Digital Enablement of Psychedelic-Assisted Therapy in Non-Clinical Settings: A Systematic Review of Safety, Efficacy, and Implementation Models
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection
2.4. Data Collection Process
2.5. Data Items
2.6. Risk of Bias Assessment
2.7. Synthesis of Results
3. Results
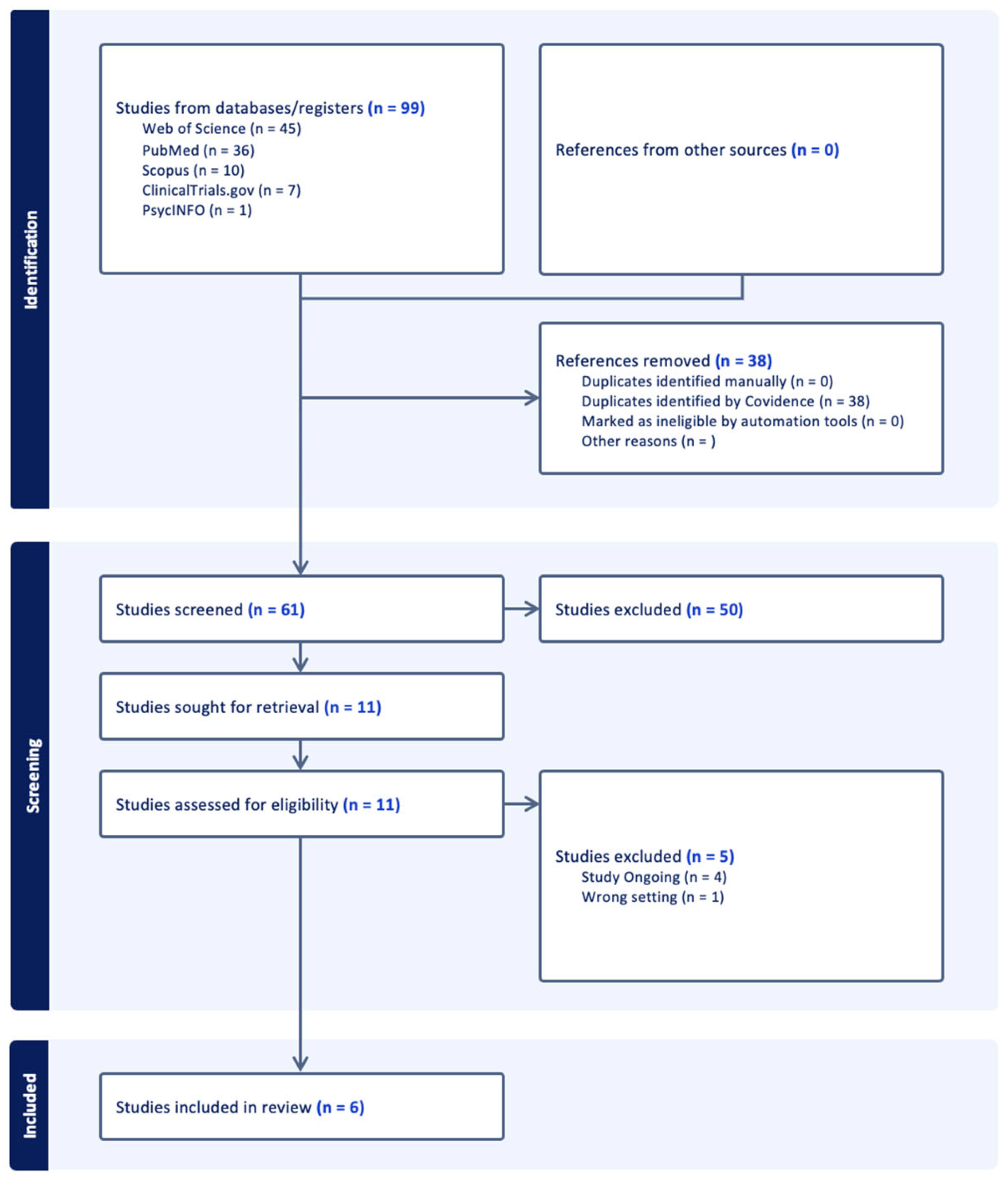
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Included Studies
3.4. Non-Randomized Studies
3.5. Qualitative Study
3.6. Case Reports
3.7. Synthesis of Results
3.8. At-Home Ketamine Therapy via Telehealth Platforms
- Efficacy: Both studies reported significant and rapid reductions in symptoms. Hull et al. (2022) [16], in a sample of 1247 patients, found that 62.8% achieved a response (≥50% reduction) on the PHQ-9 for depression, with a large effect size (d = 1.61). Similarly, 62.9% responded on the GAD-7 for anxiety (d = 1.56). Mathai et al. (2024) [15] replicated these findings in a much larger cohort of 11,441 patients, reporting a 56.4% response rate and a 28.1% remission rate for depression.
