Hormonal Influences on Psilocybin Responsivity Across the Female Lifespan: Toward Personalized Psychedelic-Assisted Therapy
Abstract
1. Introduction
2. Gender Disparities in Mental Health Disorders
3. Psilocybin: From Psychedelic Drug to Treatment Option
- P. cubensis: Abundant in tropical and subtropical regions, it is the most widely known, cultivated, and distributed for recreational and therapeutic use.
- P. semilanceata: Found in Europe, North America, and New Zealand, this species has the highest content of psilocybin. While this is the most potent, its complex growth requirement makes its distribution challenging.
- P. cyanescens: Similarly to P. semilanceata, this species is found in temperate regions, but it thrives in urban and suburban environments. It is recognized as the one with the most abundant psychoactive content, among the listed species.
- P. mexicana: This species holds historical importance, since its first use in Aztec religious practices and the several studies, including ethnomycological, that drew continued attention since the 1950s, but it is considered one of the least potent based on psilocybin content.
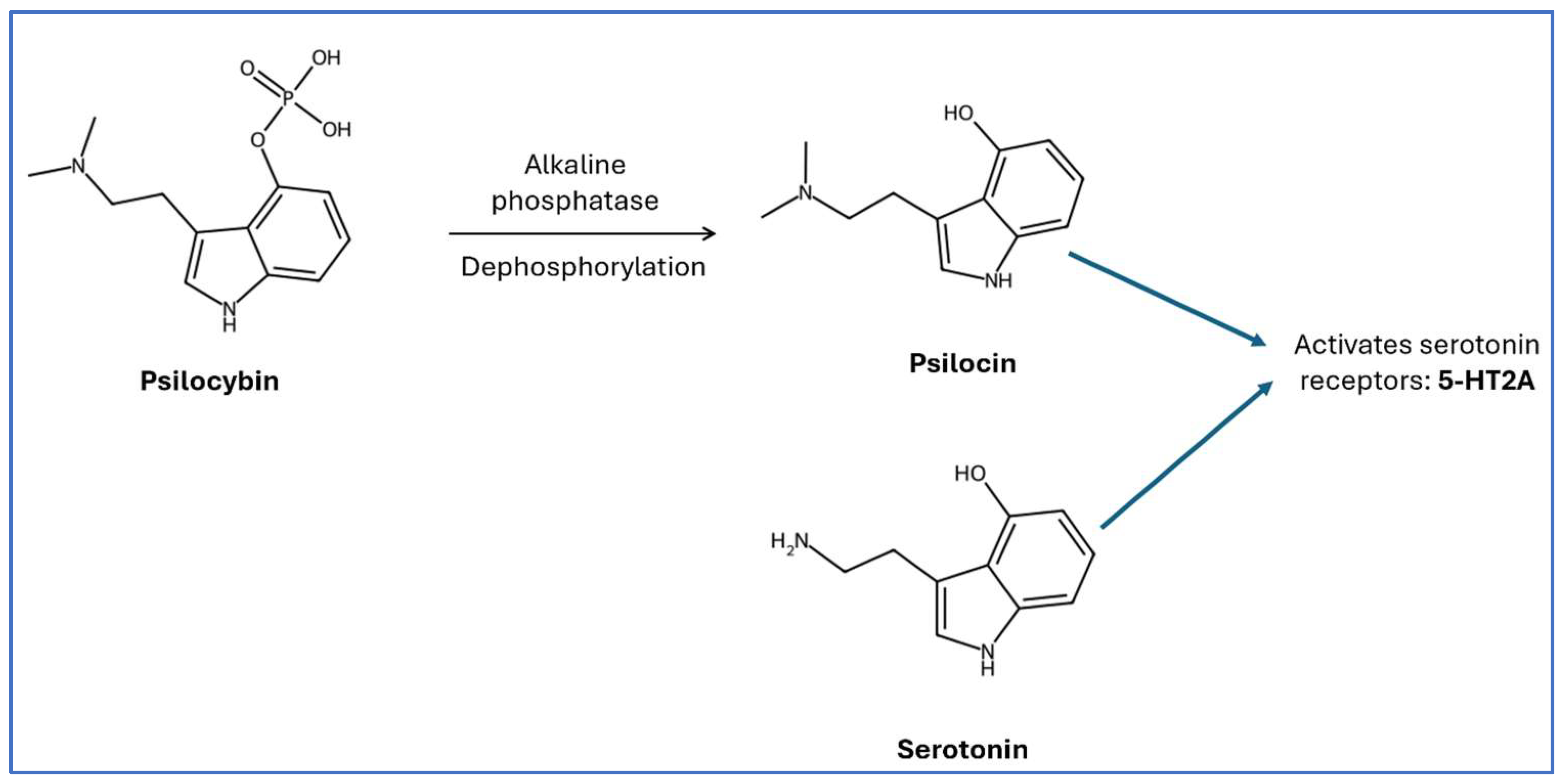
4. Psilocybin: Mechanism of Action
5. The Interplay of Sex Hormones, Serotonergic System, and Psilocin
5.1. Estrogen
5.2. Testosterone
6. The Potential Effects of Psilocin Across Different Reproductive Stages in Females
6.1. Puberty and Menstrual Cycle
6.2. Pregnancy and Contraception
6.3. Menopause
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDD | major depressive disorder |
| PTSD | post-traumatic stress disorder |
| BBB | Blood–brain barrier |
| LSD | Lysergic acid diethylamide |
| PCOS | Polycystic ovarian syndrome |
| OCD | Obsessive–compulsive disorder |
| HPLC | High-performance liquid chromatography |
| IP3 | Inositol triphosphate |
| DAG | Diacylglycerol |
| HRT | Hormonal replacement therapy |
| PLC | Phospholipase C |
| E1 | Estrone |
| E2 | 17β-estradiol |
| E3 | Estriol |
| E4 | Sterol |
| BDNF | Brain-Derived Neurotrophic Factor |
| mTOR | Mechanistic Target of Rapamycin |
| DMN | Default Mode Network |
| fMRI | Functional MRI |
| HPG | Hypothalamic-pituitary-gonadal axis |
| HC | Hormonal contraceptives |
| 5-HT | 5- hydroxytryptamine |
| 5-HT2A | Serotonergic receptor 5-HT subtype 2A |
| 5-HT1A | Serotonergic receptor 5-HT subtype 1A |
| 5-HT2C | Serotonergic receptor 5-HT subtype 2C |
| GPER | G protein-coupled estrogen receptor |
| Erα | Estrogen receptor α |
| Erβ | Estrogen receptor β |
| SSRIs | Selective serotonin reuptake inhibitors |
| SNRIs | Serotonin norepinephrine reuptake inhibitors |
| PFC | Prefrontal Cortex |
| TPH | Tryptophan hydroxylase |
References
- Nichols, D.E. Psilocybin: From ancient magic to modern medicine. J. Antibiot. 2020, 73, 679–686. [Google Scholar] [CrossRef]
- Goel, D.B.; Zilate, S. Potential Therapeutic Effects of Psilocybin: A Systematic Review. Cureus 2022, 14, e30214. [Google Scholar] [CrossRef]
- Turkia, M. Underground small-group therapy of treatment-resistant depression and complex post-traumatic stress disorder (C-PTSD) with psilocybin—A retrospective case study. Psychedelicther 2022, in press. [Google Scholar]
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol. Abuse. 2017, 43, 55–60. [Google Scholar] [CrossRef]
- Lehavot, K.; Katon, J.G.; Chen, J.A.; Fortney, J.C.; Simpson, T.L. Post-traumatic Stress Disorder by Gender and Veteran Status. Am. J. Prev. Med. 2018, 54, e1–e9. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Zheng, L.; Pang, H.; Zhang, Q.; Lou, L.; Huang, X. Sex Difference in Global Burden of Major Depressive Disorder: Findings From the Global Burden of Disease Study 2019. Front. Psychiatry 2022, 13, 789305. [Google Scholar] [CrossRef]
- Gradus, J.L.L.S.; Curreri, A.; Myers, L.G.; Ferguson, R.; Miller, M. Gender differences in substance abuse, PTSD and intentional self-harm among veterans health administration patients. Drug Alcohol Depend. 2017, 171, 66–69. [Google Scholar] [CrossRef]
- Piper, M.E.; Cook, J.W.; Schlam, T.R.; Jorenby, D.E.; Smith, S.S.; Bolt, D.M.; Loh, W.Y. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob. Res. 2010, 12, 647–657. [Google Scholar] [CrossRef]
