1. Introduction
Despite growing evidence supporting the efficacy of LSD-assisted psychotherapy in treating major depressive disorder (MDD) [
1,
2,
3], reliable biomarkers of psychopharmacological or treatment response are not yet fully identified [
4]. LSD (lysergic acid diethylamide), a classical serotonergic psychedelic, exerts its acute psychoactive effects primarily through partial agonism at the 5-HT
2A receptor, initiating a cascade of neurotransmitter and neuropeptide release [
5,
6,
7,
8]. The neuropeptide oxytocin, for instance, is released within this cascade and could be a potential psychopharmacological biomarker for LSD-assisted psychotherapy in MDD due to its implication in social bonding, stress modulation, and most importantly, flexibility [
9,
10,
11]. Indeed, theoretical models suggest that flexibility is a major mechanism underlying mood improvement following psychedelic treatment [
12,
13,
14].
The identification of robust biomarkers is crucial in psychopharmacological research, offering objective measures to understand drug action and predict therapeutic outcomes. Biomarkers, broadly defined by the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) in their Biomarkers, EndpointS, and other Tools (BEST) resource as ‘a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions’ (FDA-NIH Biomarker Working Group, Maryland, USA, 2016; [
15]), encompass a diverse range of measurable indicators. Among these, pharmacodynamic biomarkers and predictive biomarkers are particularly essential in the context of novel drug development and therapeutic optimization [
15,
16]. Pharmacodynamic biomarkers reflect the biological effects of a drug, providing insights into its mechanism of action, target engagement, and dose-response relationships. These can include changes in receptor occupancy, alterations in neurochemical levels, or specific physiological responses. A predictive biomarker is characterized by its ability to identify individuals or patient subgroups who are most likely to experience a specific effect, whether favorable or unfavorable, in response to a particular medical product or intervention. It is important to note that a single biomarker may, in certain contexts, serve both pharmacodynamic and predictive roles, providing comprehensive insights into drug efficacy and patient stratification [
15,
16]. For a biomarker to be valuable in research, it must be reproducible. Furthermore, the feasibility of biomarker measurement is a critical consideration in clinical research; ideally, biomarkers should be assessed through accessible and minimally invasive procedures, such as blood, urine, or saliva sampling, to facilitate repeated measurements and enhance patient comfort and compliance [
15,
16].
Within this framework, in healthy volunteers, plasma oxytocin concentrations consistently rise 90–180 min after oral LSD administration, subsequently declining after the peak [
5,
6,
17,
18,
19,
20]. Studies in rodents, conducted over two decades ago, demonstrated that the selective 5-HT
2A agonist (±)-2,5-dimethoxy-4-iodoamphetamine (DOI) produces a comparable oxytocin increase, which is blocked by the 5-HT
2A antagonist ritanserin [
21,
22]. Interestingly, in humans, the antagonist ketanserin attenuates LSD’s subjective effects as well as oxytocin release during LSD [
5]. Despite these convergent findings, oxytocin reactivity during psychedelic treatment has not yet been characterized in psychiatric populations. Furthermore, salivary oxytocin, a convenient and non-invasive measurement method, has not yet been assessed during LSD administration. While the methodological reliability of salivary oxytocin measures is subject to some debate [
23], evidence suggests that repeated measurements, particularly when analyzed using radioimmunoassay (RIA), can enhance the consistency and reliability of such assessments [
23,
24,
25].
Here, we report pilot data on salivary oxytocin dynamics during a single LSD-assisted psychotherapy session in patients with treatment-resistant MDD, conducted under compassionate-use approval from the Swiss Federal Office of Public Health. Our primary objective was to characterize salivary oxytocin reactivity across the acute pharmacodynamic window of a single LSD session. We also explored concurrent ratings of subjective drug intensity.
2. Materials and Methods
2.1. Design and Setting
This single-center, observational, and prospective pilot study was integrated into the routine clinical care at the Psychedelic Program of the Division of Addictology, Geneva University Hospitals (HUG), Switzerland. The study protocol was approved by the regional ethics committee (BASEC No. 2024-01122) and prospectively registered on ClinicalTrials.gov on the 15th of August 2024 (NCT06557239). All procedures adhered to the Declaration of Helsinki and Good Clinical Practice guidelines. Patients were invited to participate in this research after they had obtained individual compassionate-use authorization for LSD-assisted psychotherapy from the Swiss Federal Office of Public Health (OFSP). Following this approval, consecutive patients meeting study inclusion criteria and with scheduled LSD-assisted psychotherapy treatments were approached for participation in this study. The research protocol, which primarily involved additional salivary sample collection, did not alter the standard clinical routine. All participants provided written informed consent prior to any study procedures. Data collection for this pilot study occurred between September 2024 and February 2025.