- Safety: The safety profile was generally favorable, with a low overall rate of adverse events (AEs) reported (4.7–4.8%). However, both studies noted the occurrence of serious adverse events (SAEs), which were exclusively psychiatric in nature. Mathai et al. [15] reported six SAEs, including psychosis and suicidal behavior, and 46 patients (0.4%) discontinued treatment due to AEs.
- Feasibility/Acceptability: The large-scale implementation and high treatment completion rates reported in both studies suggest that this model is a feasible and acceptable method for increasing access to care.
3.9. At-Home (Es)ketamine with Mobile or Telemedicine Support
- Efficacy: Kim et al. (2024) [17] found that esketamine nasal spray produced a significant reduction in depressive symptoms (mean PHQ-9 score decreased from 19.69 to 14.14; p < 0.001) in 29 patients with TRD. Notably, their mobile app detected a statistically significant improvement in depressive symptoms just one day after the first dose (p = 0.049). The case report by Longpré-Poirier et al. (2020) [20] did not measure efficacy quantitatively but noted a “positive experience” for a 61-year-old patient receiving remotely supervised intranasal ketamine.
- Safety: The safety findings were mixed. The case report by Longpré-Poirier et al. (2020) [20] reported no AEs. In contrast, Kim et al. (2024) [17] reported one death by suicide and one instance of self-harm, though both were attributed to the exacerbation of pre-existing symptoms rather than the drug itself. The case report by Johnson et al. (2024) [19] detailed the most severe safety event in this review: a life-threatening, unintentional overdose of 1200 mg of sublingual ketamine at home, which resulted in hospitalization.
- Feasibility/Acceptability: These studies suggest the model is feasible. Kim et al. (2024) [17] reported high patient adherence (89.6% in the first 3 days) to the daily monitoring app. Longpré-Poirier et al. (2020) [20] concluded that remote supervision was a feasible strategy to continue care during the COVID-19 pandemic. However, the overdose reported by Johnson et al. (2024) [19] raises critical concerns about the feasibility of ensuring patient safety without robust regulation and clear provider instructions.
3.10. Online Ayahuasca Rituals
- Efficacy: Efficacy was not measured clinically. The study found that the online format fulfilled an important social function by allowing the community to maintain its practice and foster a sense of global connection during a time of social isolation.
- Safety: The primary concerns were contextual rather than medical, including a higher potential for distractions, social anxiety from being on camera, and an “in-built tension between the social and spiritual dimensions of ritual.”
- Feasibility/Acceptability: The online format was a feasible ad-hoc alternative during the pandemic. However, acceptability was mixed. While it provided a crucial social lifeline, participants also reported an “impoverished ritual experience” and expressed concerns about the commodification of the sacred ritual.
