individuals with stroke: A systematic review. NeuroRehabilitation 2019, 45, 471–481. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Romagnoli, C.; Raju, M.; Annino, G. Clinical effectiveness of focal muscle vibration on gait and postural stability in individuals with neurological disorders: A systematic review. Physiother. Res. Int. 2022, 27, e1945. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Anwer, S.; Zafar, H.; Iqbal, Z.A. Effect of localised vibration on muscle strength in healthy adults: A systematic review. Physiotherapy 2018, 104, 18–24. [Google Scholar] [CrossRef]
- Fattorini, L.; Rodio, A.; Pettorossi, V.E.; Filippi, G.M. Is the focal muscle vibration an effective motor conditioning intervention? A systematic review. J. Funct. Morphol. Kinesiol. 2021, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Germann, D.; El Bouse, A.; Shnier, J.; Abdelkader, N.; Kazemi, M. Effects of local vibration therapy on various performance parameters: A narrative literature review. J. Can. Chiropr. Assoc. 2018, 62, 170–181. [Google Scholar]
- Murillo, N.; Valls-Sole, J.; Vidal, J.; Opisso, E.; Medina, J.; Kumru, H. Focal vibration in neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2014, 50, 231–242. [Google Scholar] [PubMed]
- Paolucci, T.; Pezzi, L.; La Verde, R.; Latessa, P.M.; Bellomo, R.G.; Saggini, R. The Focal Mechanical Vibration for Balance Improvement in Elderly —A Systematic Review. Clin. Interv. Aging 2021, 16, 2009–2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chandrashekhar, R.; Rippetoe, J.; Ghazi, M. Focal Muscle Vibration for Stroke Rehabilitation: A Review of Vibration Parameters and Protocols. Appl. Sci. 2020, 10, 8270. [Google Scholar] [CrossRef]
- Sadeghi, M.; Sawatzky, B. Effects of vibration on spasticity in individuals with spinal cord injury: A scoping systematic review. Am. J. Phys. Med. Rehabil. 2014, 93, 995–1007. [Google Scholar] [CrossRef]
- Herda, T.J.; Ryan, E.D.; Smith, A.E.; Walter, A.A.; Bemben, M.G.; Stout, J.R.; Cramer, J.T. Acute effects of passive stretching vs vibration on the neuromuscular function of the plantar flexors. Scand. J. Med. Sci. Sport. 2009, 19, 703–713. [Google Scholar] [CrossRef]
- Dickerson, C.; Gabler, G.; Hopper, K.; Kirk, D.; McGregor, C.J. Immediate effects of localized vibration on hamstring and quadricep muscle performance. Int. J. Sport. Phys. Ther. 2012, 7, 381–387. [Google Scholar]
- Pamukoff, D.N.; Ryan, E.D.; Troy Blackburn, J. The acute effects of local muscle vibration frequency on peak torque, rate of torque development, and EMG activity. J. Electromyogr. Kinesiol. 2014, 24, 888–894. [Google Scholar] [CrossRef]
- Silva, H.R.; Couto, B.P.; Szmuchrowski, L.A. Effects of mechanical vibration applied in the opposite direction of muscle shortening on maximal isometric strength. J. Strength Cond. Res. 2008, 22, 1031–1036. [Google Scholar] [CrossRef]
- Iodice, P.; Bellomo, R.G.; Gialluca, G.; Fanò, G.; Saggini, R. Acute and cumulative effects of focused high-frequency vibrations on the endocrine system and muscle strength. Eur. J. Appl. Physiol. 2011, 111, 897–904. [Google Scholar] [CrossRef]
- Bosco, C.; Cardinale, M.; Tsarpela, O. Influence of vibration on mechanical power and electromyogram activity in human arm flexor muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 306–311. [Google Scholar] [CrossRef]
- Cochrane, D.J. The Acute Effect of Direct Vibration on Muscular Power Performance in Master Athletes. Int. J. Sport. Med. 2016, 37, 144–148. [Google Scholar] [CrossRef]
- Couto, B.P.; Silva, H.R.; Filho, A.G.; Da Silveira Neves, S.R.; Ramos, M.G.; Szmuchrowski, L.A.; Barbosa, M.P. Acute effects of resistance training with local vibration. Int. J. Sport. Med. 2013, 34, 814–819. [Google Scholar] [CrossRef]
- Custer, L.; Peer, K.S.; Miller, L. The Effects of Local Vibration on Balance, Power, and Self-Reported Pain After Exercise. J. Sport Rehabil. 2017, 26, 193–201. [Google Scholar] [CrossRef]
- Luo, J.; McNamara, B.; Moran, K. Effect of vibration training on neuromuscular output with ballistic knee extensions. J. Sport. Sci. 2008, 26, 1365–1373. [Google Scholar] [CrossRef]
- Luo, J.; Clarke, M.; McNamara, B.; Moran, K. Influence of Resistance Load on Neuromuscular Response to Vibration Training. J. Strength Cond. Res. 2009, 23, 420–426. [Google Scholar] [CrossRef]
- Moran, K.; McNamara, B.; Luo, J. Effect of vibration training in maximal effort (70% 1RM) dynamic bicep curls. Med. Sci. Sport. Exerc. 2007, 39, 526–533. [Google Scholar] [CrossRef]
- Souron, R.; Farabet, A.; Féasson, L.; Belli, A.; Millet, G.Y.; Lapole, T. Eight weeks of local vibration training increases dorsiflexor muscle cortical voluntary activation. J. Appl. Physiol. 2017, 122, 1504–1515. [Google Scholar] [CrossRef]
- Cannon, S.E.; Rues, J.P.; Melnick, M.E.; Guess, D. Head-erect behavior among three preschool-aged children with cerebral palsy. Phys. Ther. 1987, 67, 1198–1204. [Google Scholar] [CrossRef]
- Tardieu, G.; Tardieu, C.; Lespargot, A.; Roby, A.; Bret, M.D. Can Vibration-Induced Illusions Be Used As a Muscle Perception Test for Normal and Cerebral-Palsied Children? Dev. Med. Child Neurol. 1984, 26, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Noma, T.; Matsumoto, S.; Etoh, S.; Shimodozono, M.; Kawahira, K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients. Brain Inj. 2009, 23, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Marconi, B.; Filippi, G.M.; Koch, G.; Giacobbe, V.; Pecchioli, C.; Versace, V.; Camerota, F.; Saraceni, V.M.; Caltagirone, C. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabilit. Neural Repair 2011, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Noma, T.; Matsumoto, S.; Shimodozono, M.; Etoh, S.; Kawahira, K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients: A proof-of-principle study. J. Rehabil. Med. 2012, 44, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Caliandro, P.; Celletti, C.; Padua, L.; Minciotti, I.; Russo, G.; Granata, G.; La Torre, G.; Granieri, E.; Camerota, F. Focal muscle vibration in the treatment of upper limb spasticity: A pilot randomized controlled trial in patients with chronic stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1656–1661. [Google Scholar] [CrossRef]
- Lee, S.W.; Cho, K.H.; Lee, W.H. Effect of a local vibration stimulus training programme on postural sway and gait in chronic stroke patients: A randomized controlled trial. Clin. Rehabil. 2013, 27, 921–931. [Google Scholar] [CrossRef]
- Tavernese, E.; Paoloni, M.; Mangone, M.; Mandic, V.; Sale, P.; Franceschini, M.; Santilli, V. Segmental muscle vibration improves reaching movement in patients with chronic stroke. A randomized controlled trial. NeuroRehabilitation 2013, 32, 591–599. [Google Scholar] [CrossRef]
- Casale, R.; Damiani, C.; Maestri, R.; Fundarò, C.; Chimento, P.; Foti, C. Localized 100 Hz vibration improves function and reduces upper limb spasticity: A double-blind controlled study. Eur. J. Phys. Rehabil. Med. 2014, 50, 495–504. [Google Scholar]
- Costantino, C.; Galuppo, L.; Romiti, D. Efficacy of mechano-acoustic vibration on strength, pain, and function in poststroke rehabilitation: A pilot study. Top. Stroke Rehabil. 2014, 21, 391–399. [Google Scholar] [CrossRef]
- Costantino, C.; Galuppo, L.; Romiti, D. Short-term effect of local muscle vibration treatment versus sham therapy on upper limb in chronic post-stroke patients: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 32–40. [Google Scholar] [CrossRef]
- Bonan, I.; Butet, S.; Jamal, K.; Yelnik, A.; Tasseel Ponche, S.; Leplaideur, S. Difference between individuals with left and right hemiparesis in the effect of gluteus medius vibration on body weight shifting. Neurophysiol. Clin. 2017, 47, 419–426. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Milardi, D.; Leo, A.; Filoni, S.; Trinchera, A.; Bramanti, P. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS ONE 2017, 12, e0185936. [Google Scholar] [CrossRef]
- Celletti, C.; Sinibaldi, E.; Pierelli, F.; Monari, G.; Camerota, F. Focal Muscle Vibration and Progressive Modular Rebalancing with neurokinetic facilitations in post- stroke recovery of upper limb. Clin. Ter. 2017, 168, 33–36. [Google Scholar] [CrossRef]
- Jung, S.M. The effects of vibratory stimulation employed to forearm and arm flexor muscles on upper limb function in patients with chronic stroke. J. Phys. Ther. Sci. 2017, 29, 1620–1622. [Google Scholar] [CrossRef][Green Version]
- Choi, W.H. Effects of repeated vibratory stimulation of wrist and elbow flexors on hand dexterity, strength, and sensory function in patients with chronic stroke: A pilot study. J. Phys. Ther. Sci. 2017, 29, 605–608. [Google Scholar] [CrossRef][Green Version]
- Toscano, M.; Celletti, C.; Viganò, A.; Altarocca, A.; Giuliani, G.; Jannini, T.B.; Mastria, G.; Ruggiero, M.; Maestrini, I.; Vicenzini, E.; et al. Short-term effects of focal muscle vibration on motor recovery after acute stroke: A pilot randomized sham-controlled study. Front. Neurol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Annino, G.; Alashram, A.R.; Alghwiri, A.A.; Romagnoli, C.; Messina, G.; Tancredi, V.; Padua, E.; Mercuri, N.B. Effect of segmental muscle vibration on upper extremity functional ability poststroke: A randomized controlled trial. Med. (United States) 2019, 98, 1–5. [Google Scholar] [CrossRef]
- Ayvat, F.; Özçakar, L.; Ayvat, E.; Aksu Yıldırım, S.; Kılınç, M. Effects of low vs. high frequency local vibration on mild-moderate muscle spasticity: Ultrasonographical and functional evaluation in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 51. [Google Scholar] [CrossRef]