2.2. Participants
Participants were eligible if they met the following inclusion criteria: major depressive disorder according to the DSM-5 [
26], with criteria of treatment resistance (two or more failed antidepressant treatments of different classes, each administered for at least 6 to 8 weeks at a therapeutic dose and with documented adherence [
27]), aged between 18 and 55 years, were receiving their first or second LSD treatment, with 100 µg being the standard first-cycle dose, and 100 µg or 150 µg used in the second cycle depending on clinical therapeutic choice. There were no restrictions related to ongoing antidepressant medication, menstrual cycle phase, or hormonal contraception use, due to the pilot nature of the study. Exclusion criteria, which are part of the routine protocol, were as follows: current psychotic or bipolar disorder, imminent suicide risk, severe cardiovascular, hepatic or neurological disease, pregnancy or breastfeeding, and systemic corticosteroid use.
Twelve participants (5 females and 7 males), aged between 25 and 55 years (mean: 43.9, SD: 8.79), signed informed consent and agreed to comply with the protocol. As this was a pilot investigation, we targeted 10 participants for full data analysis, a sample size pragmatically determined by resource and budget limitations, leading to a convenience sample. Although 12 participants were initially enrolled, we encountered problems with salivary sampling for two participants (1 male and 1 female), for whom we did not obtain enough saliva at multiple time points, thus impeding the analysis of reactivity. Consistent with our protocol’s anticipation of potential missing data, these issues resulted in 10 participants with complete, analyzable repeated measurements. As the protocol involved a single-day assessment, all participants completed the study session, and there were no dropouts.
2.3. Procedures
The standard clinical procedure for psychedelic-assisted psychotherapy in our unit involves an initial screening session, during which a detailed clinical history is collected and eligibility for the program is checked. This comprehensive assessment typically includes a review of medical and psychiatric comorbidities, a thorough medication history, and an evaluation of any contraindications for psychedelic treatment. During this session, the rationale for LSD-assisted psychotherapy, potential risks and benefits, and the overall treatment trajectory are discussed in detail with the patient. Following this, an application is sent to the federal office of public health to obtain approval for the compassionate use of psychedelics to treat depression. This rigorous approval process ensures regulatory oversight and patient safety for the use of non-approved compounds. Once this approval is obtained, patients have a preparation appointment typically held 1 to 3 weeks before the day of the scheduled treatment. During this meeting, patients are invited to participate in the current research protocol. If a patient declines participation, their clinical treatment proceeds as originally planned without inclusion in the research. For those who consent to participate, beyond the standard clinical preparation for LSD-assisted psychotherapy, additional details regarding the salivary sampling procedure are explained.
The treatment is scheduled for 9:00 AM, and patients are required to be present beforehand to finalize preparation details. Upon arrival, patients undergo a brief medical check, including vital signs assessment (such as heart rate, blood pressure, and body temperature), to ensure their well-being before drug administration. Any last-minute questions or concerns are addressed. At 9:00 AM, they receive their LSD treatment orally as a 1 mL liquid solution containing 0.1 mg/mL of LSD d-tartrate-certified solution for compassionate use from Biel, Switzerland. In cases where an additional half dose was administered, participants received two separate 1 mL solutions, each containing 0.025 mg/mL of LSD. Then, they rest in a quiet room under the supervision of trained psychiatric nurses with over three years of experience in the field, operating within a well-established clinical structure. The setting is designed to be therapeutic and supportive within a sober medical environment. Nurses maintain a continuous presence, monitoring the patient’s physiological and psychological state throughout the acute drug effects. Specific safety protocols are in place to manage any unexpected or challenging experiences. Participants experienced only minor, transient adverse events typically associated with psychedelic administration (blurred vision, dizziness, and nausea), with no need to discontinue the protocol.
The first saliva measure was taken before LSD administration, and subsequent samples were collected following the timeline described below. Although not part of the current research protocol’s primary objectives, it is important to note that patients receive clinical follow-up the day after LSD administration and again one month later. These follow-up appointments collect valuable clinical information that could be utilized in future studies assessing longitudinal treatment outcomes.