3.11. Certainty and Bias from Missing Data
4. Discussion
4.1. Summary of Principal Findings
4.2. Interpretation of Findings
4.3. Limitations of the Evidence
4.4. Limitations of This Review
4.5. Implications for Clinical Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and psychedelic-assisted psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Barry, J.J.; Bennett, J.C.; Brown, L.C.; Carpenter, L.L.; Corlett, P.R.; Creech, S.K.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Schenberg, E.E. Psychedelic-assisted psychotherapy: A paradigm shift in psychiatric research and development. Front. Pharmacol. 2018, 9, 733. [Google Scholar] [CrossRef]
- Askariyan, K.; Joghataei, M.T.; Dehghan, S.; Khodagholi, F.; Karimi-Hajishirzi, M.; Safakhah, H.A.; Nazari-Robati, M.; Haghparast, A. An overview of psilocybin, LSD, MDMA, and ketamine in revitalizing psychedelic-assisted therapy: Insights, limitations and future directions. Prog. Neuropsychopharmacol. Biol. Psychiatry, 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.S.; Aaronson, S.T. The Emerging Field of Psychedelic Psychotherapy. Curr. Psychiatry Rep. 2022, 24, 583–590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, M.L.; Korevaar, D.; Harvey, R.; Rucker, J.J.H.; Erritzoe, D.; Nutt, D. Translating psychedelic therapies from clinical trials to community clinics: Building bridges and addressing potential challenges ahead. Front. Psychiatry 2021, 12, 737738. [Google Scholar] [CrossRef]
- Guinart, D.; Marcy, P.; Hauser, M.; Dwyer, M.; Kane, J.M. Mental health care providers’ attitudes toward telepsychiatry: A systemwide, multisite survey during the COVID-19 pandemic. Psychiatr. Serv. 2021, 72, 704–707. [Google Scholar] [CrossRef]
- Monaghesh, E.; Hajizadeh, A. The role of telehealth during COVID-19 outbreak: A systematic review based on current evidence. BMC Public Health 2020, 20, 1193. [Google Scholar] [CrossRef]
- Bucci, S.; Schwannauer, M.; Berry, N. The digital revolution and its impact on mental health care. Psychol. Psychother. 2019, 92, 277–297. [Google Scholar] [CrossRef]
- Torous, J.; Jän Myrick, K.; Rauseo-Ricupero, N.; Firth, J. Digital Mental Health and COVID-19: Using Technology Today to Accelerate the Curve on Access and Quality Tomorrow. JMIR Ment. Health 2020, 7, e18848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalman, J.L.; Burkhardt, G.; Samochowiec, J.; Pacchiarotti, I.; Bernardo, M.; Van Ameringen, M.; Vieta, E.; Wasserman, D.; Winkler, P.; Zohar, J.; et al. Digitalising mental health care: Practical recommendations from the European Psychiatric Association. Eur. Psychiatry 2023, 67, e4. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Critical Appraisal Skills Programme (CASP). CASP (Qualitative) Checklist. CASP UK. 2018. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 1 August 2025).