- Camerota, F.; Celletti, C.; Suppa, A.; Galli, M.; Cimolin, V.; Filippi, G.M.; La Torre, G.; Albertini, G.; Stocchi, F.; De Pandis, M.F. Focal Muscle Vibration Improves Gait in Parkinson’s Disease: A Pilot Randomized, Controlled Trial. Mov. Disord. Clin. Pract. 2016, 3, 559–566. [Google Scholar] [CrossRef]
- Özvar, G.B.; Ayvat, E.; Kılınç, M. Immediate Effects of Local Vibration and Whole-body Vibration on Postural Control in Patients with Ataxia: An Assessor-Blind, Cross-over randomized trial. Cerebellum 2021, 20, 83–91. [Google Scholar] [CrossRef]
- Calancie, B.; Broton, J.G.; John Klose, K.; Traad, M.; Difini, J.; Ram Ayyar, D. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials 1993, 89, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Ashby, P.; Verrier, M. Neurophysiological changes following traumatic spinal lesions in man. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Hilgevoord, A.A.J.; Bour, L.J.; Koelman, J.H.T.M.; Ongerboer De Visser, B.W. The relationship between the soleus H-reflex amplitude and vibratory inhibition in controls and spastic subjects. II. Computer model. J. Electromyogr. Kinesiol. 1996, 6, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Celletti, C.; Fattorini, L.; Camerota, F.; Ricciardi, D.; La Torre, G.; Landi, F.; Filippi, G.M. Focal muscle vibration as a possible intervention to prevent falls in elderly women: A pragmatic randomized controlled trial. Aging Clin. Exp. Res. 2015, 27, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, H.; Mohler, J.; Marlinski, V.; Rashedi, E.; Toosizadeh, N. The influence of mechanical vibration on local and central balance control. J. Biomech. 2018, 71, 59–66. [Google Scholar] [CrossRef]
- Filippi, G.M.; Brunetti, O.; Botti, F.M.; Panichi, R.; Roscini, M.; Camerota, F.; Cesari, M.; Pettorossi, V.E. Improvement of Stance Control and Muscle Performance Induced by Focal Muscle Vibration in Young-Elderly Women: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2009, 90, 2019–2025. [Google Scholar] [CrossRef]
- Rabini, A.; De Sire, A.; Marzetti, E.; Gimigliano, R.; Ferriero, G.; Piazzini, D.B.; Iolascon, G.; Gimigliano, F. Effects of focal muscle vibration on physical functioning in patients with knee osteoarthritis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 513–520. [Google Scholar]
- Wanderley, F.S.; Alburquerque-Sendn, F.; Parizotto, N.A.; Rebelatto, J.R. Effect of plantar vibration stimuli on the balance of older women: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2011, 92, 199–206. [Google Scholar] [CrossRef]
- Yu, M.; Piao, Y.J.; Kim, S.H.; Kim, D.W.; Kim, N.G. Effects of tendon vibration during one- legged and two-legged stance in elderly individuals. Int. J. Precis. Eng. Manuf. 2010, 11, 969–977. [Google Scholar] [CrossRef]
- Kamada, K.; Shimodozono, M.; Hamada, H.; Kawahira, K. Effects of 5 minutes of neck-muscle vibration immediately before occupational therapy on unilateral spatial neglect. Disabil. Rehabil. 2011, 33, 2322–2328. [Google Scholar] [CrossRef]
- Paoloni, M.; Giovannelli, M.; Mangone, M.; Leonardi, L.; Tavernese, E.; Di Pangrazio, E.; Bernetti, A.; Santilli, V.; Pozzilli, C. Does giving segmental muscle vibration alter the response to botulinum toxin injections in the treatment of spasticity in people with multiple sclerosis? A single-blind randomized controlled trial. Clin. Rehabil. 2013, 27, 803–812. [Google Scholar] [CrossRef]
- Novak, P.; Novak, V. Effect of step-synchronized vibration stimulation of soles on gait in Parkinson’s disease: A pilot study. J. NeuroEngineering Rehabil. 2006, 3, 1–7. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Butler, K.; Williamon, A.; Rothwell, J.C. Regaining motor control in musician’s dystonia by restoring sensorimotor organization. J. Neurosci. 2009, 29, 14627–14636. [Google Scholar] [CrossRef]
- Fattorini, L.; Ferraresi, A.; Rodio, A.; Azzena, G.B.; Filippi, G.M. Motor performance changes induced by muscle vibration. Eur. J. Appl. Physiol. 2006, 98, 79–87. [Google Scholar] [CrossRef]
- Casale, R.; Ring, H.; Rainoldi, A. High frequency vibration conditioning stimulation centrally reduces myoelectrical manifestation of fatigue in healthy subjects. J. Electromyogr. Kinesiol. 2009, 19, 998–1004. [Google Scholar] [CrossRef]
- Lapole, T.; Pérot, C. Effects of repeated Achilles tendon vibration on triceps surae force production. J. Electromyogr. Kinesiol. 2010, 20, 648–654. [Google Scholar] [CrossRef]
- Brunetti, O.; Botti, F.M.; Roscini, M.; Brunetti, A.; Panichi, R.; Filippi, G.M.; Biscarini, A.; Pettorossi, V.E. Focal vibration of quadriceps muscle enhances leg power and decreases knee joint laxity in female volleyball players. J. Sport. Med. Phys. Fit. 2012, 52, 596–605. [Google Scholar]
- Brunetti, O.; Botti, F.M.; Brunetti, A.; Biscarini, A.; Scarponi, A.M.; Filippi, G.M.; Pettorossi, V.E. Effects of focal vibration on bone mineral density and motor performance of postmenopausal osteoporotic women. J. Sport. Med. Phys. Fit. 2015, 55, 118–127. [Google Scholar]
- Filippi, G.M.; Fattorini, L.; Summa, A.; Zagaglia, A.; Rodio, A. Effects of focal vibration on power and work in multiple wingate tests. Biol. Sport 2020, 37, 25–31. [Google Scholar] [CrossRef]
- Ritzmann, R.; Stark, C.; Krause, A. Vibration therapy in patients with cerebral palsy: A systematic review. Neuropsychiatr. Dis. Treat. 2018, 14, 1607–1625. [Google Scholar] [CrossRef]
- Marazzi, S.; Kiper, P.; Palmer, K.; Agostini, M.; Turolla, A. Effects of vibratory stimulation on balance and gait in Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2021, 57, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Avvantaggiato, C.; Casale, R.; Cinone, N.; Facciorusso, S.; Turitto, A.; Stuppiello, L.; Picelli, A.; Ranieri, M.; Intiso, D.; Fiore, P.; et al. Localized muscle vibration in the treatment of motor impairment and spasticity in post-stroke patients: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Mortaza, N.; Abou-Setta, A.M.; Zarychanski, R.; Loewen, H.; Rabbani, R.; Glazebrook, C.M. Upper limb tendon/muscle vibration in persons with subacute and chronic stroke: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2019, 55, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Etoom, M.; Khraiwesh, Y.; Lena, F.; Hawamdeh, M.; Hawamdeh, Z.; Centonze, D.; Foti, C. Effectiveness of Physiotherapy Interventions on Spasticity in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2018, 97, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Celletti, C.; Suppa, A.; Bianchini, E.; Lakin, S.; Toscano, M.; La Torre, G.; Di Piero, V.; Camerota, F. Promoting post-stroke recovery through focal or whole body vibration: Criticisms and prospects from a narrative review. Neurol. Sci. 2020, 41, 11–24. [Google Scholar] [CrossRef]
- Grünewald, R.A.; Yoneda, Y.; Shipman, J.M.; Sagar, H.J. Idiopathic focal dystonia: A disorder of muscle spindle afferent processing? Brain 1997, 120, 2179–2185. [Google Scholar] [CrossRef]
- Yoneda, Y.; Rome, S.; Sagar, H.J.; Grünewald, R.A. Abnormal perception of the tonic vibration reflex in idiopathic focal dystonia. Eur. J. Neurol. 2000, 7, 529–533. [Google Scholar] [CrossRef]
- Trompetto, C.; Currà, A.; Buccolieri, A.; Suppa, A.; Abbruzzese, G.; Berardelli, A. Botulinum toxin changes intrafusal feedback in dystonia: A study with the tonic vibration reflex. Mov. Disord. 2006, 21, 777–782. [Google Scholar] [CrossRef]
- Conte, A.; Defazio, G.; Hallett, M.; Fabbrini, G.; Berardelli, A. The role of sensory information in the pathophysiology of focal dystonias. Nat. Rev. Neurol. 2019, 15, 224–233. [Google Scholar] [CrossRef]
- Kaji, R.; Rothwell, J.C.; Katayama, M.; Ikeda, T.; Kubori, T.; Kohara, N.; Mezaki, T.; Shibasaki, H.; Kimura, J. Tonic vibration reflex and muscle afferent block in writer’s cramp. Ann. Neurol. 1995, 38, 155–162. [Google Scholar] [CrossRef]
- Boychuk, J.A.; Schwerin, S.C.; Thomas, N.; Roger, A.; Silvera, G.; Liverpool, M.; Adkins, D.L.; Kleim, J.A. Enhanced Motor Recovery after Stroke with Combined Cortical Stimulation and Rehabilitative Training Is Dependent on Infarct Location. Neurorehabilit. Neural Repair 2016, 30, 173–181. [Google Scholar] [CrossRef]
- Ekblom, M.M.N.; Thorstensson, A. Effects of prolonged vibration on H-Reflexes, muscle activation, and dynamic strength. Med. Sci. Sport. Exerc. 2011, 43, 1933–1939. [Google Scholar] [CrossRef]
- Xu, L.; Cardinale, M.; Rabotti, C.; Beju, B.; Mischi, M. Eight-Week Vibration Training of the Elbow Flexors by Force Modulation. J. Strength Cond. Res. 2016, 30, 739–746. [Google Scholar] [CrossRef]
- Lundborg, G.; Dahlin, L.B.; Hansson, H.A.; Kanje, M.; Necking, L.E. Vibration exposure and peripheral nerve fiber damage. J. Hand Surg. 1990, 15, 346–351. [Google Scholar] [CrossRef]
- Chang, K.Y.; Ho, S.T.; Yu, H.S. Vibration induced neurophysiological and electron microscopical changes in rat peripheral nerves. Occup. Environ. Med. 1994, 51, 130–135. [Google Scholar] [CrossRef]
- Uryash, A.; Wu, H.; Bassuk, J.; Kurlansky, P.; Sackner, M.A.; Adams, J.A. Low-amplitude pulses to the circulation through periodic acceleration induces endothelial-dependent vasodilatation. J. Appl. Physiol. 2009, 106, 1840–1847. [Google Scholar] [CrossRef]
- Martínez, A.; Arias, J.; Bassuk, J.A.; Wu, H.; Kurlansky, P.; Adams, J.A. Adrenomedullin is increased by pulsatile shear stress on the vascular endothelium via periodic acceleration (pGz). Peptides 2008, 29, 73–78. [Google Scholar] [CrossRef]
- White, C.R.; Haidekker, M.A.; Stevens, H.Y.; Frangos, J.A. Extracellular signal-regulated kinase activation and endothelin-1 production in human endothelial cells exposed to vibration. J. Physiol. 2004, 555, 565–572. [Google Scholar] [CrossRef]