- Agabio, R.C.I.; Pisanu, C.; Gessa, G.L.; Franconi, F. Sex differences in substance use disorders: Focus on side effects. Addict Biol. 2016, 21, 1030–1042. [Google Scholar] [CrossRef]
- Shadani, S.; Conn, K.; Andrews, Z.B.; Foldi, C.J. Potential Differences in Psychedelic Actions Based on Biological Sex. Endocrinology 2024, 165, bqae083. [Google Scholar] [CrossRef]
- Krystal, J.H.; Neumeister, A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009, 1293, 13–23. [Google Scholar] [CrossRef]
- Pannu, A. RKG Serotonin and Depression: Scrutiny of New Targets for Future Anti- Depressant Drug Development. Curr. Drug Targets. 2023, 24, 816–837. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benetatos, J.; Lewis, V.; Bonniwell, E.M.; Jaster, A.M.; Moliner, R.; Castrén, E.; McCorvy, J.D.; Palner, M.; Aguilar-Valles, A. Beyond the 5-HT(2A) Receptor: Classic and Nonclassic Targets in Psychedelic Drug Action. J. Neurosci. 2023, 43, 7472–7482. [Google Scholar] [CrossRef]
- Picco, L.; Subramaniam, M.; Abdin, E.; Vaingankar, J.A.; Chong, S.A. Gender differences in major depressive disorder: Findings from the Singapore Mental Health Study. Singap. Med. J. 2017, 58, 649–655. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Cai, Y.; Zheng, L.; Pang, H.; Lou, L. Sex difference in incidence of major depressive disorder: An analysis from the Global Burden of Disease Study 2019. Ann. Gen. Psychiatry 2023, 22, 53. [Google Scholar] [CrossRef]
- Cheng, Y.; Fang, Y.; Zheng, J.; Guan, S.; Wang, M.; Hong, W. The burden of depression, anxiety and schizophrenia among the older population in ageing and aged countries: An analysis of the Global Burden of Disease Study 2019. Gen. Psychiatr. 2024, 37, e101078. [Google Scholar] [CrossRef]
- Villarroel, M.A.; Terlizzi, E.P. Symptoms of Depression Among Adults: United States, 2019. NCHS Data Brief. 2020, 379, 1–8. [Google Scholar]
- Kundakovic, M.; Rocks, D. Sex hormone fluctuation and increased female risk for depression and anxiety disorders: From clinical evidence to molecular mechanisms. Front. Neuroendocrinol. 2022, 66, 101010. [Google Scholar] [CrossRef]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- Towers, E.B.; Hsu, K.A.; Qillawala, E.I.; Fraser, S.D.; Lynch, W.J. Sex Differences in the Development of an Opioid Addiction-Like Phenotype: A Focus on the Telescoping Effect. Biol. Psychiatry Glob. Open Sci. 2024, 4, 100373. [Google Scholar] [CrossRef]
- Bobzean, S.A.; DeNobrega, A.K.; Perrotti, L.I. Sex differences in the neurobiology of drug addiction. Exp. Neurol. 2014, 259, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Zakiniaeiz, Y.; Potenza, M.N. Gender-related differences in addiction: A review of human studies. Curr. Opin. Behav. Sci. 2018, 23, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Zywiak, W.H.; Stout, R.L.; Trefry, W.B.; Glasser, I.; Connors, G.J.; Maisto, S.A.; Westerberg, V.S. Alcohol relapse repetition, gender, and predictive validity. J. Subst. Abus. Treat. 2006, 30, 349–353. [Google Scholar] [CrossRef]
- Miller, L.J.; Girgis, C.; Gupta, R. Depression and related disorders during the female reproductive cycle. Womens Health 2009, 5, 577–587. [Google Scholar] [CrossRef]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef]
- Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam. Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef]
- Marazziti, D.; Mucci, F.; Tripodi, B.; Carbone, M.G.; Muscarella, A.; Falaschi, V.; Baroni, S. Emotional Blunting, Cognitive Impairment, Bone Fractures, and Bleeding as Possible Side Effects of Long-Term Use of SSRIs. Clin. Neuropsychiatry 2019, 16, 75–85. [Google Scholar]
- Healy, D.; Mangin, D. Post-SSRI sexual dysfunction: Barriers to quantifying incidence and prevalence. Epidemiol. Psychiatr. Sci. 2024, 33, e40. [Google Scholar] [CrossRef]
- Dattu, D.S. Magic Mushrooms: A Comprehensive Review on Pharmacognosy and Therapeutic Potential. Int. J. Res. Publ. Rev. 2024, 5, 2615–2619. [Google Scholar]
- Van Court, R.; Wiseman, M.; Meyer, K.; Ballhorn, D.; Amses, K.; Slot, J.; Dentinger, B.; Garibay-Orijel, R.; Uehling, J. Diversity, biology, and history of psilocybin-containing fungi: Suggestions for research and technological development. Fungal. Biol. 2022, 126, 308–319. [Google Scholar] [CrossRef]