2.4. Salivary Oxytocin
Saliva samples were collected using Salivette
® synthetic swabs (Sarstedt, Germany) at four distinct time-points on the day of LSD administration: Pre-LSD (−10 min before taking the substance, representing baseline), then 60 min, 90 min, and 180 min post-LSD, based on the time-frame reported by previous studies [
5,
17] and LSD plasma concentrations [
28,
29]. Regardless of a minor delay in treatment start (up to 20 min from the 9 AM schedule), this baseline measurement was always taken before LSD intake, and subsequent samples were consistently collected according to the protocol at +60, +90, and +180 min post-LSD administration. Due to initial challenges in obtaining sufficient sample volumes, patients were carefully instructed to chew the Salivette swab for at least one minute, or longer if they perceived that the swab was not sufficiently saturated with saliva, as adequate sample volume and saturation are critical for reliable oxytocin quantification and to ensure the reproducibility of analyses. To further enhance sampling reliability and participant familiarity, all participants had the opportunity to practice using the Salivette
® during their pre-treatment inclusion visit. The choice of salivary oxytocin measurement was based on its non-invasive nature, which enhances patient comfort and facilitates repeated sampling within a clinical setting, making it particularly suitable for vulnerable populations and acute drug administration protocols. All participants fully complied with salivary sampling instructions, as collection was closely supervised by a trained psychiatric nurse. Samples were temporarily stored at −20 °C, then centrifuged and stored at −80 °C. Aliquots were subsequently shipped on dry ice to RIAgnosis (Germany), where oxytocin concentrations (expressed in pg/mL) were quantified by radioimmunoassay (RIA). The specific assay procedures are consistent with those described in previous studies, including a study led by our group [
30,
31]. Thus, each 300 µL saliva sample underwent evaporation (Eppendorf Concentrator, Wesseling-Berzdorf, Germany). Following evaporation, 50 µL of assay buffer was introduced, along with 50 µL of oxytocin-specific antibody (raised in rabbits). The detection limit for this radioimmunoassay (RIA) ranged from 0.1 to 0.5 pg/sample. Assay precision was confirmed by intra- and inter-assay coefficients of variation consistently below 10%. To minimize batch effects, all saliva samples were assayed concurrently.
2.5. Subjective Measures
At each salivary oxytocin collection time-point after LSD intake (60, 90, and 180 min post-LSD), participants provided momentary ratings of subjective drug intensity ranging between 0 (none) and 10 (maximal intensity). This home-made scale is regularly used within the clinical routine.
2.6. Statistical Analyses
Analyses were performed in IBM SPSS Statistics 28. A linear mixed model (LMM) was employed to investigate the effect of time on oxytocin levels. The assumption of normality of residuals was visually inspected via QQ-plots and found to be sufficiently met for the purpose of this analysis. Oxytocin levels were designated as the dependent variable. Time, a categorical variable with four levels (Pre-LSD, 60 min post-LSD, 90 min post-LSD, and 180 min post-LSD), was included as a fixed effect. To account for the repeated measures nature of the data and individual variability, participant-specific random intercepts were included in the model, with participant ID as the subject grouping variable. An Autoregressive First-Order (AR1) covariance structure was specified for the within-subject repeated measures of oxytocin. The model was estimated using Restricted Maximum Likelihood (REML). It should be noted that the two participants with insufficient repeated salivary samples (as detailed in the
Section 2.2.) were inherently excluded from this LMM analysis, as these models require multiple time-points for repeated measures. For any remaining sparse missing data points, these were considered at random and were treated within the LMM. A Friedman ANOVA was employed to assess differences in the perceived intensity of psychedelic effects across three repeated measurements (60 min post-LSD, 90 min post-LSD, and 180 min post-LSD). This non-parametric test was chosen due to the ordinal nature of the perceived intensity scale (0–10) and to account for the repeated measures design.