- Johnson, P.K.; Feland, J.B.; Johnson, A.W.; Mack, G.W.; Mitchell, U.H. Effect of whole body vibration on skin blood flow and nitric oxide production. J. Diabetes Sci. Technol. 2014, 8, 889–894. [Google Scholar] [CrossRef]
- Chien, S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292. [Google Scholar] [CrossRef]
- Ueda, A.; Koga, M.; Ikeda, M.; Kudo, S.; Tanishita, K. Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am. J. Physiol.-Heart Circ. Physiol. 2004, 287. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Christensen, L.P.; Tomanek, R.J. Differential effects of cyclic and static stretch on coronary microvascular endothelial cell receptors and vasculogenic/angiogenic responses. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295. [Google Scholar] [CrossRef]
- Ruehle, M.A.; Eastburn, E.A.; Labelle, S.A.; Krishnan, L.; Weiss, J.A.; Boerckel, J.D.; Wood, L.B.; Guldberg, R.E.; Willett, N.J. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci. Adv. 2020, 6, eabb6351. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Polte, T.; Huang, S.; Shi, B.; Alsberg, E.; Sunday, M.E.; Ingber, D.E. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. 2005, 232, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Connor, K.M.; Mammoto, T.; Yung, C.W.; Huh, D.; Aderman, C.M.; Mostoslavsky, G.; Smith, L.E.; Ingber, D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009, 457, 1103–1108. [Google Scholar] [CrossRef]
- Wu, Y.; Al-Ameen, M.A.; Ghosh, G. Integrated Effects of Matrix Mechanics and Vascular Endothelial Growth Factor (VEGF) on Capillary Sprouting. Ann. Biomed. Eng. 2014, 42, 1024–1036. [Google Scholar] [CrossRef]
- Huang, S.; Ingber, D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999, 1, E131–E138. [Google Scholar] [CrossRef]
- Folkman, J.; Moscona, A. Role of cell shape in growth control. Nature 1978, 273, 345–349. [Google Scholar] [CrossRef]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial Cilia Are Fluid Shear Sensors That Regulate Calcium Signaling and Nitric Oxide Production Through Polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef]
- Bromann, P.A.; Korkaya, H.; Courtneidge, S.A. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 2004, 23, 7957–7968. [Google Scholar] [CrossRef]
- Conway, D.; Schwartz, M.A. Lessons from the endothelial junctional mechanosensory complex. F1000 Biol. Rep. 2012, 4, 2–7. [Google Scholar] [CrossRef]
- Hood, J.D.; Meininger, C.J.; Ziche, M.; Granger, H.J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 1998, 274, H1054–H1058. [Google Scholar] [CrossRef]
- Van Der Zee, R.; Murohara, T.; Luo, Z.; Zollmann, F.; Passeri, J.; Lekutat, C.; Isner, J.M. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation 1997, 95, 1030–1037. [Google Scholar] [CrossRef]
- Cooke, J.P.; Losordo, D.W. Nitric Oxide and Angiogenesis. Circulation 2002, 105, 2133–2135. [Google Scholar] [CrossRef]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef]
- Losordo, D.W.; Isner, J.M. Vascular endothelial growth factor-induced angiogenesis: Crouching tiger or hidden dragon? J. Am. Coll. Cardiol. 2001, 37, 2131–2135. [Google Scholar] [CrossRef]
- Friedrich, E.E.; Hong, Z.; Xiong, S.; Zhong, M.; Di, A.; Rehman, J.; Komarova, Y.A.; Malik, A.B. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl. Acad. Sci. USA 2019, 116, 12980–12985. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The Endothelial-Specific MicroRNA miR-126 Governs Vascular Integrity and Angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Chamorro-Jorganes, A.; Lee, M.Y.; Araldi, E.; Landskroner-Eiger, S.; Fernández-Fuertes, M.; Sahraei, M.; Quiles Del Rey, M.; Van Solingen, C.; Yu, J.; Fernández-Hernando, C.; et al. VEGF-Induced Expression of miR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016, 118, 38–47. [Google Scholar] [CrossRef]
- Hong, S.G.; Shin, J.; Aldokhayyil, M.; Brown, M.D.; Park, J.Y. Mitochondrial and Metabolic Adaptations to Exercise-Induced Fluid Shear Stress in Endothelial Cells. Exerc. Sport Sci. Rev. 2022, 50, 145–155. [Google Scholar] [CrossRef]
- Chiu, J.J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Ingber, D.E. Mechanical control of tissue and organ development. Development 2010, 137, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Lucitti, J.L.; Jones, E.A.V.; Huang, C.; Chen, J.; Fraser, S.E.; Dickinson, M.E. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 2007, 134, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Munn, L.L. Fluid forces control endothelial sprouting. Proc. Natl. Acad. Sci. USA 2011, 108, 15342–15347. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J.M.; Kolega, J.; Meng, H. High wall shear stress and spatial gradients in vascular pathology: A review. Ann. Biomed. Eng. 2013, 41, 1411–1427. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.C.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.S.; Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- Iring, A.; Jin, Y.J.; Albarrán-Juárez, J.; Siragusa, M.; Wang, S.P.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Davies, P.F.; Spaan, J.A.; Krams, R. Shear stress biology of the endothelium. Ann. Biomed. Eng. 2005, 33, 1714–1718. [Google Scholar] [CrossRef]
- Balligand, J.L.; Feron, O.; Dessy, C. eNOS Activation by Physical Forces: From Short-Term Regulation of Contraction to Chronic Remodeling of Cardiovascular Tissues. Physiol. Rev. 2009, 89, 481–534. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Masini, E.; Amerini, S.; Granger, H.J.; Maggi, C.A.; Geppetti, P.; Ledda, F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994, 94, 2036–2044. [Google Scholar] [CrossRef]
- Morley, L.C.; Shi, J.; Gaunt, H.J.; Hyman, A.J.; Webster, P.J.; Williams, C.; Forbes, K.; Walker, J.J.; Simpson, N.A.B.; Beech, D.J. Piezo1 channels are mechanosensors in human fetoplacental endothelial cells. Mol. Hum. Reprod. 2018, 24, 510–520. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Zhang, D.Y.; Zhu, J.; Zhang, T.; Wang, C. MicroRNA 126 inhibits the transition of endothelial progenitor cells to mesenchymal cells via the PIK3R2-PI3K/Akt signalling pathway. PLoS ONE 2013, 8, e83294. [Google Scholar] [CrossRef]
- Png, K.J.; Halberg, N.; Yoshida, M.; Tavazoie, S.F. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 2012, 481, 190–196. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.; Zhang, C.; Yang, W.; Li, C.; Gao, Z.; Pei, K.; Li, Y. Piezo1 mediates endothelial atherogenic inflammatory responses via regulation of YAP/TAZ activation. Hum. Cell 2022, 35, 51–62. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef]
- Doma’nska-Senderowska, D.; Laguette, M.J.N.; Jegier, A.; Cieszczyk, P.; September, A.V.; Brzezia’nska-Lasota, E. MicroRNA Profile and Adaptive Response to Exercise Training: A Review. Int. J. Sport. Med. 2019, 40, 227–235. [Google Scholar] [CrossRef]
- Sabirin, R.M.; Yunus, J.; Agustiningsih, D. The effects of aerobic exercise on blood plasma microRNA level in healthy adults: A systematic review and meta-analysis. Sport Sci. for Health 2022, 1–16. [Google Scholar] [CrossRef]
- Celic, T.; Meuth, V.; Six, I.; Massy, Z.; Metzinger, L. The mir-221/222 Cluster is a Key Player in Vascular Biology via the Fine-Tuning of Endothelial Cell Physiology. Curr. Vasc. Pharmacol. 2016, 15, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Song, B.W.; Oh, S.; Chang, W. Multiplexed targeting of microRNA in stem cell-derived extracellular vesicles for regenerative medicine. BMB Rep. 2022, 55, 65–71. [Google Scholar] [CrossRef]
- Esposito, E.; Hayakawa, K.; Maki, T.; Arai, K.; Lo, E.H. Effects of Postconditioning on Neurogenesis and Angiogenesis during the Recovery Phase after Focal Cerebral Ischemia. Stroke 2015, 46, 2691–2694. [Google Scholar] [CrossRef]
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and Neurogenic Rejuvenation of the Aging Mouse Brain by Young Systemic Factors. Science 2014, 344, 630–634. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; He, G.; Tan, X.; Jin, X.; Li, C. Transcutaneous auricular vagus nerve stimulation regulates expression of growth differentiation factor 11 and activin-like kinase 5 in cerebral ischemia/reperfusion rats. J. Neurol. Sci. 2016, 369, 27–35. [Google Scholar] [CrossRef]
- Carmeliet, P. Blood vessels and nerves: Common signals, pathways and diseases. Nat. Rev. Genet. 2003, 4, 710–720. [Google Scholar] [CrossRef]
- Plummer, P.N.; Freeman, R.; Taft, R.J.; Vider, J.; Sax, M.; Umer, B.A.; Gao, D.; Johns, C.; Mattick, J.S.; Wilton, S.D.; et al. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013, 73, 341–352. [Google Scholar] [CrossRef]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef]
- Heusschen, R.; van Gink, M.; Griffioen, A.W.; Thijssen, V.L. MicroRNAs in the tumor endothelium: Novel controls on the angioregulatory switchboard. Biochim. Et Biophys. Acta-Rev. Cancer 2010, 1805, 87–96. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, A.; Li, X.; Zhang, S.; Liu, S.; Zhao, H.; Wu, S.; Chen, L.; Ma, C.; Zhao, H. MiR-21-5p regulates extracellular matrix degradation and angiogenesis in TMJOA by targeting Spry1. Arthritis Res. Ther. 2020, 22, 1–17. [Google Scholar] [CrossRef]
- Suárez, Y.; Fernández-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Semo, J.; Sharir, R.; Afek, A.; Avivi, C.; Barshack, I.; Maysel-Auslender, S.; Krelin, Y.; Kain, D.; Entin-Meer, M.; Keren, G.; et al. The 106b∼25 microRNA cluster is essential for neovascularization after hindlimb ischaemia in mice. Eur. Heart J. 2014, 35, 3212–3223. [Google Scholar] [CrossRef]