- Strauss, D.; Ghosh, S.; Murray, Z.; Gryzenhout, M. An Overview on the Taxonomy, Phylogenetics and Ecology of the Psychedelic Genera Psilocybe, Panaeolus, Pluteus and Gymnopilus. Rev. Front. For. Glob. Chang. 2022, 5, 813998. [Google Scholar] [CrossRef]
- Sharma, P.; Nguyen, Q.A.; Matthews, S.J.; Carpenter, E.; Mathews, D.B.; A Patten, C.; Hammond, C.J. Psilocybin history, action and reaction: A narrative clinical review. J. Psychopharmacol. 2023, 37, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Isbell, H.; Miner, E.J.; Logan, C.R. Relationships of psychotomimetic to anti-serotonin potencies of congeners of lysergic acid diethylamide (LSD-25). Psychopharmacologia 1959, 1, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Holze, F.; Ley, L.; Müller, F.; Becker, A.M.; Straumann, I.; Vizeli, P.; Kuehne, S.S.; Roder, M.A.; Duthaler, U.; Kolaczynska, K.E.; et al. Direct comparison of the acute effects of lysergic acid diethylamide and psilocybin in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology 2022, 47, 1180–1187. [Google Scholar] [CrossRef]
- Hofmann, A. My Problem Child; Oxford University Press: Oxford, MS, USA, 2013. [Google Scholar]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.C.; Strassman, R.J. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Cosimano, M.P.; Griffiths, R.R. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014, 28, 983–992. [Google Scholar] [CrossRef]
- Adeyinka, D.; Forsyth, D.; Currie, S.; Faraone, N. Neurobiology of psilocybin: A comprehensive overview and comparative analysis of experimental models. Front. Syst. Neurosci. 2025, 19, 1585367. [Google Scholar] [CrossRef]
- Daniel, J.; Haberman, M. Clinical potential of psilocybin as a treatment for mental health conditions. Ment. Health Clin. 2017, 7, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Galdino, T.P.; Oliveira, L.C.; Luz, M.A.; Jesus, R.A.; Lima, E.P.N.; Torres, M.C.M.; Sivieri, K.; Afonso, V.I.; Delgado, J.M.P.Q.; Lima, A.G.B.; et al. Extraction Yields of Psilocybin and Psilocin: A Short Review of Current Methods and Their Implications. Pharmaceuticals 2025, 18, 380. [Google Scholar] [CrossRef]
- Serreau, R.; Amirouche, A.; Benyamina, A.; Berteina-Raboin, S. A Review of Synthetic Access to Therapeutic Compounds Extracted from Psilocybe. Pharmaceuticals 2023, 16, 40. [Google Scholar] [CrossRef]
- Shirota, O.; Hakamata, W.; Goda, Y. Concise large-scale synthesis of psilocin and psilocybin, principal hallucinogenic constituents of “magic mushroom”. J. Nat. Prod. 2003, 66, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Thomann, J.; Kolaczynska, K.E.; Stoeckmann, O.V.; Rudin, D.; Vizeli, P.; Hoener, M.C.; Pryce, C.R.; Vollenweider, F.X.; Liechti, M.E.; Duthaler, U. In vitro and in vivo metabolism of psilocybin’s active metabolite psilocin. Front. Pharmacol. 2024, 15, 1391689. [Google Scholar] [CrossRef]
- Horita, A.; Weber, L.J. The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochem. Pharmacol. 1961, 7, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Horita, A.; Weber, L.J. Dephosphorylation of psilocybin to psilocin by alkaline phosphatase. Proc. Soc. Exp. Biol. Med. 1961, 106, 32–34. [Google Scholar] [CrossRef]
- Nichols, D.; Johnson, M.; Nichols, C. Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Therapeutics. 2017, 101, 209–219. [Google Scholar] [CrossRef]
- Nichols, D.E.N.C. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Raote, I.; Bhattacharya, A.; Panicker, M.M. Serotonin Receptors in Neurobiology. In Serotonin 2A (5-HT2A) Receptor Function: Ligand-Dependent Mechanisms and Pathways Book; Chattopadhyay, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; Chapter 6. [Google Scholar]
- Xu, T.; Pandey, S.C. Cellular localization of serotonin2A (5HT2A) receptors in the rat brain. Brain Res. Bulletin. 2000, 51, 499–505. [Google Scholar] [CrossRef]
- Chisamore, N.; Kaczmarek, E.; Le, G.H.; Wong, S.; Orsini, D.K.; Mansur, R.; McIntyre, R.S.; Rosenblat, J.D. Neurobiology of the Antidepressant Effects of Serotonergic Psychedelics: A Narrative Review. Curr. Treat. Options Psychiatry 2024, 11, 90–105. [Google Scholar] [CrossRef]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Bécamel, C.; Berthoux, C.; Barre, A.; Marin, P. Growing Evidence for Heterogeneous Synaptic Localization of 5-HT2A Receptors. ACS Chem. Neurosci. 2017, 8, 897–899. [Google Scholar] [CrossRef]