3. Results
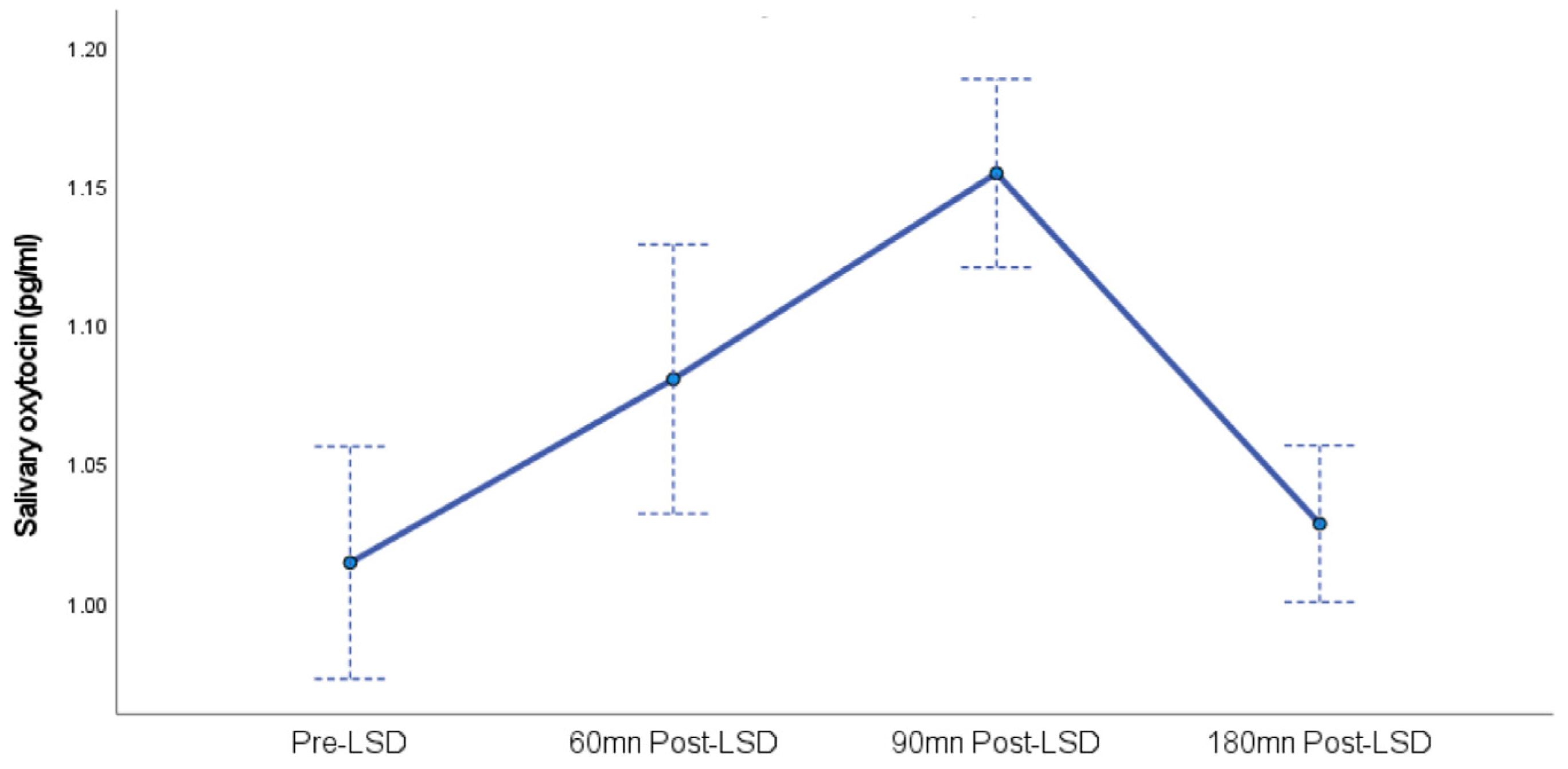
A linear mixed model analysis revealed a significant main effect of time on oxytocin levels, F (3, 19.8) = 14.9,
p < 0.001. This indicates that oxytocin levels changed significantly across the four measurement points (Pre-LSD, 60 min post-LSD, 90 min post-LSD, and 180 min post-LSD). The estimated marginal means for oxytocin levels and standard errors at each time point were Pre-LSD (0.98 ± 0.05 pg/mL), 60 min post-LSD (1.08 ± 0.27 pg/mL), 90 min post-LSD (1.12 ± 0.04 pg/mL), and 180 min post-LSD (1.02 ± 0.03 pg/mL). These values are also presented in
Figure 1.
Regarding the random effects, the estimated variance for participant-specific random intercepts was 0.004 (SE = 0.01). The test of this variance component against zero was not significant (Z = 0.40, p = 0.690), suggesting no detectable significant individual differences in participants’ overall oxytocin levels in this pilot study. The 95% confidence interval for the random intercept variance was [~0.000, 0.587], with the lower bound indicating a boundary solution where the estimated variance is effectively zero.
The estimated AR1 parameter for the within-subject covariance structure was 0.009 (SE = 0.011, 95% CI = [0.001, 0.097]). This parameter was also not significantly different from zero (Z = 0.81, p = 0.418), suggesting no significant autocorrelation between oxytocin levels at adjacent time points within individuals in this sample.