- Gallach, S.; Calabuig-Fariñas, S.; Jantus-Lewintre, E.; Camps, C. MicroRNAs: Promising new antiangiogenic targets in cancer. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Anand, S.; Majeti, B.K.; Acevedo, L.M.; Murphy, E.A.; Mukthavaram, R.; Scheppke, L.; Huang, M.; Shields, D.J.; Lindquist, J.N.; Lapinski, P.E.; et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 2010, 16, 909–914. [Google Scholar] [CrossRef]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef]
- Li, H.; Ouyang, X.P.; Jiang, T.; Zheng, X.L.; He, P.P.; Zhao, G.J. MicroRNA-296: A promising target in the pathogenesis of atherosclerosis? Mol. Med. 2018, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Deng, Z.; Wang, C.H.; Yang, B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef] [PubMed]
- Floriano, J.F.; Emanueli, C.; Vega, S.; Barbosa, A.M.P.; Oliveira, R.G.D.; Floriano, E.A.F.; Graeff, C.F.D.O.; Abbade, J.F.; Herculano, R.D.; Sobrevia, L.; et al. Pro-angiogenic approach for skeletal muscle regeneration. Biochim. Et Biophys. Acta-Gen. Subj. 2022, 1866. [Google Scholar] [CrossRef] [PubMed]
- Gorenoi, V.; Brehm, M.U.; Koch, A.; Hagen, A. Growth factors for angiogenesis in peripheral arterial disease. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Nascimento, A.I.; Mar, F.M.; Sousa, M.M. The intriguing nature of dorsal root ganglion neurons: Linking structure with polarity and function. Prog. Neurobiol. 2018, 168, 86–103. [Google Scholar] [CrossRef]
- Rigoni, M.; Negro, S. Signals Orchestrating Peripheral Nerve Repair. Cells 2020, 9, 1768. [Google Scholar] [CrossRef]
- Ziv, N.E.; Spira, M.E. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J. Neurophysiol. 1995, 74, 2625–2637. [Google Scholar] [CrossRef]
- Ziv, N.E.; Spira, M.E. Localized and transient elevations of intracellular Ca2+ induce the dedifferentiation of axonal segments into growth cones. J. Neurosci. 1997, 17, 3568–3579. [Google Scholar] [CrossRef]
- Cho, Y.; Sloutsky, R.; Naegle, K.M.; Cavalli, V. Injury-Induced HDAC5 nuclear export is essential for axon regeneration. Cell 2013, 155, 894. [Google Scholar] [CrossRef]
- Rishal, I.; Fainzilber, M. Axon-soma communication in neuronal injury. Nat. Rev. Neurosci. 2014, 15, 32–42. [Google Scholar] [CrossRef]
- Yoo, S.; van Niekerk, E.A.; Merianda, T.T.; Twiss, J.L. Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp. Neurol. 2010, 223, 19–27. [Google Scholar] [CrossRef][Green Version]
- Czogalla, A.; Sikorski, A.F. Spectrin and calpain: A ’target’ and a ’sniper’ in the pathology of neuronal cells. Cell. Mol. Life Sci. 2005, 62, 1913–1924. [Google Scholar] [CrossRef]
- Yoo, S.; Nguyen, M.P.; Fukuda, M.; Bittner, G.D.; Fishman, H.M. Plasmalemmal Sealing of Transected Mammalian Neurites Is a Gradual Process Mediated by Ca2+-Regulated Proteins. J. Neurosci. Res. 2003, 74, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Gitler, D.; Spira, M.E. Real time imaging of calcium-induced localized proteolytic activity after axotomy and its relation to growth cone formation. Neuron 1998, 20, 1123–1135. [Google Scholar] [CrossRef]
- Gitler, D.; Spira, M.E. Short window of opportunity for calpain induced growth cone formation after axotomy of Aplysia neurons. J. Neurobiol. 2002, 52, 267–279. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann cells: Development and role in nerve repair. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–15. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front. Bioeng. Biotechnol. 2019, 7, 1–30. [Google Scholar] [CrossRef]
- Evans, G.R.D.; Brandt, K.; Katz, S.; Chauvin, P.; Otto, L.; Bogle, M.; Wang, B.; Meszlenyi, R.K.; Lu, L.; Mikos, A.G.; et al. Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 2002, 23, 841–848. [Google Scholar] [CrossRef]
- Zochodne, D.W. The challenges and beauty of peripheral nerve regrowth. J. Peripher. Nerv. Syst. 2012, 17, 1–18. [Google Scholar] [CrossRef]
- Toth, C.C.; Willis, D.; Twiss, J.L.; Walsh, S.; Martinez, J.A.; Liu, W.Q.; Midha, R.; Zochodne, D.W. Locally synthesized calcitonin gene-related peptide has a critical role in peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2009, 68, 326–337. [Google Scholar] [CrossRef]
- Höke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [PubMed]
- Court, F.A.; Hendriks, W.T.J.; MacGillavry, H.D.; Alvarez, J.; Van Minnen, J. Schwann cell to axon transfer of ribosomes: Toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 2008, 28, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.; Kim, H.; Santerre, M.; Krupka, A.J.; Han, S.B.; Zhai, J.; Cho, J.Y.; Park, R.; Harris, M.; Kim, S.; et al. YAP/TAZ initiate and maintain Schwann cell myelination. eLife 2017, 6, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.T.; Bevan, S.; Ritchie, J.M. High conductance anion-selective channels in rat cultured Schwann cells. Proc. R. Soc. London. Ser. B. Biol. Sci. 1984, 221, 395–409. [Google Scholar] [CrossRef]
- Barres, B.A.; Chun, L.L.Y.; Corey, D.P. Ion Channels in Vertebrate Glia. Annu. Rev. Neurosci. 1990, 13, 441–474. [Google Scholar] [CrossRef]
- Belin, S.; Zuloaga, K.L.; Poitelon, Y. Influence of mechanical stimuli on schwann cell biology. Front. Cell. Neurosci. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Chen, Z.L.; Yu, W.M.; Strickland, S. Peripheral regeneration. Annu. Rev. Neurosci. 2007, 30, 209–233. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; MacKenzie, F.E.; Hoving, J.J.A.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Andreone, B.J.; Lacoste, B.; Gu, C. Neuronal and Vascular Interactions. Annu. Rev. Neurosci. 2015, 38, 25–46. [Google Scholar] [CrossRef]
- Muratori, L.; Gnavi, S.; Fregnan, F.; Mancardi, A.; Raimondo, S.; Perroteau, I.; Geuna, S. Evaluation of Vascular Endothelial Growth Factor (VEGF) and Its Family Member Expression After Peripheral Nerve Regeneration and Denervation. Anat. Rec. 2018, 301, 1646–1656. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of Hepatocyte Growth Factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.R.; Lee, J.; Lee, D.; Nho, B.; Kim, S. Hepatocyte Growth Factor (HGF) Promotes Peripheral Nerve Regeneration by Activating Repair Schwann Cells. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hromada, C.; Hartmann, J.; Oesterreicher, J.; Stoiber, A.; Daerr, A.; Schädl, B.; Priglinger, E.; Teuschl-Woller, A.H.; Holnthoner, W.; Heinzel, J.; et al. Occurrence of Lymphangiogenesis in Peripheral Nerve Autografts Contrasts Schwann Cell-Induced Apoptosis of Lymphatic Endothelial Cells In Vitro. Biomolecules 2022, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.P.; McCleskey, E.W. Cell damage excites nociceptors through release of cytosolic ATP. Pain 2002, 95, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Barr, T.; Gee, L.; Vickers, J.; Wymer, J.; Borsani, E.; Rodella, L.; Getsios, S.; Burdo, T.; Eisenberg, E.; et al. Keratinocyte expression of calcitonin gene-related peptide β: Implications for neuropathic and inflammatory pain mechanisms. Pain 2011, 152, 2036–2051. [Google Scholar] [CrossRef]
- Bigliardi-Qi, M.; Sumanovski, L.T.; Büchner, S.; Rufli, T.; Bigliardi, P.L. Mu-Opiate Receptor and Beta-Endorphin Expression in Nerve Endings and Keratinocytes in Human Skin. Dermatology 2004, 209, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem. 1992, 267, 24675–24680. [Google Scholar] [CrossRef]
- Pincelli, C.; Sevignani, C.; Manfredini, R.; Grande, A.; Fantini, F.; Bracci-Laudiero, L.; Aloe, L.; Ferrari, S.; Cossarizza, A.; Giannetti, A. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J. Investig. Dermatol. 1994, 103, 13–18. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Clark, J.D.; Kingery, W.S. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul. Pept. 2013, 186, 92–103. [Google Scholar] [CrossRef]
- Chateau, Y.; Misery, L. Connections between nerve endings and epidermal cells: Are they synapses? Exp. Dermatol. 2004, 13, 2–4. [Google Scholar] [CrossRef]
- Talagas, M.; Lebonvallet, N.; Leschiera, R.; Elies, P.; Marcorelles, P.; Misery, L. Intra-epidermal nerve endings progress within keratinocyte cytoplasmic tunnels in normal human skin. Exp. Dermatol. 2020, 29, 387–392. [Google Scholar] [CrossRef]
- Talagas, M.; Lebonvallet, N.; Leschiera, R.; Sinquin, G.; Elies, P.; Haftek, M.; Pennec, J.P.; Ressnikoff, D.; La Padula, V.; Le Garrec, R.; et al. Keratinocytes Communicate with Sensory Neurons via Synaptic-like Contacts. Ann. Neurol. 2020, 88, 1205–1219. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Yi, S. Identification of critical growth factors for peripheral nerve regeneration. RSC Adv. 2019, 9, 10760–10765. [Google Scholar] [CrossRef]
- Freidin, M.; Asche, S.; Bargiello, T.A.; Bennett, M.V.L.; Abrams, C.K. Connexin 32 increases the proliferative response of Schwann cells to neuregulin-1 (Nrg1). Proc. Natl. Acad. Sci. USA 2009, 106, 3567–3572. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, H.; Sun, F.; Lu, Z.; Reed-Maldonado, A.; Lee, Y.C.; Wang, G.; Banie, L.; Lue, T.F. Brain-derived neurotrophic factor promotes nerve regeneration by activating the JAK/STAT pathway in Schwann cells. Transl. Androl. Urol. 2016, 5, 167–175. [Google Scholar] [CrossRef]
- Shakhbazau, A.; Kawasoe, J.; Hoyng, S.A.; Kumar, R.; van Minnen, J.; Verhaagen, J.; Midha, R. Early regenerative effects of NGF-transduced Schwann cells in peripheral nerve repair. Mol. Cell. Neurosci. 2012, 50, 103–112. [Google Scholar] [CrossRef]
- Mamet, J.; Lazdunski, M.; Voilley, N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J. Biol. Chem. 2003, 278, 48907–48913. [Google Scholar] [CrossRef]
- Xu, P.; Rosen, K.M.; Hedstrom, K.; Rey, O.; Guha, S.; Hart, C.; Corfas, G. Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in schwann cells through purinergic signaling and the PKC-PKD pathway. Glia 2013, 61, 1029–1040. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Belluscio, L.; Squinto, S.; Ip, N.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. Neurotrophin-3: Related to Neurotrophic Factor. Science 1990, 247, 1446–1451. [Google Scholar] [CrossRef]