- Ekins, T.G.; Rybicki-Kler, C.; Deng, T.; Brooks, I.A.W.; Jedrasiak-Cape, I.; Donoho, E.; Ahmed, O.J. Psychedelic neuroplasticity of cortical neurons lacking 5-HT2A receptors. Mol. Psychiatry 2025. [Google Scholar] [CrossRef]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef]
- Gersh, F.; O’Keefe, J.H.; Elagizi, A.; Lavie, C.J.; Laukkanen, J.A. Estrogen and cardiovascular disease. Prog. Cardiovasc. Dis. 2024, 84, 60–67. [Google Scholar] [CrossRef]
- Hariri, L.; Rehman, A. Estradiol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Delgado, B.J.; Patel, P.; Lopez-Ojeda, W. Estrogen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Lastupdated 9 August 2025. [Google Scholar]
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology 2003, 144, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, R.; Handa, R.J. Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J. Neurochem. 2013, 127, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.; Handa, R.J. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience 2009, 163, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Summer, B.E.; Fink, G. Estrogen increases the density of 5-hydroxytryptamine(2A) receptors in cerebral cortex and nucleus accumbens in the female rat. J. Steroid. Biochem. Mol. Biol. 1995, 54, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Cyr, M.; Landry, M.; Di Paolo, T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacol. 2000, 23, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; Sumner, B.E.; Rosie, R.; Grace, O.; Quinn, J.P. Estrogen control of central neurotransmission: Effect on mood, mental state, and memory. Cell Mol. Neurobiol. 1996, 16, 325–344. [Google Scholar] [CrossRef]
- Biegon, A.; McEwen, B.S. Modulation by estradiol of serotonin receptors in brain. J. Neurosci. 1982, 2, 199–205. [Google Scholar] [CrossRef]
- Kugaya, A.; Epperson, C.N.; Zoghbi, S.; van Dyck, C.H.; Hou, Y.; Fujita, M.; Staley, J.K.; Garg, P.K.; Seibyl, J.P.; Innis, R.B. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am. J. Psychiatry 2003, 160, 1522–1524. [Google Scholar] [CrossRef]
- Moses-Kolko, E.L.; Berga, S.L.; Greer, P.J.; Smith, G.; Cidis Meltzer, C.; Drevets, W.C. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil. Steril. 2003, 80, 554–559. [Google Scholar] [CrossRef]
- Birzniece, V.; Johansson, I.M.; Wang, M.D.; Bäckström, T.; Olsson, T. Ovarian hormone effects on 5-hydroxytryptamine(2A) and 5-hydroxytryptamine(2C) receptor mRNA expression in the ventral hippocampus and frontal cortex of female rats. Neurosci. Lett. 2002, 319, 157–161. [Google Scholar] [CrossRef]
- Albert, K.; Pruessner, J.; Newhouse, P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinol. 2015, 59, 14–24. [Google Scholar] [CrossRef]
- Cohen, Z.Z.; Blest-Hopley, G. Females in Psychedelic Research: A Perspective for Advancing Research and Practice. ACS Pharmacol. Transl. Sci. 2025, 8, 1837–1846. [Google Scholar] [CrossRef]
- LiCausi, F.; Hartman, N.W. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018, 19, 1544. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef]
- An, X.; Yao, X.; Li, B.; Yang, W.; Cui, R.; Zhao, G.; Jin, Y. Role of BDNF-mTORC1 Signaling Pathway in Female Depression. Neural Plast. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Gattuso, J.J.; Perkins, D.; Ruffell, S.; Lawrence, A.J.; Hoyer, D.; Jacobson, L.H.; Timmermann, C.; Castle, D.; Rossell, S.L.; Downey, L.A.; et al. Default Mode Network Modulation by Psychedelics: A Systematic Review. Int. J. Neuropsychopharmacol. 2023, 26, 155–188. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Roseman, L.; Bolstridge, M.; Demetriou, L.; Pannekoek, J.N.; Wall, M.B.; Tanner, M.; Kaelen, M.; McGonigle, J.; Murphy, K.; et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 2017, 7, 13187. [Google Scholar] [CrossRef]
- Siegel, J.S.; Subramanian, S.; Perry, D.; Kay, B.P.; Gordon, E.M.; Laumann, T.O.; Reneau, T.R.; Metcalf, N.V.; Chacko, R.V.; Gratton, C.; et al. Psilocybin desynchronizes the human brain. Nature 2024, 632, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.; Magnesa, A.; Gambini, M.; Gurrieri, R.; Annuzzi, E.; Elefante, C.; Perugi, G.; Marazziti, D. Psychedelic-Induced Neural Plasticity: A Comprehensive Review and a Discussion of Clinical Implications. Brain Sci. 2025, 15, 117. [Google Scholar] [CrossRef] [PubMed]