- Mathai, D.S.; Hull, T.D.; Vando, L.; Malgaroli, M. At-home, telehealth-supported ketamine treatment for depression: Findings from longitudinal, machine learning and symptom network analysis of real-world data. J. Affect. Disord. 2024, 361, 198–208. [Google Scholar] [CrossRef]
- Hull, T.D.; Malgaroli, M.; Gazzaley, A.; Akiki, T.J.; Madan, A.; Vando, L.; Arden, K.; Swain, J.; Klotz, M.; Paleos, C. At-home, sublingual ketamine telehealth is a safe and effective treatment for moderate to severe anxiety and depression: Findings from a large, prospective, open-label effectiveness trial. J. Affect. Disord. 2022, 314, 59–67. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.-H.; Shin, C.; Han, K.-M.; Cho, S.J.; Hong, N.; Han, C. A Multi-Center, Open-Label, Single-Arm Study to Investigate the Early Effectiveness of Esketamine Nasal Spray in Patients with Treatment-Resistant Depression Using a Mobile Self-Monitoring Application. Pharmaceuticals 2024, 17, 1143. [Google Scholar] [CrossRef]
- Hartogsohn, I. Virtual Daime: When Psychedelic Ritual Migrates Online. Front. Psychol. 2022, 13, 819994. [Google Scholar] [CrossRef]
- Johnson, B.E.; Borges, E.S.; Gaspari, R.J.; Galletta, G.M.; Lai, J.T. Unintentional Ketamine Overdose Via Telehealth. Am. J. Psychiatry 2024, 181, 81–82. [Google Scholar] [CrossRef]
- Longpré-Poirier, C.; Desbeaumes Jodoin, V.; Miron, J.-P.; Lespérance, P. Remote Monitoring of Intranasal Ketamine Self-Administration as Maintenance Therapy in Treatment-Resistant Depression (TRD): A Novel Strategy for Vulnerable and At-Risk Populations to COVID-19? Am. J. Geriatr. Psychiatry 2020, 28, 892–893. [Google Scholar] [CrossRef]
- American Psychiatric Association. Practice Guideline for the Assessment and Treatment of Patients with Suicidal Behaviors. Am. J. Psychiatry 2003, 160, 1–60. [Google Scholar]
- Moran, M. Joint Commission Issues Update for Suicide Prevention. Psychiatr. News 2019, 54. [Google Scholar] [CrossRef]
- Posner, K.; Brown, G.K.; Stanley, B.; Brent, D.A.; Yershova, K.V.; Oquendo, M.A.; Currier, G.W.; Melvin, G.A.; Greenhill, L.; Shen, S.; et al. The Columbia–Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings from Three Multisite Studies with Adolescents and Adults. Am. J. Psychiatry 2011, 168, 1266–1277. [Google Scholar] [CrossRef]
- NPSG.15.01.01 EP 2 Requires Use of a Validated Screening Tool for Patients Being Evaluated or Treated for Behavioral Health Conditions as Their Primary Reason for Care. Is Screening Required for All Patients and What Is Meant by a Validated Screening Tool? (Effective: July 1, 2019). Available online: https://www.jointcommission.org/en/knowledge-library/support-center/standards-interpretation/standards-faqs/000002240 (accessed on 1 August 2025).
- Sanacora, G.; Frye, M.A.; McDonald, W.; Mathew, S.J.; Turner, M.S.; Schatzberg, A.F.; Summergrad, P.; Nemeroff, C.B.; for the American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry 2017, 74, 399–405. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. Guidance on the Safe Use of Ketamine Outside of Acute Care Settings. American Society of Anesthesiologists. 2025. Available online: https://www.asahq.org/advocating-for-you/guidance/ketamine-safe-use (accessed on 1 August 2025).
- Janssen Pharmaceuticals. SPRAVATO (Esketamine) Nasal Spray, CIII: Prescribing Information; Janssen Pharmaceuticals: Titusville, NJ, USA, 2025; Available online: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SPRAVATO-pi.pdf (accessed on 1 August 2025).
- U.S. Food and Drug Administration. Supplement Approval. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2025/211243Orig1s013ltr.pdf (accessed on 1 August 2025).
- U.S. Food and Drug Administration. Digital Health Technologies for Remote Data Acquisition in Clinical Investigations. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/digital-health-technologies-remote-data-acquisition-clinical-investigations (accessed on 1 August 2025).