- Mendell, L.M.; Johnson, R.D.; Munson, J.B. Neurotrophin modulation of the monosynaptic reflex after peripheral nerve transection. J. Neurosci. 1999, 19, 3162–3170. [Google Scholar] [CrossRef]
- Sahenk, Z.; Nagaraja, H.N.; Mccracken, B.S.; King, W.M.; Freimer, M.L.; Cedarbaum, J.M.; Mendell, J.R. NT-3 promotes nerve regeneration and sensory improvement in CMT1A mouse models and in patients. Neurology 2005, 65, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Ning, N.; Hu, J.F.; Yuan, Y.H.; Zhang, X.Y.; Dai, J.G.; Chen, N.H. Huperzine A derivative M3 protects PC12 cells against sodium nitroprusside-induced apoptosis. Acta Pharmacol. Sin. 2012, 33, 34–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; Costa, L.D.F.; Guck, J.; et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Bray, D. Axonal growth in response to experimentally applied mechanical tension. Dev. Biol. 1984, 102, 379–389. [Google Scholar] [CrossRef]
- Kerstein, P.C.; Nichol, R.H.; Gomez, T.M. Mechanochemical regulation of growth cone motility. Front. Cell. Neurosci. 2015, 9, 1–16. [Google Scholar] [CrossRef]
- Weiss, P. Nerve patterns: The mechanics of nerve growth. Growth: Third Growth Symp. 1941, 5, 163–203. [Google Scholar] [CrossRef]
- Li, F.; Lo, T.Y.; Miles, L.; Wang, Q.; Noristani, H.N.; Li, D.; Niu, J.; Trombley, S.; Goldshteyn, J.I.; Wang, C.; et al. The Atr-Chek1 pathway inhibits axon regeneration in response to Piezo-dependent mechanosensation. Nat. Commun. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Moreno-López, B. Local isoform-specific NOS inhibition: A promising approach to promote motor function recovery after nerve injury. J. Neurosci. Res. 2010, 88, 1846–1857. [Google Scholar] [CrossRef]
- Zhou, S.; Shen, D.; Wang, Y.; Gong, L.; Tang, X.; Yu, B.; Gu, X.; Ding, F. microRNA-222 Targeting PTEN Promotes Neurite Outgrowth from Adult Dorsal Root Ganglion Neurons following Sciatic Nerve Transection. PLoS ONE 2012, 7, e44768. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.J.; Morgan, C.C.; Adamowicz, M.; Gomez-Sanchez, J.A.; Fazal, S.V.; Beucher, A.; Razzaghi, B.; Mirsky, R.; Jessen, K.R.; Aitman, T.J. Changes in the Coding and Non-coding Transcriptome and DNA Methylome that Define the Schwann Cell Repair Phenotype after Nerve Injury. Cell Rep. 2017, 20, 2719–2734. [Google Scholar] [CrossRef]
- Chang, W.; Kanda, H.; Ikeda, R.; Ling, J.; DeBerry, J.J.; Gu, J.G. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc. Natl. Acad. Sci. USA 2016, 113, E5491–E5500. [Google Scholar] [CrossRef]
- Borger, A.; Stadlmayr, S.; Haertinger, M.; Semmler, L.; Supper, P.; Millesi, F.; Radtke, C. How miRNAs Regulate Schwann Cells during Peripheral Nerve Regeneration—A Systemic Review. Int. J. Mol. Sci. 2022, 23, 3440. [Google Scholar] [CrossRef]
- Hussein, M.; Magdy, R. MicroRNAs in central nervous system disorders: Current advances in pathogenesis and treatment. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Yi, S.; Liu, Q.; Zhang, R.; Wang, P.; Qian, T.; Li, S. The microRNAs let-7 and miR-9 down-regulate the axon-guidance genes Ntn1 and Dcc during peripheral nerve regeneration. J. Biol. Chem. 2019, 294, 3489–3500. [Google Scholar] [CrossRef]
- Yi, S.; Yuan, Y.; Chen, Q.; Wang, X.; Gong, L.; Liu, J.; Gu, X.; Li, S. Regulation of Schwann cell proliferation and migration by MIR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.P.; Xu, P.; Guo, C.X.; Luo, Z.R.; Zhu, J.; Mou, F.F.; Cai, H.; Wang, C.; Ye, X.C.; Shao, S.J.; et al. miR-1b overexpression suppressed proliferation and migration of RSC96 and increased cell apoptosis. Neurosci. Lett. 2018, 687, 137–145. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Wang, J.; Zhang, Z.; Fu, S.; Wang, S.; Li, X. MiR-1b up-regulation inhibits rat neuron proliferation and regeneration yet promotes apoptosis via targeting KLF7. Folia Neuropathol. 2021, 59, 67–80. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, R.; Hu, W.; Qian, T.; Wang, N.; Ding, G.; Ding, F.; Yu, B.; Gu, X. MiR-9 inhibits schwann cell migration by targeting cthrc1 following sciatic nerve injury. J. Cell Sci. 2014, 127, 967–976. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Gu, Y.; Chen, C.; Wang, Y.; Liu, J.; Hu, W.; Yu, B.; Wang, Y.; Ding, F.; et al. Let-7 microRNAs regenerate peripheral nerve regeneration by targeting nerve growth factor. Mol. Ther. 2015, 23, 423–433. [Google Scholar] [CrossRef]
- Gökbuget, D.; Pereira, J.A.; Bachofner, S.; Marchais, A.; Ciaudo, C.; Stoffel, M.; Schulte, J.H.; Suter, U. The Lin28/let-7 axis is critical for myelination in the peripheral nervous system. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.J.; Lu, X.H.; Luo, J.C.; Chen, C.; Gao, Q.; Li, Z.Y.; Wang, H. Molecular mechanism of microRNA-21 promoting Schwann cell proliferation and axon regeneration during injured nerve repair. RNA Biol. 2020, 17, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- López-Leal, R.; Díaz-Viraqué, F.; Catalán, R.J.; Saquel, C.; Enright, A.; Iraola, G.; Court, F.A. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J. Cell Sci. 2020, 133, jcs239004. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Wang, Q.H.; Zhao, L.L.; Qin, J.; Wang, Y.X.; Yu, B.; Zhou, S.L. MiR-30c promotes Schwann cell remyelination following peripheral nerve injury. Neural Regen. Res. 2017, 12, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zhou, X.; Liu, J.; Zhao, Y.; Jiang, X. MiR-34a regulates Schwann cell proliferation and migration by targeting CNTN2. NeuroReport 2020, 31, 1180–1188. [Google Scholar] [CrossRef]
- Viader, A.; Chang, L.W.; Fahrner, T.; Nagarajan, R.; Milbrandt, J. MicroRNAs modulate schwann cell response to nerve injury by reinforcing transcriptional silencing of dedifferentiation-related genes. J. Neurosci. 2011, 31, 17358–17369. [Google Scholar] [CrossRef]
- Nagata, K.; Hama, I.; Kiryu-Seo, S.; Kiyama, H. MicroRNA-124 is down regulated in nerve-injured motor neurons and it potentially targets mRNAs for KLF6 and STAT3. Neuroscience 2014, 256, 426–432. [Google Scholar] [CrossRef]
- Zhu, H.; Xue, C.; Yao, M.; Wang, H.; Zhang, P.; Qian, T.; Zhou, S.; Li, S.; Yu, B.; Wang, Y.; et al. MIR-129 controls axonal regeneration via regulating insulin-like growth factor-1 in peripheral nerve injury. Cell Death Dis. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Yao, C.; Shi, X.; Zhang, Z.; Zhou, S.; Qian, T.; Wang, Y.; Ding, F.; Gu, X.; Yu, B. Hypoxia-Induced Upregulation of miR-132 Promotes Schwann Cell Migration After Sciatic Nerve Injury by Targeting PRKAG3. Mol. Neurobiol. 2016, 53, 5129–5139. [Google Scholar] [CrossRef]
- Sullivan, T.; Robert, L.; Teebagy, P.; Morgan, S.; Beatty, E.; Cicuto, B.; Nowd, P.; Rieger-Christ, K.M.; Bryan, D. Spatiotemporal microRNA profile in peripheral nerve regeneration: MiR-138 targets vimentin and inhibits Schwann cell migration and proliferation. Neural Regen. Res. 2018, 13, 1253–1262. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhang, W.T.; Cheng, Y.X.; Liu, Y.C.; Zhai, F.G.; Sun, P.; Li, H.T.; Deng, L.X.; Zhu, X.F.; Wang, Y. Inhibition of KLF7-Targeting MicroRNA 146b Promotes Sciatic Nerve Regeneration. Neurosci. Bull. 2018, 34, 419–437. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, S.; Wang, Y.; Qian, T.; Ding, G.; Ding, F.; Gu, X. Mir-221 and mir-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. J. Cell Sci. 2012, 125, 2675–2683. [Google Scholar] [CrossRef]
- Liu, X.; Cui, X.; Guan, G.; Dong, Y.; Zhang, Z. microRNA-192-5p is involved in nerve repair in rats with peripheral nerve injury by regulating XIAP. Cell Cycle 2020, 19, 326–338. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, X.; Qiu, J.; Zhang, Y.; Gong, F. MicroRNA-210 contributes to peripheral nerve regeneration through promoting the proliferation and migration of Schwann cells. Exp. Ther. Med. 2017, 14, 2809–2816. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.; Yuan, Y.; Li, P.; Pan, J.; Qin, J.; Liu, Y.; Zhang, Y.; Tian, F.; Yu, B.; Zhou, S. miR-221-3p Inhibits Schwann Cell Myelination. Neuroscience 2018, 379, 239–245. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Yuan, Y.; Yi, S.; Chen, Q.; Gong, L.; Liu, J.; Ding, F.; Cao, Z.; Gu, X. MiR-340 Regulates Fibrinolysis and Axon Regrowth Following Sciatic Nerve Injury. Mol. Neurobiol. 2017, 54, 4379–4389. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Li, A.; Wu, G. miR-485-5p suppresses Schwann cell proliferation and myelination by targeting cdc42 and Rac1. Exp. Cell Res. 2020, 388, 111803. [Google Scholar] [CrossRef]
- Wang, P.; He, J.; Wang, S.; Wang, X.; Liu, Q.; Peng, W.; Qian, T. miR-3075 Inhibited the Migration of Schwann Cells by Targeting Cntn2. Neurochem. Res. 2018, 43, 1879–1886. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Miao, Y.; Wang, X.H.; Wang, P.; Cheng, Z.C.; Qian, T.M. Increased levels of miR-3099 induced by peripheral nerve injury promote Schwann cell proliferation and migration. Neural Regen. Res. 2019, 14, 525–531. [Google Scholar] [CrossRef]
- Motz, C.T.; Kabat, V.; Saxena, T.; Bellamkonda, R.V.; Zhu, C. Neuromechanobiology: An Expanding Field Driven by the Force of Greater Focus. Adv. Healthc. Mater. 2021, 10, 1–25. [Google Scholar] [CrossRef]
- De Vincentiis, S.; Falconieri, A.; Scribano, V.; Ghignoli, S.; Raffa, V. Manipulation of Axonal Outgrowth via Exogenous Low Forces. Int. J. Mol. Sci. 2020, 21, 8009. [Google Scholar] [CrossRef] [PubMed]
- Fallon, J.B.; Bent, L.R.; McNulty, P.A.; Macefield, V.G. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J. Neurophysiol. 2005, 94, 3795–3804. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.S.; Herr, H. A cutaneous mechanoneural interface for neuroprosthetic feedback. Nature Biomedical Engineering 2021. [Google Scholar] [CrossRef] [PubMed]