- Banushi, B.; Polito, V. A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics. Biology 2023, 12, 1380. [Google Scholar] [CrossRef]
- Kashuba, A.D.; Nafziger, A.N. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin. Pharmacokinet. 1998, 34, 203–218. [Google Scholar] [CrossRef]
- Erkizia-Santamaría, I.; Alles-Pascual, R.; Horrillo, I.; Meana, J.J.; Ortega, J.E. Serotonin 5-HT(2A), 5-HT(2c) and 5-HT(1A) receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed. Pharmacother. 2022, 154, 113612. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Heisler, L.K.; Zhou, L.; Bajwa, P.; Hsu, J.; Tecott, L.H. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007, 6, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Jiang, Y.; Hu, Z.; Yang, Y.; Du, X.; Botchway, B.O.A.; Fang, M. Serotonin receptors 2A and 1A modulate anxiety-like behavior in post-traumatic stress disordered mice. Am. J. Transl. Res. 2019, 11, 2288–2303. [Google Scholar]
- Braun, D.; Rosenberg, A.M.; Rabaniam, E.; Haruvi, R.; Malamud, D.; Barbara, R.; Aiznkot, T.; Levavi-Sivan, B.; Kawashima, T. High-resolution tracking of unconfined zebrafish behavior reveals stimulatory and anxiolytic effects of psilocybin. Mol. Psychiatry 2024, 29, 1046–1062. [Google Scholar] [CrossRef]
- Del Río, J.P.; Alliende, M.I.; Molina, N.; Serrano, F.G.; Molina, S.; Vigil, P. Steroid Hormones and Their Action in Women’s Brains: The Importance of Hormonal Balance. Front. Public. Health. 2018, 6, 141. [Google Scholar] [CrossRef]
- Hwang, W.J.; Lee, T.Y.; Kim, N.S.; Kwon, J.S. The Role of Estrogen Receptors and Their Signaling across Psychiatric Disorders. Int. J. Mol. Sci. 2021, 22, 373. [Google Scholar] [CrossRef]
- Rojas-Zambrano, J.G.; Rojas-Zambrano, A.R.; Rojas-Zambrano, A.F.; Barahona-Cueva, G.E. Benefits of Testosterone Hormone in the Human Body: A Systematic Review. Cureus 2025, 17, e78785. [Google Scholar] [CrossRef]
- Heany, S.J.; van Honk, J.; Stein, D.J.; Brooks, S.J. A quantitative and qualitative review of the effects of testosterone on the function and structure of the human social-emotional brain. Metab. Brain Dis. 2016, 31, 157–167. [Google Scholar] [CrossRef]
- Hammond, J.; Le, Q.; Goodyer, C.; Gelfand, M.; Trifiro, M.; LeBlanc, A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001, 77, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Sarkey, S.; Azcoitia, I.; Garcia-Segura, L.M.; Garcia-Ovejero, D.; DonCarlos, L.L. Classical androgen receptors in non-classical sites in the brain. Horm. Behav. 2008, 53, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-Y.; Huang, Y.-L.; Chang, C.; Kang, H.-Y. Deficiency in Androgen Receptor Aggravates the Depressive-Like Behaviors in Chronic Mild Stress Model of Depression. Cells 2019, 8, 1021. [Google Scholar] [CrossRef]
- Azcoitia, I.; Mendez, P.; Garcia-Segura, L.M. Aromatase in the Human Brain. Androg. Clin. Res. Ther. 2021, 2, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sareddy, G.R.; Wang, J.; Zhang, Q.; Tang, F.-L.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Neuron-Derived Estrogen Is Critical for Astrocyte Activation and Neuroprotection of the Ischemic Brain. J. Neurosci. 2020, 40, 7355–7374. [Google Scholar] [CrossRef]
- Reddy, D.S.; Jian, K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J. Pharmacol. Exp. Ther. 2010, 334, 1031–1041. [Google Scholar] [CrossRef]
- Frye, C.A.; Walf, A.A. Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiol. Behav. 2009, 97, 266–269. [Google Scholar] [CrossRef]
- Garcia-Argibay, M.; Hiyoshi, A.; Fall, K.; Montgomery, S. Association of 5α-Reductase Inhibitors With Dementia, Depression, and Suicide. JAMA Netw. Open 2022, 5, e2248135. [Google Scholar] [CrossRef]
- Meyer, J.H. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J. Psychiatry Neurosci. 2007, 32, 86–102. [Google Scholar] [CrossRef]
- Meyer, J.H. Neuroimaging markers of cellular function in major depressive disorder: Implications for therapeutics, personalized medicine, and prevention. Clin. Pharmacol. Ther. 2012, 91, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Gryglewski, G.; Lanzenberger, R.; Kranz, G.S.; Cumming, P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood Flow. Metab. 2014, 34, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Staley, J.K.; Sanacora, G.; Tamagnan, G.; Maciejewski, P.K.; Malison, R.T.; Berman, R.M.; Vythilingam, M.; Kugaya, A.; Baldwin, R.M.; Seibyl, J.P.; et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol. Psychiatry 2006, 59, 40–47. [Google Scholar] [CrossRef]