- Substance Abuse and Mental Health Services Administration (SAMHSA). National Guidelines for Behavioral Health Crisis Care: Best Practice Toolkit. Available online: https://bja.ojp.gov/sites/g/files/xyckuh186/files/media/document/samsha-national-guidelines.pdf (accessed on 1 August 2025).
| Study | Study Design | Population (N, Condition) | Intervention Details | Digital Integration | Key Outcomes Measured |
|---|---|---|---|---|---|
| [18] | Qualitative Study | N = 12 Santo Daime members | Ayahuasca administered during online group rituals. | Public video streaming platforms (Zoom, YouTube, Facebook Live). | Qualitative experiences, challenges, and benefits of online rituals. |
| [16] | Prospective, open-label effectiveness trial | N = 1247 Adults with moderate-to-severe depression and anxiety | At-home sublingual ketamine (300–450 mg) over 4 weeks. | Comprehensive telehealth platform (Mindbloom) with video consults, remote monitoring, and behavioral coaching. | Depression (PHQ-9), Anxiety (GAD-7), and Adverse Events. |
| [19] | Case Report | N = 1 Adult with PTSD | At-home sublingual ketamine (1200 mg). | Telehealth platform for prescribing and instruction. | Clinical presentation of an unintentional overdose. |
| [17] | Multi-center, open-label, single-arm study | N = 29 Adults with Treatment-Resistant Depression (TRD) | At-home esketamine nasal spray (starting at 56 mg) twice weekly. | “EsCARe” mobile application for daily self-monitoring of symptoms. | Depression (PHQ-9, HAMD), Anxiety (GAD-7), Adverse Events, and App Adherence. |
| [20] | Case Report | N = 1 Adult with TRD | At-home intranasal ketamine (dose not specified). | Telemedicine for remote supervision by a registered nurse. | Feasibility and safety of remote supervision. |
| [15] | Longitudinal, real-world data analysis | N = 11,441 Adults with moderate-to-severe depression | At-home sublingual ketamine (avg. dose 590 mg) over 4 weeks. | Comprehensive telehealth platform (Mindbloom) with video consults, remote monitoring, and behavioral coaching. | Depression (PHQ-9), Anxiety (GAD-7), and Adverse Events. |
| Study | Study Design | Tool Used | Overall Judgment | Key Sources of Potential Bias |
|---|---|---|---|---|
| [18] | Qualitative Study | CASP Checklist | High Quality | Lack of researcher reflexivity statement; unclear data analysis process. |
| [16] | Non-Randomized Study | ROBINS-I | Serious Risk | No control group (confounding); self-selected paying sample (selection bias); >55% missing follow-up data (attrition bias). |
| [19] | Case Report | Quality Checklist | High Quality | Not applicable (case report); findings are not generalizable. |
| [17] | Non-Randomized Study | ROBINS-I | Moderate to Serious Risk | No control group (confounding); unstandardized use of concurrent medications (deviation from intervention). |
| [20] | Case Report | Quality Checklist | Moderate Quality | Dose of ketamine not specified; subjective outcome measure. |
| [15] | Non-Randomized Study | ROBINS-I | Moderate Risk | Retrospective design (risk of bias from deviations); unclear handling of missing data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driscoll, B.; Lakhan, S.E. Digital Enablement of Psychedelic-Assisted Therapy in Non-Clinical Settings: A Systematic Review of Safety, Efficacy, and Implementation Models. Psychoactives 2025, 4, 35. https://doi.org/10.3390/psychoactives4040035
Driscoll B, Lakhan SE. Digital Enablement of Psychedelic-Assisted Therapy in Non-Clinical Settings: A Systematic Review of Safety, Efficacy, and Implementation Models. Psychoactives. 2025; 4(4):35. https://doi.org/10.3390/psychoactives4040035
Chicago/Turabian StyleDriscoll, Brendan, and Shaheen E. Lakhan. 2025. "Digital Enablement of Psychedelic-Assisted Therapy in Non-Clinical Settings: A Systematic Review of Safety, Efficacy, and Implementation Models" Psychoactives 4, no. 4: 35. https://doi.org/10.3390/psychoactives4040035
APA StyleDriscoll, B., & Lakhan, S. E. (2025). Digital Enablement of Psychedelic-Assisted Therapy in Non-Clinical Settings: A Systematic Review of Safety, Efficacy, and Implementation Models. Psychoactives, 4(4), 35. https://doi.org/10.3390/psychoactives4040035