- Mosabbir, A.; Almeida, Q.J.; Ahonen, H. The effects of long-term 40-hz physioacoustic vibrations on motor impairments in parkinson’s disease: A double-blinded randomized control trial. Healthcare 2020, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Vuong, V.; Mosabbir, A.; Paneduro, D.; Picard, L.; Faghfoury, H.; Evans, M.; Gordon, A.; Bartel, L. Effects of Rhythmic Sensory Stimulation on Ehlers-Danlos Syndrome: A Pilot Study. Pain Res. Manag. 2020, 2020, 3586767. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Clements-Cortes, A.; Ahonen, H.; Evans, M.; Freedman, M.; Bartel, L. Short-Term Effects of Rhythmic Sensory Stimulation in Alzheimer’s Disease: An Exploratory Pilot Study. J. Alzheimer’s Dis. 2016, 52, 651–660. [Google Scholar] [CrossRef]
- King, L.K.; Almeida, Q.J.; Ahonen, H. Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. NeuroRehabilitation 2009, 25, 297–306. [Google Scholar] [CrossRef]
- Wolf, E.J.; Cruz, T.H.; Emondi, A.A.; Langhals, N.B.; Naufel, S.; Peng, G.C.; Schulz, B.W.; Wolfson, M. Advanced technologies for intuitive control and sensation of prosthetics. Biomed. Eng. Lett. 2020, 10, 119–128. [Google Scholar] [CrossRef]
- Botvinick, M.; Cohen, J.D. Rubber hand ’feels’ what eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef]
- Jones, L.A. Motor illusions: What do they reveal about proprioception? Psychol. Bull. 1988, 103, 72–86. [Google Scholar] [CrossRef]
- Synofzik, M.; Vosgerau, G.; Newen, A. I move, therefore I am: A new theoretical framework to investigate agency and ownership. Conscious. Cogn. 2008, 17, 411–424. [Google Scholar] [CrossRef]
- Beauchamp, M.S.; Yasar, N.E.; Kishan, N.; Ro, T. Human MST but not MT responds to tactile stimulation. J. Neurosci. 2007, 27, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.T.; Hsiao, S.S.; Johnson, K.O. Neural coding mechanisms in tactile pattern recognition: The relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness. J. Neurosci. 1997, 17, 7480–7489. [Google Scholar] [CrossRef] [PubMed]
- Hagen, M.C.; Franzén, O.; McGlone, F.; Essick, G.; Dancer, C.; Pardo, J.V. Tactile motion activates the human middle temporal/V5 (MT/V5) complex. Eur. J. Neurosci. 2002, 16, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.O.; Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 2002, 415, 429–433. [Google Scholar] [CrossRef]
- Van Den Bos, E.; Jeannerod, M. Sense of body and sense of action both contribute to self-recognition. Cognition 2002, 85, 177–187. [Google Scholar] [CrossRef]
- Kalckert, A.; Henrik Ehrsson, H. Moving a rubber hand that feels like your own: A dissociation of ownership and agency. Front. Hum. Neurosci. 2012, 6, 1–14. [Google Scholar] [CrossRef]
- Rognini, G.; Petrini, F.M.; Raspopovic, S.; Valle, G.; Granata, G.; Strauss, I.; Solcà, M.; Bello-Ruiz, J.; Herbelin, B.; Mange, R.; et al. Multisensory bionic limb to achieve prosthesis embodiment and reduce distorted phantom limb perceptions. J. Neurol. Neurosurg. Psychiatry 2019, 90, 833–836. [Google Scholar] [CrossRef]
- Hill, A. Phantom limb pain: A review of the literature on attributes and potential mechanisms. J. Pain Symptom Manag. 1999, 17, 125–142. [Google Scholar] [CrossRef]
- Alahakone, A.U.; Senanayake, S.M.N.A. Vibrotactile feedback systems: Current trends in rehabilitation, sports and information display. In Proceedings of the 2009 IEEE/ASME International Conference on Advanced Intelligent Mechatronics, Singapore, 14–17 July 2009; pp. 1148–1153. [Google Scholar] [CrossRef]
- Bramati, I.E.; Rodrigues, E.C.; Simões, E.L.; Melo, B.; Höfle, S.; Moll, J.; Lent, R.; Tovar-Moll, F. Lower limb amputees undergo long-distance plasticity in sensorimotor functional connectivity. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Kaas, J.H.; Nelson, R.J.; Sur, M.; Dykes, R.W.; Merzenich, M.M. The somatotopic organization of the ventroposterior thalamus of the squirrel monkey, Saimiri sciureus. J. Comp. Neurol. 1984, 226, 111–140. [Google Scholar] [CrossRef]
- Ackerley, R.; Hassan, E.; Curran, A.; Wessberg, J.; Olausson, H.; McGlone, F. An fMRI study oncortical responses during active self-touch and passive touch from others. Front. Behav. Neurosci. 2012, 6, 1–9. [Google Scholar] [CrossRef]
- Disbrow, E.; Roberts, T.; Krubitzer, L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: Evidence for SII and PV. J. Comp. Neurol. 2000, 418, 1–21. [Google Scholar] [CrossRef]
- Francis, S.T.; Kelly, E.F.; Bowtell, R.; Dunseath, W.J.R.; Folger, S.E.; McGlone, F. fMRI of the responses to vibratory stimulation of digit tips. NeuroImage 2000, 11, 188–202. [Google Scholar] [CrossRef]
- Ruben, J.; Schwiemann, J.; Deuchert, M.; Meyer, R.; Krause, T.; Curio, G.; Villringer, K.; Kurth, R.; Villringer, A. Somatotopic organization of human secondary somatosensory cortex. Cereb. Cortex 2001, 11, 463–473. [Google Scholar] [CrossRef]
- Penfield, W.; Boldrey, E. Somatic Motor And Sensory Representation In The Cerebral Cortex Of Man As Studied By Electrical Stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Llinás, R.R.; Ribary, U.; Jeanmonod, D.; Kronberg, E.; Mitra, P.P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA 1999, 96, 15222–15227. [Google Scholar] [CrossRef] [PubMed]
- Hara, E.S.; Witzel, A.L.; de Luca, C.E.P.; Ballester, R.Y.; Kuboki, T.; Bolzan, M.C. A novel vibratory stimulation-based occlusal splint for alleviation of TMD painful symptoms: A pilot study. J. Oral Rehabil. 2013, 40, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.A.; Hollins, M.; Maixner, W. Reduction of TMD pain by high-frequency vibration: A spatial and temporal analysis. Pain 2003, 101, 267–274. [Google Scholar] [CrossRef]
- List, E.B.; Krijgh, D.D.; Martin, E.; Coert, J.H. Prevalence of residual limb pain and symptomatic neuromas after lower extremity amputation: A systematic review and meta-analysis. Pain 2021, 162, 1906–1913. [Google Scholar] [CrossRef]
- Meade, Z.S.; Likens, A.D.; Kent, J.A.; Takahashi, K.Z.; Wurdeman, S.R.; Jacobsen, A.L.; Hernandez, M.E.; Stergiou, N. Subthreshold Vibration Influences Standing Balance but Has Unclear Impact on Somatosensation in Persons With Transtibial Amputations. Front. Physiol. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Cochrane, D.J.; Cochrane, F.; Roake, J.A. An exploratory study of vibration therapy on muscle function in patients with peripheral artery disease. J. Vasc. Surg. 2020, 71, 1340–1345. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.P.; Sánchez-Herrera-Baeza, P.; Cano-de-la Cuerda, R.; Camacho-Montaño, L.R.; Serrada-Tejeda, S.; Pérez-de Heredia-Torres, M. Effects of Intensive Vibratory Treatment with a Robotic System on the Recovery of Sensation and Function in Patients with Subacute and Chronic Stroke: A Non-Randomized Clinical Trial. J. Clin. Med. 2022, 11, 3572. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Ghilardi, M.F.; Poizner, H.; Ghez, C. Control of limb dynamics in normal subjects and patients without proprioception. J. Neurophysiol. 1995, 73, 820–835. [Google Scholar] [CrossRef]
- Resnik, L.; Meucci, M.R.; Lieberman-Klinger, S.; Fantini, C.; Kelty, D.L.; Disla, R.; Sasson, N. Advanced upper limb prosthetic devices: Implications for upper limb prosthetic rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 710–717. [Google Scholar] [CrossRef]
- Graczyk, E.L.; Resnik, L.; Schiefer, M.A.; Schmitt, M.S.; Tyler, D.J. Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Biddiss, E.; Chau, T. Upper-limb prosthetics: Critical factors in device abandonment. Am. J. Phys. Med. Rehabil. 2007, 86, 977–987. [Google Scholar] [CrossRef]
- Petrini, F.M.; Bumbasirevic, M.; Valle, G.; Ilic, V.; Mijović, P.; Čvančara, P.; Barberi, F.; Katic, N.; Bortolotti, D.; Andreu, D.; et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 2019, 25, 1356–1363. [Google Scholar] [CrossRef]
- Raspopovic, S.; Capogrosso, M.; Petrini, F.M.; Bonizzato, M.; Rigosa, J.; Di Pino, G.; Carpaneto, J.; Controzzi, M.; Boretius, T.; Fernandez, E.; et al. Restoring Natural Sensory Feedback in Real-Time Bidirectional Hand Prostheses. Sci. Transl. Med. 2014, 6, 222ra19. [Google Scholar] [CrossRef]
- Oddo, C.M.; Raspopovic, S.; Artoni, F.; Mazzoni, A.; Spigler, G.; Petrini, F.; Giambattistelli, F.; Vecchio, F.; Miraglia, F.; Zollo, L.; et al. Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. eLife 2016, 5, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.E.; Dragomir, A.; Betthauser, J.L.; Hunt, C.L.; Nguyen, H.H.; Kaliki, R.R.; Thakor, N.V. Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain. Sci. Robot. 2018, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valle, G.; Mazzoni, A.; Iberite, F.; D’Anna, E.; Strauss, I.; Granata, G.; Controzzi, M.; Clemente, F.; Rognini, G.; Cipriani, C.; et al. Biomimetic Intraneural Sensory Feedback Enhances Sensation Naturalness, Tactile Sensitivity, and Manual Dexterity in a Bidirectional Prosthesis. Neuron 2018, 100, 37–45.e7. [Google Scholar] [CrossRef] [PubMed]
- George, J.A.; Kluger, D.T.; Davis, T.S.; Wendelken, S.M.; Okorokova, E.V.; He, Q.; Duncan, C.C.; Hutchinson, D.T.; Thumser, Z.C.; Beckler, D.T.; et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 2019, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.W.; Schiefer, M.A.; Keith, M.W.; Anderson, J.R.; Tyler, J.; Tyler, D.J. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 2014, 6, 257ra138. [Google Scholar] [CrossRef]