- Fink, G.; Sumner, B.; Rosie, R.; Wilson, H.; McQueen, J. Androgen actions on central serotonin neurotransmission: Relevance for mood, mental state and memory. Behav. Brain Res. 1999, 105, 53–68. [Google Scholar] [CrossRef]
- Herrera-Pérez, J.J.; Fernández-Guasti, A.; Martínez-Mota, L. Brain SERT Expression of Male Rats Is Reduced by Aging and Increased by Testosterone Restitution. Neurosci. J. 2013, 2013, 201909. [Google Scholar] [CrossRef] [PubMed]
- Kranz, G.S.; Wadsak, W.; Kaufmann, U.; Savli, M.; Baldinger, P.; Gryglewski, G.; Haeusler, D.; Spies, M.; Mitterhauser, M.; Kasper, S.; et al. High-Dose Testosterone Treatment Increases Serotonin Transporter Binding in Transgender People. Biol. Psychiatry 2015, 78, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.J.; Wilson, C.; Li, S.; Hannan, A.J.; Renoir, T. Mice lacking the serotonin transporter do not respond to the behavioural effects of psilocybin. Eur. J. Pharmacol. 2025, 991, 177304. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016, 26, 1327–1337. [Google Scholar] [CrossRef]
- Steiner, M.; Dunn, E.; Born, L. Hormones and mood: From menarche to menopause and beyond. J. Affect. Disord. 2003, 74, 67–83. [Google Scholar] [CrossRef]
- Carlson, L.J.; Shaw, N.D. Development of Ovulatory Menstrual Cycles in Adolescent Girls. J. Pediatr. Adolesc. Gynecol. 2019, 32, 249–253. [Google Scholar] [CrossRef]
- Shirtcliff, E.A.; Dahl, R.E.; Pollak, S.D. Pubertal development: Correspondence between hormonal and physical development. Child. Dev. 2009, 80, 327–337. [Google Scholar] [CrossRef]
- Hazell, P.; Balzer, B.W.R.; Garden, F.; Handelsman, D.J.; Paxton, K.; Hawke, C.; Ivers, R.; Skinner, S.R.; Luscombe, G.; Steinbeck, K.S. Association of urinary sex hormones with mood and behavior changes in a community adolescent cohort. PLoS ONE 2023, 18, e0293040. [Google Scholar] [CrossRef]
- Copeland, W.E.; Worthman, C.; Shanahan, L.; Costello, E.J.; Angold, A. Early Pubertal Timing and Testosterone Associated With Higher Levels of Adolescent Depression in Girls. J. Am. Acad. Child. Adolesc. Psychiatry 2019, 58, 1197–1206. [Google Scholar] [CrossRef]
- Cardoos, S.L.; Ballonoff Suleiman, A.; Johnson, M.; van den Bos, W.; Hinshaw, S.P.; Dahl, R.E. Social status strategy in early adolescent girls: Testosterone and value-based decision making. Psychoneuroendocrinology 2017, 81, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Spielberg, J.M.; Forbes, E.E.; Ladouceur, C.D.; Worthman, C.M.; Olino, T.M.; Ryan, N.D.; Dahl, R.E. Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc. Cogn. Affect. Neurosci. 2015, 10, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Izmi, N.; Carhart-Harris, R.L.; Kettner, H. Psychological effects of psychedelics in adolescents. Front. Child. Adolesc. Psychiatry 2024, 3, 1364617. [Google Scholar] [CrossRef]
- Shah, K.T.C.; Kamrai, D.; Akbar, M.; Tankersley, W. Association of Psilocybin Use in Adolescents with Major Depressive Episode. Eur. Psychiatry 2022, 65 (Suppl. 1), s329. [Google Scholar] [CrossRef]
- Zylko, A.; Rakoczy, R.; Roberts, B.; Wilson, M.; Powell, A.; Page, A.; Heitkamp, M.; Feist, D.; Jones, J.; McMurray, M. Age- and estrous-dependent effects of psilocybin in rats. Neuropharmacology 2025, 279, 110619. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.; Gangooly, S.; Muttukrishna, S. The normal menstrual cycle in women. Anim. Reprod. Sci. 2011, 124, 229–236. [Google Scholar] [CrossRef]
- Carlson, K.; Mughal, S.; Azhar, Y.; Siddiqui, W. Perinatal Depression. In NCBI Bookshelf A service of the National Library of Medicine, National Institutes of Health StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Aswathi, A.; Rajendiren, S.; Nimesh, A.; Philip, R.R.; Kattimani, S.; Jayalakshmi, D.; Ananthanarayanan, P.; Dhiman, P. High serum testosterone levels during postpartum period are associated with postpartum depression. Asian J. Psychiatr. 2015, 17, 85–88. [Google Scholar] [CrossRef]
- Hohlagschwandtner, M.; Husslein, P.; Klier, C.; Ulm, B. Correlation between serum testosterone levels and peripartal mood states. Acta Obstet. Gynecol. Scand. 2001, 80, 326–330. [Google Scholar] [CrossRef]
- Soares, C.N. Mood disorders in midlife women: Understanding the critical window and its clinical implications. Menopause 2014, 21, 198–206. [Google Scholar] [CrossRef]
- Skalkidou, A.; Hellgren, C.; Comasco, E.; Sylvén, S.; Sundström Poromaa, I. Biological aspects of postpartum depression. Womens Health 2012, 8, 659–672. [Google Scholar] [CrossRef]
- Gukasyan, N.; Narayan, S.K. Menstrual Changes and Reversal of Amenorrhea Induced by Classic Psychedelics: A Case Series. J. Psychoact. Drugs 2024, 56, 50–55. [Google Scholar] [CrossRef]
- Yin, X.; Sun, N.; Jiang, N.; Xu, X.; Gan, Y.; Zhang, J.; Qiu, L.; Yang, C.; Shi, X.; Chang, J.; et al. Prevalence and associated factors of antenatal depression: Systematic reviews and meta-analyses. Clin. Psychol. Rev. 2021, 83, 101932. [Google Scholar] [CrossRef]
- Law, F.P.G.; Chui, Y.C.; He, S. 14C-psilocin tissue distribution in pregnant rats after intravenous administration. FFDH 2014, 4, 232. [Google Scholar] [CrossRef]
- Mandeville, J.B.; Sander, C.Y.; Jenkins, B.G.; Hooker, J.M.; Catana, C.; Vanduffel, W.; Alpert, N.M.; Rosen, B.R.; Normandin, M.D. A receptor-based model for dopamine-induced fMRI signal. Neuroimage. 2013, 75, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhou, Z.; Xiang, F.; Hu, W.; Cao, X. Global prevalence of depression in menopausal women: A systematic review and meta-analysis. J. Affect. Disord. 2024, 358, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, J.T.; Schott, L.L.; Kravitz, H.M.; Sowers, M.; Avis, N.E.; Gold, E.B.; Randolph, J.F.; Matthews, K.A. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: Results from the Study of Women’s Health Across the Nation (SWAN). Arch. Gen. Psychiatry 2010, 67, 598–607. [Google Scholar] [CrossRef]
- Hemachandra, C.; Islam, R.M.; Bell, R.J.; Sultana, F.; Davis, S.R. The association between testosterone and depression in postmenopausal women: A systematic review of observational studies. Maturitas 2023, 168, 62–70. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Murphy, J.H.; Haq, N.; Danaceau, M.A.; St Clair, L. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology 2002, 27, 907–920. [Google Scholar] [CrossRef]
- Burger, H.G.; Dudley, E.C.; Cui, J.; Dennerstein, L.; Hopper, J.L. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J. Clin. Endocrinol. Metab. 2000, 85, 2832–2838. [Google Scholar] [CrossRef]
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef] [PubMed]
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [CrossRef] [PubMed]
- Maki, P.M.; Henderson, V.W. Hormone therapy, dementia, and cognition: The Women’s Health Initiative 10 years on. Climacteric 2012, 15, 256–262. [Google Scholar] [CrossRef]
- Greendale, G.A.; Huang, M.-H.; Wight, R.G.; Seeman, T.; Luetters, C.; Avis, N.E.; Johnston, J.; Karlamangla, A.S. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 2009, 72, 1850–1857. [Google Scholar] [CrossRef]
- Syed, O.A.; Petranker, R.; Fewster, E.C.; Sobolenko, V.; Beidas, Z.; Husain, M.I.; Lake, S.; Lucas, P. Global Trends in Psychedelic Microdosing: Demographics, Substance Testing Behavior, and Patterns of Use. J. Psychoact. Drugs 2024, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Balzer, B.W.; Duke, S.A.; Hawke, C.I.; Steinbeck, K.S. The effects of estradiol on mood and behavior in human female adolescents: A systematic review. Eur. J. Pediatr. 2015, 174, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N.; Zitek, B. Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? J. Psychiatry Neurosci. 2008, 33, 331–343. [Google Scholar] [CrossRef]
- Bui, H.N.; Sluss, P.M.; Blincko, S.; Knol, D.L.; Blankenstein, M.A.; Heijboer, A.C. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids 2013, 78, 96–101. [Google Scholar] [CrossRef]
- Cohen, L.S.; Altshuler, L.L.; Harlow, B.L.; Nonacs, R.; Newport, D.J.; Viguera, A.C.; Suri, R.; Burt, V.K.; Hendrick, V.; Reminick, A.M.; et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006, 295, 499–507. [Google Scholar] [CrossRef]
- Esscher, A.; Essén, B.; Innala, E.; Papadopoulos, F.C.; Skalkidou, A.; Sundström-Poromaa, I.; Högberg, U. Suicides during pregnancy and 1 year postpartum in Sweden, 1980-2007. Br. J. Psychiatry 2016, 208, 462–469. [Google Scholar] [CrossRef]