- Oddsson, L.I.E.; Bisson, T.; Cohen, H.S.; Jacobs, L.; Khoshnoodi, M.; Kung, D.; Lipsitz, L.A.; Manor, B.; McCracken, P.; Rumsey, Y.; et al. The Effects of a Wearable Sensory Prosthesis on Gait and Balance Function After 10 Weeks of Use in Persons With Peripheral Neuropathy and High Fall Risk—The Walk2Wellness Trial. Front. Aging Neurosci. 2020, 12, 1–21. [Google Scholar] [CrossRef]
- Koehler-McNicholas, S.R.; Danzl, L.; Cataldo, A.Y.; Oddsson, L.I.E. Neuromodulation to improve gait and balance function using a sensory neuroprosthesis in people who report insensate feet—A randomized control crossover study. PLoS ONE 2019, 14, e216212. [Google Scholar] [CrossRef]
- Ortiz-Catalan, M.; Håkansson, B.; Brånemark, R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci. Transl. Med. 2014, 6, 257re6. [Google Scholar] [CrossRef]
- Page, D.M.; George, J.A.; Kluger, D.T.; Duncan, C.; Wendelken, S.; Davis, T.; Hutchinson, D.T.; Clark, G.A. Motor Control and Sensory Feedback Enhance Prosthesis Embodiment and Reduce Phantom Pain After Long-Term Hand Amputation. Front. Hum. Neurosci. 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Clippinger, F.W.; Avery, R.; Titus, B.R. A sensory feedback system for an upper-limb amputation prosthesis. Bull. Prosthetics Res. 1974, 1974, 247–258. [Google Scholar]
- Clippinger, F.W.; Seaber, A.V.; McElhaney, J.H.; Harrelson, J.M.; Maxwell, G.M. Afferent sensory feedback for lower extremity prosthesis. Clin. Orthop. Relat. Res. 1982, 169, 202–208. [Google Scholar] [CrossRef]
- Markovic, M.; Schweisfurth, M.A.; Engels, L.F.; Bentz, T.; Wüstefeld, D.; Farina, D.; Dosen, S. The clinical relevance of advanced artificial feedback in the control of a multi-functional myoelectric prosthesis. J. NeuroEngineering Rehabil. 2018, 15, 28. [Google Scholar] [CrossRef]
- Wendelken, S.; Page, D.M.; Davis, T.; Wark, H.A.C.; Kluger, D.T.; Duncan, C.; Warren, D.J.; Hutchinson, D.T.; Clark, G.A. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves. J. NeuroEngineering Rehabil. 2017, 14, 1–17. [Google Scholar] [CrossRef]
- Schiefer, M.; Tan, D.; Sidek, S.M.; Tyler, D.J. Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J. Neural Eng. 2016, 13, 016001. [Google Scholar] [CrossRef]
- Osborn, L.E.; Ding, K.; Hays, M.A.; Bose, R.; Iskarous, M.M.; Dragomir, A.; Tayeb, Z.; Lévay, G.M.; Hunt, C.L.; Cheng, G.; et al. Sensory stimulation enhances phantom limb perception and movement decoding. J. Neural Eng. 2020, 17, 056006. [Google Scholar] [CrossRef]
- Schultz, A.E.; Marasco, P.D.; Kuiken, T.A. Vibrotactile detection thresholds for chest skin of amputees following targeted reinnervation surgery. Brain Res. 2009, 1251, 121–129. [Google Scholar] [CrossRef]
- Marasco, P.D.; Schultz, A.E.; Kuiken, T.A. Sensory capacity of reinnervated skin after redirection of amputated upper limb nerves to the chest. Brain 2009, 132, 1441–1448. [Google Scholar] [CrossRef]
- Hebert, J.S.; Chan, K.M.; Dawson, M.R. Cutaneous sensory outcomes from three transhumeral targeted reinnervation cases. Prosthetics Orthot. Int. 2016, 40, 303–310. [Google Scholar] [CrossRef]
- Hebert, J.S.; Olson, J.L.; Morhart, M.J.; Dawson, M.R.; Marasco, P.D.; Kuiken, T.A.; Chan, K.M. Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 765–773. [Google Scholar] [CrossRef]
- Marasco, P.D.; Kim, K.; Colgate, J.E.; Peshkin, M.A.; Kuiken, T.A. Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain 2011, 134, 747–758. [Google Scholar] [CrossRef]
- Hebert, J.S.; Elzinga, K.; Chan, K.M.; Olson, J.; Morhart, M. Updates in Targeted Sensory Reinnervation for Upper Limb Amputation. Curr. Surg. Rep. 2014, 2, 1–9. [Google Scholar] [CrossRef]
- Marasco, P.D.; Hebert, J.S.; Sensinger, J.W.; Beckler, D.T.; Thumser, Z.C.; Shehata, A.W.; Williams, H.E.; Wilson, K.R. Neurorobotic fusion of prosthetic touch, kinesthesia, and movement in bionic upper limbs promotes intrinsic brain behaviors. Sci. Robot. 2021, 6, abf3368. [Google Scholar] [CrossRef] [PubMed]
- Gardetto, A.; Baur, E.M.; Prahm, C.; Smekal, V.; Jeschke, J.; Peternell, G.; Pedrini, M.T.; Kolbenschlag, J. Reduction of phantom limb pain and improved proprioception through a tsr-based surgical technique: A case series of four patients with lower limb amputation. J. Clin. Med. 2021, 10, 4029. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Di Iorio, R.; Miraglia, F.; Caulo, M.; Iodice, F.; Vecchio, F.; Valle, G.; Strauss, I.; D’Anna, E.; Iberite, F.; et al. Brain reactions to the use of sensorized hand prosthesis in amputees. Brain Behav. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Dietrich, C.; Walter-Walsh, K.; Preißler, S.; Hofmann, G.O.; Witte, O.W.; Miltner, W.H.R.; Weiss, T. Sensory feedback prosthesis reduces phantom limb pain: Proof of a principle. Neurosci. Lett. 2012, 507, 97–100. [Google Scholar] [CrossRef]
- Serino, A.; Akselrod, M.; Salomon, R.; Martuzzi, R.; Blefari, M.L.; Canzoneri, E.; Rognini, G.; Van Der Zwaag, W.; Iakova, M.; Luthi, F.; et al. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain 2017, 140, 2993–3011. [Google Scholar] [CrossRef]
- Dietrich, C.; Nehrdich, S.; Seifert, S.; Blume, K.R.; Miltner, W.H.R.; Hofmann, G.O.; Weiss, T. Leg prosthesis with somatosensory feedback reduces phantom limb pain and increases functionality. Front. Neurol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Münte, T.F.; Jöbges, E.M.; Wieringa, B.M.; Klein, S.; Schubert, M.; Johannes, S.; Dengler, R. Human evoked potentials to long duration vibratory stimuli: Role of muscle afferents. Neurosci. Lett. 1996, 216, 163–166. [Google Scholar] [CrossRef]
- Bark, K.; Wheeler, J.W.; Premakumar, S.; Cutkosky, M.R. Comparison of skin stretch and vibrotactile stimulation for feedback of proprioceptive information. In Proceedings of the 2008 Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systemsm, Reno, NV, USA, 13–14 March 2008; pp. 71–78. [Google Scholar] [CrossRef]
- Meyers, E.C.; Kasliwal, N.; Solorzano, B.R.; Lai, E.; Bendale, G.; Berry, A.; Ganzer, P.D.; Romero-Ortega, M.; Rennaker, R.L.; Kilgard, M.P.; et al. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Ganzer, P.D.; Darrow, M.J.; Meyers, E.C.; Solorzano, B.R.; Ruiz, A.D.; Robertson, N.M.; Adcock, K.S.; James, J.T.; Jeong, H.S.; Becker, A.M.; et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. eLife 2018, 7, 1–19. [Google Scholar] [CrossRef]
- Meyers, E.C.; Solorzano, B.R.; James, J.; Ganzer, P.D.; Lai, E.S.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke 2018, 49, 710–717. [Google Scholar] [CrossRef]
- Porter, B.A.; Khodaparast, N.; Fayyaz, T.; Cheung, R.J.; Ahmed, S.S.; Vrana, W.A.; Rennaker, R.L.; Kilgard, M.P. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb. Cortex 2012, 22, 2365–2374. [Google Scholar] [CrossRef]
- Engineer, N.D.; Kimberley, T.J.; Prudente, C.N.; Dawson, J.; Tarver, W.B.; Hays, S.A. Targeted vagus nerve stimulation for rehabilitation after stroke. Front. Neurosci. 2019, 13, 1–18. [Google Scholar] [CrossRef]
- Darrow, M.J.; Mian, T.M.; Torres, M.; Haider, Z.; Danaphongse, T.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. Restoration of Somatosensory Function by Pairing Vagus Nerve Stimulation with Tactile Rehabilitation. Ann. Neurol. 2020, 87, 194–205. [Google Scholar] [CrossRef]
- Kilgard, M.P.; Rennaker, R.L.; Alexander, J.; Dawson, J. Vagus nerve stimulation paired with tactile training improved sensory function in a chronic stroke patient. NeuroRehabilitation 2018, 42, 159–165. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Li, S.; Niu, L.; Ma, J.; Wen, L.; Zhang, L.; Li, C. 7nAchR mediates transcutaneous auricular vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by enhancing axonal plasticity. Neurosci. Lett. 2020, 730, 135031. [Google Scholar] [CrossRef]
- Lu, X.X.; Hong, Z.Q.; Tan, Z.; Sui, M.H.; Zhuang, Z.Q.; Liu, H.H.; Zheng, X.Y.; Yan, T.B.; Geng, D.F.; Jin, D.M. Nicotinic acetylcholine receptor alpha7 subunit mediates vagus nerve stimulation-induced neuroprotection in acute permanent cerebral ischemia by a7nAchR/JAK2 pathway. Med Sci. Monit. 2017, 23, 6072–6081. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, Q.; Zhou, X.; Wen, L.; Ma, J.; Niu, L.; Li, C. PPAR-γ Mediates Ta-VNS-Induced Angiogenesis and Subsequent Functional Recovery after Experimental Stroke in Rats. BioMed Res. Int. 2020, 2020, 8163789. [Google Scholar] [CrossRef]
- Shields, R.W. Functional Anatomy of the Autonomic Nervous System. J. Clin. Neurophysiol. 1993, 10, 2–13. [Google Scholar] [CrossRef]
- Pembroke, P. The anatomy of the vagus nerve in the cervical area in man. J. Anat. 1971, 110, 502. [Google Scholar] [PubMed]
- George, M.S.; Aston-Jones, G. Noninvasive techniques for probing neurocircuitry and treating illness: Vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology 2010, 35, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Stocker, S.D.; Sved, A.F.; Andresen, M.C. Missing pieces of the Piezo1/Piezo2 baroreceptor hypothesis: An autonomic perspective. J. Neurophysiol. 2019, 122, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.; Figueroa, A.; Robinson-Papp, J. Structural and functional connections between the autonomic nervous system, hypothalamic–pituitary–adrenal axis, and the immune system: A context and time dependent stress response network. Neurol. Sci. 2022, 43, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. Basic Clin. 2000, 85, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jänig, W. Sympathetic nervous system and inflammation: A conceptual view. Auton. Neurosci. Basic Clin. 2014, 182, 4–14. [Google Scholar] [CrossRef]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Howland, R.H. Vagus Nerve Stimulation. Curr. Behav. Neurosci. Rep. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part III. Headache: J. Head Face Pain 2016, 56, 479–490. [Google Scholar] [CrossRef]