- Norhayati, M.N.; Hazlina, N.H.; Asrenee, A.R.; Emilin, W.M. Magnitude and risk factors for postpartum symptoms: A literature review. J. Affect. Disord. 2015, 175, 34–52. [Google Scholar] [CrossRef]
- Stuenkel, C.A. What is menopause? Menopause 2024, 31, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Vesga-López, O.; Blanco, C.; Keyes, K.; Olfson, M.; Grant, B.F.; Hasin, D.S. Psychiatric disorders in pregnant and postpartum women in the United States. Arch. Gen. Psychiatry 2008, 65, 805–815. [Google Scholar] [CrossRef]
- Sander, B.; Muftah, A.; Sykes Tottenham, L.; Grummisch, J.A.; Gordon, J.L. Testosterone and depressive symptoms during the late menopause transition. Biol. Sex Differ. 2021, 12, 44. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Wallach, J.; Cao, A.B.; Calkins, M.M.; Heim, A.J.; Lanham, J.K.; Bonniwell, E.M.; Hennessey, J.J.; Bock, H.A.; Anderson, E.I.; Sherwood, A.M.; et al. Identification of 5-HT(2A) receptor signaling pathways associated with psychedelic potential. Nat. Commun. 2023, 14, 8221. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Barros, R.P.A.; Sugiyama, N.; Krishnan, V.; Yaden, B.C.; Kim, H.-J.; Warner, M.; Gustafsson, J. Involvement of estrogen receptor β in maintenance of serotonergic neurons of the dorsal raphe. Mol. Psychiatry 2013, 18, 674–680. [Google Scholar] [CrossRef] [PubMed]
| Anxiety Disorder | Prevalence in Women (%) | Prevalence in Men (%) |
|---|---|---|
| Panic disorder | 5.0 | 2.0 |
| Agoraphobia | 7.0 | 3.5 |
| Specific phobia | 15.7 | 6.7 |
| Social anxiety disorder | 15.5 | 11.1 |
| Generalized anxiety disorder | 6.6 | 3.6 |
| Posttraumatic stress disorder (PTSD) | 10.4 | 5.0 |
| Stages of Development | Hormonal Changes | Mood Changes | References |
|---|---|---|---|
| Puberty | Fluctuations in E2 Increase in Testosterone | Risk for depression. | [113,140] |
| Menstrual | Decrease in estrogen and progesterone levels Increased testosterone (especially during the ovulatory phase) | Increased risk for depression. | [120,141,142] |
| Pregnancy | Increased estrogen and progesterone levels Increased testosterone | E2 and progesterone are protective against severe depression. Elevated testosterone linked to increased risk for depression. | [123,143,144,145] |
| Postpartum | Deficiency in estrogen and progesterone levels Decrease in testosterone | Lower E2 and progesterone causes an increased risk for depression/psychiatric disorders. If testosterone increases, then possibility of depression. | [122,123,124,125,126] |
| Menopausal | Decline in ovarian estradiol production Gradual decline in testosterone | Mood swings If testosterone increases then possibility of depression | [131,146] |
| Perimenopausal | Decline in progesterone levels, Estrogen deficiency (mostly) Gradual decline in testosterone | Depressive symptoms, sleep disruption. If testosterone increases then greater risk for depression. | [124,125,147,148] |
| Postmenopausal | Estrogen shifts from estradiol to estrone. Testosterone low and stable | Increased risk for depression. Weak/Absent link between testosterone and depression. | [124,132,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekoh, F.; Rerrie, S.; Angud, J.; Mirabelli, E. Hormonal Influences on Psilocybin Responsivity Across the Female Lifespan: Toward Personalized Psychedelic-Assisted Therapy. Psychoactives 2025, 4, 39. https://doi.org/10.3390/psychoactives4040039
Ekoh F, Rerrie S, Angud J, Mirabelli E. Hormonal Influences on Psilocybin Responsivity Across the Female Lifespan: Toward Personalized Psychedelic-Assisted Therapy. Psychoactives. 2025; 4(4):39. https://doi.org/10.3390/psychoactives4040039
Chicago/Turabian StyleEkoh, Faith, Shanice Rerrie, James Angud, and Ersilia Mirabelli. 2025. "Hormonal Influences on Psilocybin Responsivity Across the Female Lifespan: Toward Personalized Psychedelic-Assisted Therapy" Psychoactives 4, no. 4: 39. https://doi.org/10.3390/psychoactives4040039
APA StyleEkoh, F., Rerrie, S., Angud, J., & Mirabelli, E. (2025). Hormonal Influences on Psilocybin Responsivity Across the Female Lifespan: Toward Personalized Psychedelic-Assisted Therapy. Psychoactives, 4(4), 39. https://doi.org/10.3390/psychoactives4040039