- Mohanta, S.K.; Peng, L.; Li, Y.; Lu, S.; Sun, T.; Carnevale, L.; Perrotta, M.; Ma, Z.; Förstera, B.; Stanic, K.; et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 2022, 605, 152–159. [Google Scholar] [CrossRef]
- Ammons, W.S.; Blair, R.W.; Foreman, R.D. Vagal afferent inhibition of primate thoracic spinothalamic neurons. J. Neurophysiol. 1983, 50, 926–940. [Google Scholar] [CrossRef]
- Randich, A.; Gebhart, G.F. Vagal afferent modulation of nociception. Brain Res. Rev. 1992, 17, 77–99. [Google Scholar] [CrossRef]
- Hummel, T.; Sengupta, J.N.; Meller, S.T.; Gebhart, G.F. Responses of T2-4 spinal cord neurons to irritation of the lower airways in the rat. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, R1147–R1157. [Google Scholar] [CrossRef]
- Dorr, A.E.; Debonnel, G. Effect of Vagus Nerve Stimulation on Serotonergic and Noradrenergic Transmission. J. Pharmacol. Exp. Ther. 2006, 318, 890–898. [Google Scholar] [CrossRef]
- Manta, S.; Dong, J.; Debonnel, G.; Blier, P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psychiatry Neurosci. 2009, 34, 272–280. [Google Scholar]
- Mesquita, R.N.O.; Taylor, J.L.; Trajano, G.S.; Škarabot, J.; Holobar, A.; Gonçalves, B.A.M.; Blazevich, A.J. Effects of reciprocal inhibition and whole-body relaxation on persistent inward currents estimated by two different methods. J. Physiol. 2022, 600, 2765–2787. [Google Scholar] [CrossRef]
- Thorstensen, J.R. Persistent inward currents in spinal motoneurones: How can we study them in human participants? J. Physiol. 2022. [Google Scholar] [CrossRef]
- Burke, S.D.; Jordan, J.; Harrison, D.G.; Ananth Karumanchi, S. Solving baroreceptor mystery: Role of PIEZO ion channels. J. Am. Soc. Nephrol. 2019, 30, 911–913. [Google Scholar] [CrossRef]
- Dampney, R.A.L.; Horiuchi, J.; Tagawa, T.; Fontes, M.A.P.; Potts, P.D.; Polson, J.W. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol. Scand. 2003, 177, 209–218. [Google Scholar] [CrossRef]
- Moruzzi, G.; Magoun, H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1949, 1, 455–473. [Google Scholar] [CrossRef]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Luccarini, P.; Gahery, Y.; Pompeiano, O. Cholinoceptive pontine reticular structures modify the postural adjustments during the limb movements induced by cortical stimulation. Arch. Ital. De Biol. 1990, 128, 19–45. [Google Scholar]
- Quessy, S.; Freedman, E.G. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis: I. Characteristics of evoked head movements. Exp. Brain Res. 2004, 156, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; Drew, T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J. Neurophysiol. 2006, 96, 2229–2252. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Chiba, R.; Nozu, T.; Okumura, T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J. Neural Transm. 2016, 123, 695–729. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Barton, E.J.; Gandhi, N.J.; Nelson, J. Studies of the role of the paramedian pontine reticular formation in the control of head-restrained and head-unrestrained gaze shifts. Ann. N. Y. Acad. Sci. 2002, 956, 85–98. [Google Scholar] [CrossRef]
- Fung, S.J.; Chan, J.Y.H.; Manzoni, D.; White, S.R.; Lai, Y.Y.; Strahlendorf, H.K.; Zhuo, H.; Liu, R.H.; Reddy, V.K.; Barnes, C.D. Cotransmitter-mediated locus coeruleus action on motoneurons. Brain Res. Bull. 1994, 35, 423–432. [Google Scholar] [CrossRef]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef]
- Pompeiano, O.; Horn, E.; D’Ascanio, P. Locus Coeruleus and Dorsal Pontine Reticular Influences on the Gain of Vestibulospinal Reflexes; Elsevier B.V.: Amsterdam, The Netherlands, 1991; Volume 88, pp. 435–462. [Google Scholar] [CrossRef]
- van Neerven, J.; Pompeiano, O.; Collewijn, H. Effects of GABAergic and Noradrenergic Injections into the Cerebellar Flocculus on Vestibulo-Ocular Reflexes in the Rabbit; Elsevier B.V.: Amsterdam, The Netherlands, 1991; Volume 88, pp. 485–497. [Google Scholar] [CrossRef]
- Pompeiano, O.; Manzoni, D.; D’ascanio, P.; Andre, P. Noradrenergic agents in the cerebellar vermis affect adaptation of the vestibulospinal reflex gain. Brain Res. Bull. 1994, 35, 433–444. [Google Scholar] [CrossRef]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Sara, S.J. Locus Coeruleus in time with the making of memories. Curr. Opin. Neurobiol. 2015, 35, 87–94. [Google Scholar] [CrossRef]
- Sara, S.J.; Bouret, S. Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron 2012, 76, 130–141. [Google Scholar] [CrossRef]
- Jurič, D.M.; Miklič, Š.; Čarman-Kržan, M. Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res. 2006, 1108, 54–62. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and memory processing. Neuropharmacology 2014, 76, 677–683. [Google Scholar] [CrossRef]
- McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016, 165, 291–299. [Google Scholar] [CrossRef]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef]
- Steriade, M. Brain activation, then (1949) and now: Coherent fast rhythms in corticothalamic networks. Arch. Ital. De Biol. 1995, 134, 5–20. [Google Scholar]
- Uhlhaas, P.J.; Pipa, G.; Lima, B.; Melloni, L.; Neuenschwander, S.; Nikolić, D.; Singer, W. Neural synchrony in cortical networks: History, concept and current status. Front. Integr. Neurosci. 2009, 3, 1–19. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A NeurOpsychological Theory.; Wiley: Oxford, UK, 1949; p. 335. [Google Scholar]
- Löwel, S.; Singer, W. Selection of Intrinsic Horizontal Connections in the Visual Cortex by Correlated Neuronal Activity. Science 1992, 255, 209–212. [Google Scholar] [CrossRef]
- Tyler, M.E.; Kaczmarek, K.A.; Rust, K.L.; Subbotin, A.M.; Skinner, K.L.; Danilov, Y.P. Non-invasive neuromodulation to improve gait in chronic multiple sclerosis: A randomized double blind controlled pilot trial. J. NeuroEngineering Rehabil. 2014, 11, 1–10. [Google Scholar] [CrossRef]
- Tramonti Fantozzi, M.P.; Artoni, F.; Di Galante, M.; Briscese, L.; De Cicco, V.; Bruschini, L.; D’Ascanio, P.; Manzoni, D.; Faraguna, U.; Carboncini, M.C. Effect of the Trigeminal Nerve Stimulation on Auditory Event-Related Potentials. Cereb. Cortex Commun. 2021, 2, 1–14. [Google Scholar] [CrossRef]
- D’Arcy, R.C.N.; Greene, T.; Greene, D.; Frehlick, Z.; Fickling, S.D.; Campbell, N.; Etheridge, T.; Smith, C.; Bollinger, F.; Danilov, Y.; et al. Portable neuromodulation induces neuroplasticity to re-activate motor function recovery from brain injury: A high-density MEG case study. J. NeuroEngineering Rehabil. 2020, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Mao, Y.R.; Yuan, T.F.; Xu, D.S.; Cheng, L.M. Multimodal treatment for spinal cord injury: A sword of neuroregeneration upon neuromodulation. Neural Regen. Res. 2020, 15, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Makin, T.R.; Scholz, J.; Filippini, N.; Henderson Slater, D.; Tracey, I.; Johansen-Berg, H. Phantom pain is associated with preserved structure and function in the former hand area. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.L.; Bramati, I.; Rodrigues, E.; Franzoi, A.; Moll, J.; Lent, R.; Tovar-Moll, F. Functional expansion of sensorimotor representation and structural reorganization of callosal connections in lower limb amputees. J. Neurosci. 2012, 32, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H.; Rosén, B.; Stockselius, A.; Ragnö, C.; Köhler, P.; Lundborg, G. Upper limb amputees can be induced to experience a rubber hand as their own. Brain 2008, 131, 3443–3452. [Google Scholar] [CrossRef]
- Shokur, S.; O’Doherty, J.E.; Winans, J.A.; Bleuler, H.; Lebedev, M.A.; Nicolelis, M.A.L. Expanding the primate body schema in sensorimotor cortex by virtual touches of an avatar. Proc. Natl. Acad. Sci. USA 2013, 110, 15121–15126. [Google Scholar] [CrossRef]
- Beech, D.J. Endothelial Piezo1 channels as sensors of exercise. J. Physiol. 2018, 596, 979–984. [Google Scholar] [CrossRef]
- Ghazi, M.; Rippetoe, J.; Chandrashekhar, R.; Wang, H. Focal vibration therapy: Vibration parameters of effective wearable devices. Appl. Sci. 2021, 11, 2969. [Google Scholar] [CrossRef]
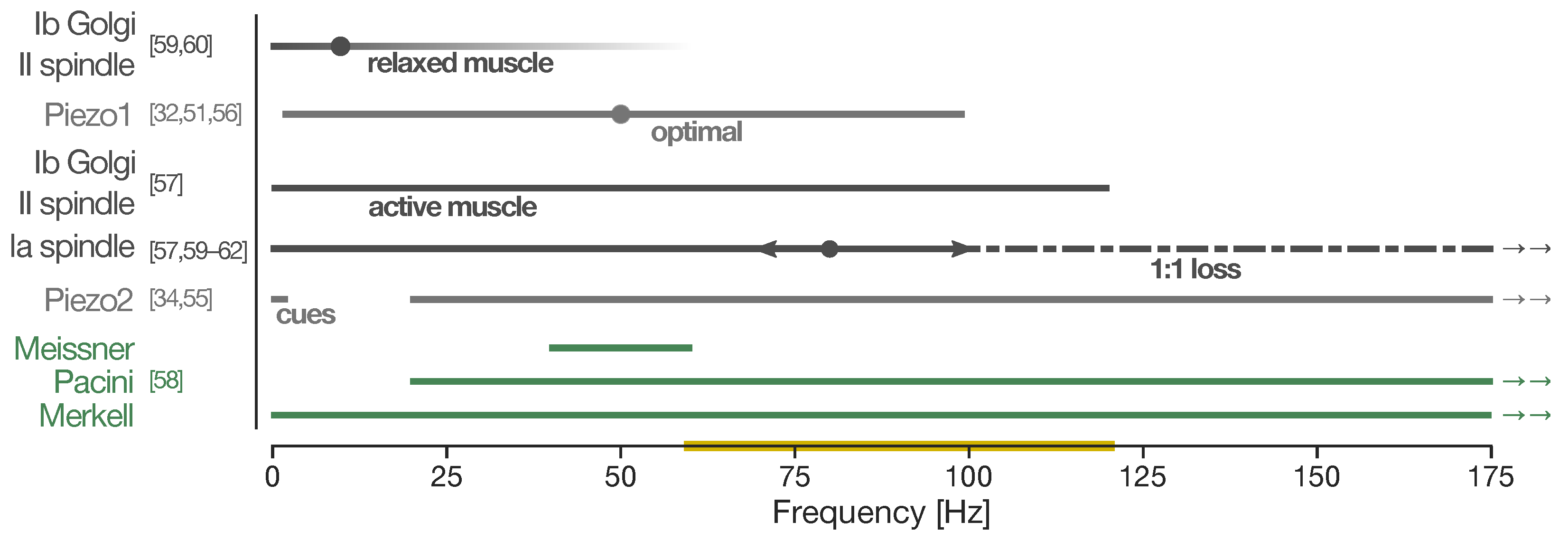

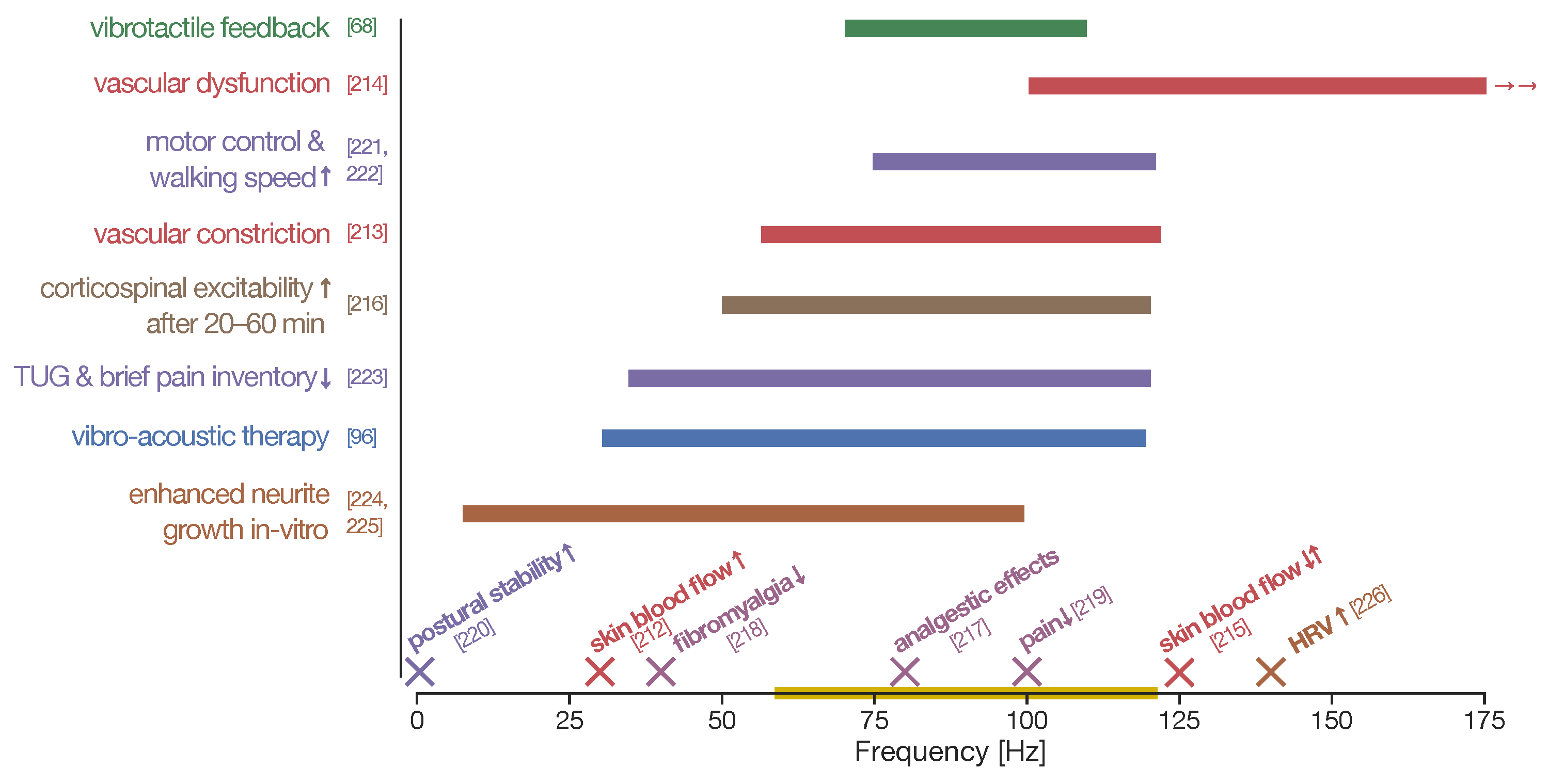
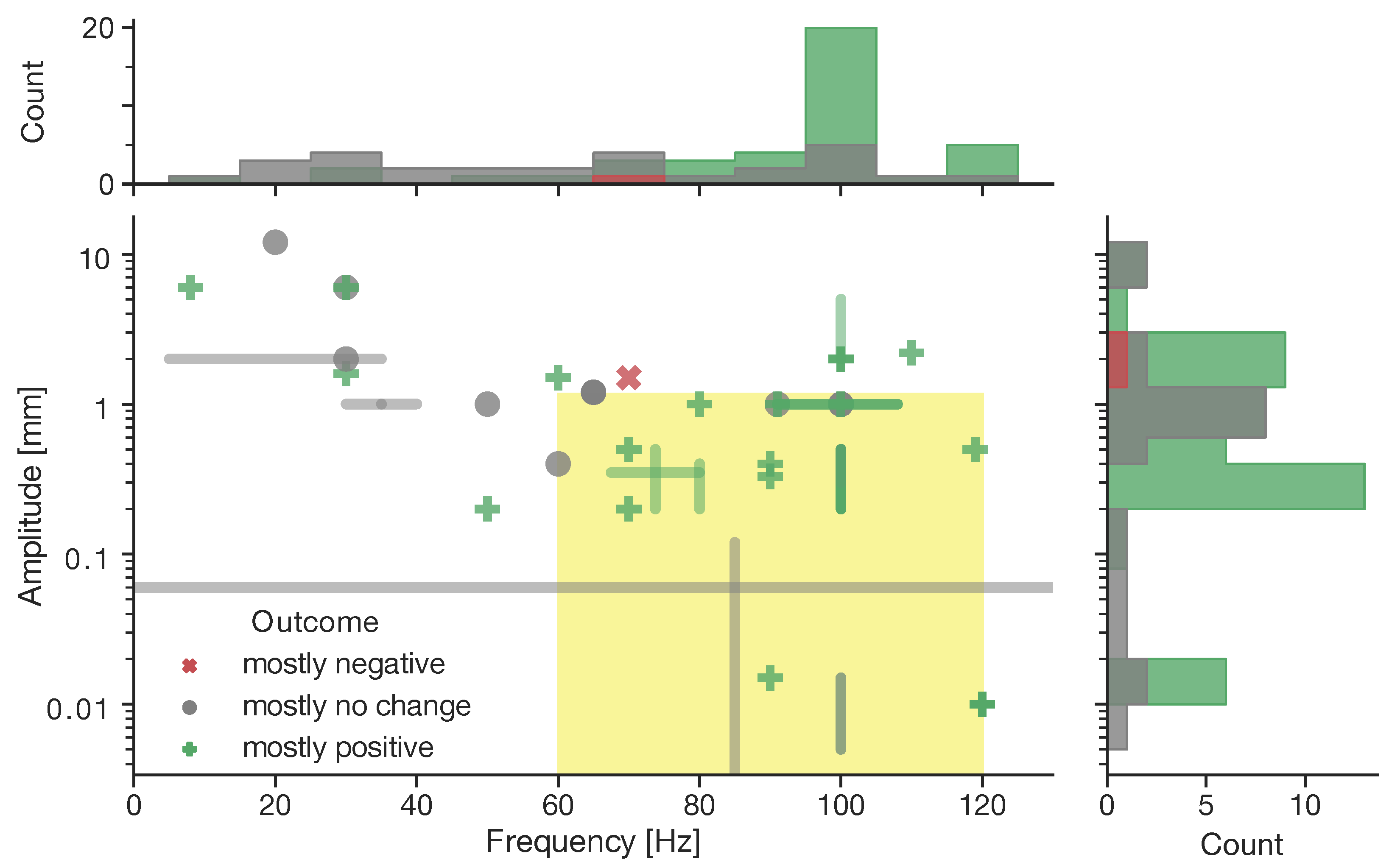
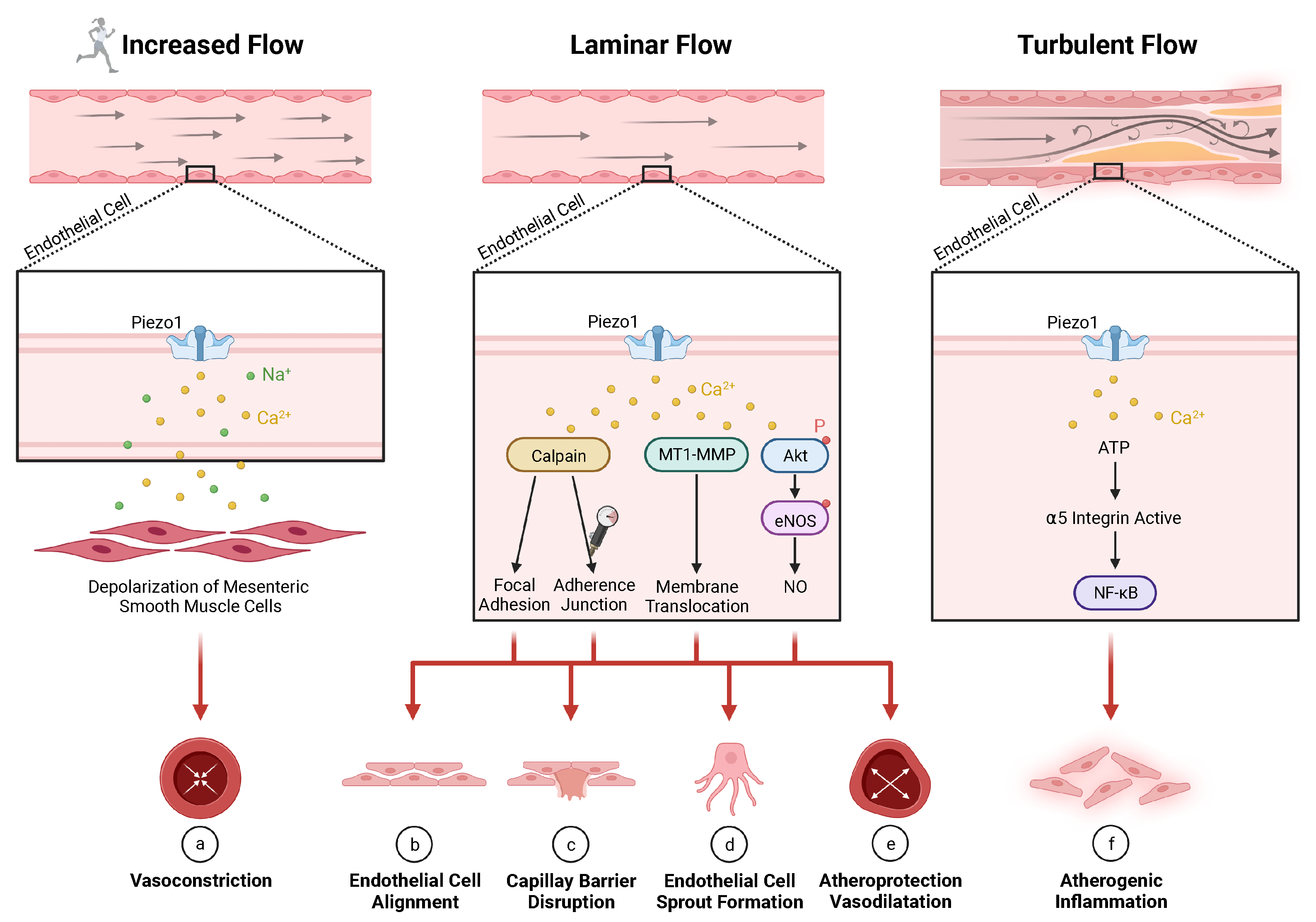
| Location | Activation | Function | |
|---|---|---|---|
| [2,12,15,32,75,76,77,78,79,80,81,82,83] | [2,17,18,19,20,21,22,23,28,32,40,85,86,87,88,89,90] | [15,18,28,32,33,34,35,36,40,41,48,51,80,86,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] | |
|
|
|
|
| Location | Activation | Function | |
|---|---|---|---|
| [2,10,12,32,55,86,125,126,127,128,129,130,131] | [10,12,31,32,34,50,51,55,132] | [10,12,13,31,33,54,55,74,130,133,134,135,136] | |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penasso, H.; Petersen, F.; Peternell, G. Vascular and Neural Response to Focal Vibration, Sensory Feedback, and Piezo Ion Channel Signaling. J. Vasc. Dis. 2023, 2, 42-90. https://doi.org/10.3390/jvd2010006
Penasso H, Petersen F, Peternell G. Vascular and Neural Response to Focal Vibration, Sensory Feedback, and Piezo Ion Channel Signaling. Journal of Vascular Diseases. 2023; 2(1):42-90. https://doi.org/10.3390/jvd2010006
Chicago/Turabian StylePenasso, Harald, Frederike Petersen, and Gerfried Peternell. 2023. "Vascular and Neural Response to Focal Vibration, Sensory Feedback, and Piezo Ion Channel Signaling" Journal of Vascular Diseases 2, no. 1: 42-90. https://doi.org/10.3390/jvd2010006
APA StylePenasso, H., Petersen, F., & Peternell, G. (2023). Vascular and Neural Response to Focal Vibration, Sensory Feedback, and Piezo Ion Channel Signaling. Journal of Vascular Diseases, 2(1), 42-90. https://doi.org/10.3390/jvd2010006