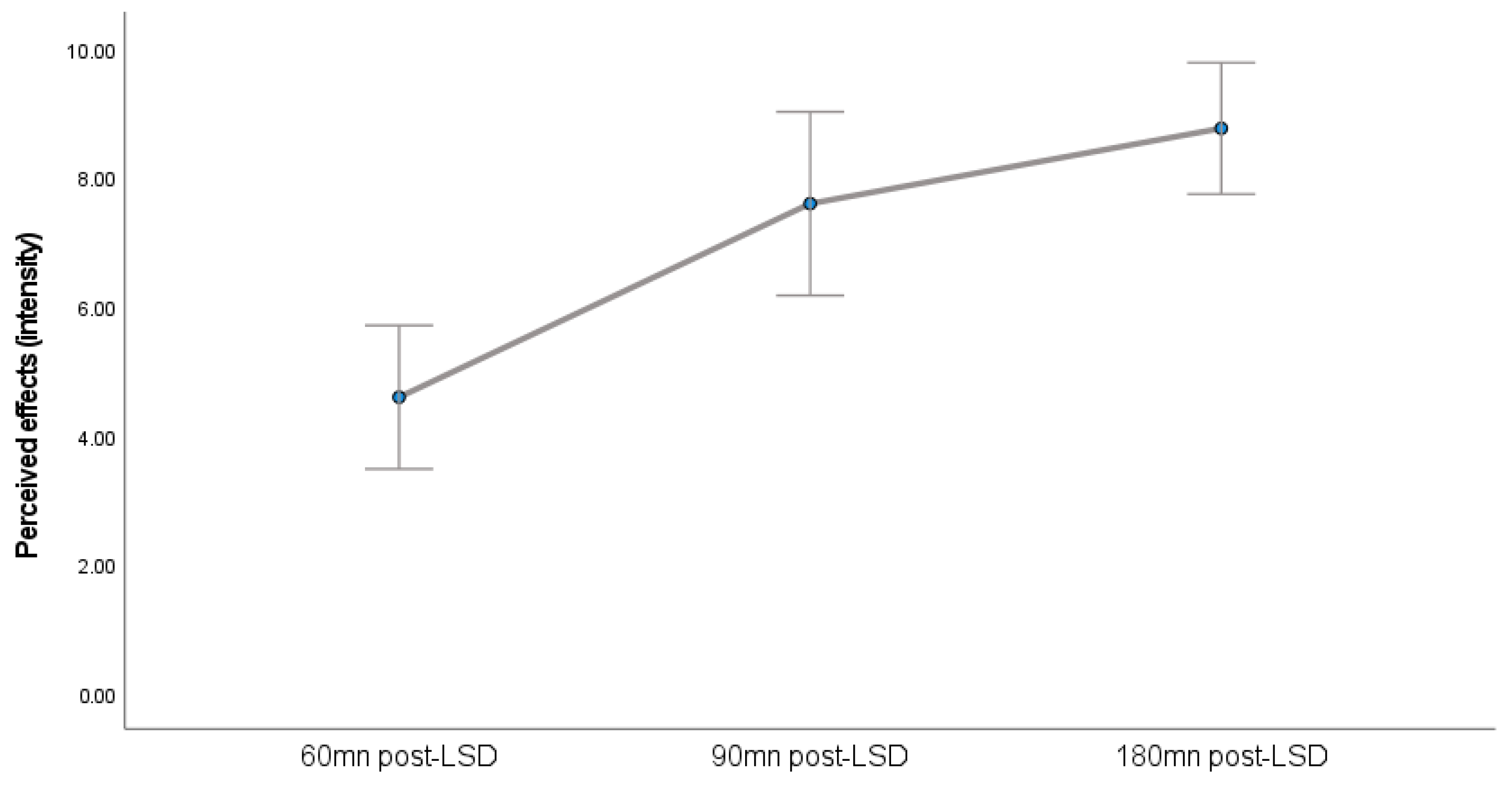
A Friedman ANOVA indicated a statistically significant effect of time on the perceived intensity of psychedelic effects (
Figure 2), χ
2 (2, N = 12) = 21.273,
p < 0.001. This suggests that the perceived intensity of effects varied significantly across the three measurement points (60, 90, and 180 min). For descriptive purposes, the median perceived intensity scores (with minimum and maximum values) were 60 min post-LSD (Median = 5 (Range = 2–7)), 90 min post-LSD (Median = 8 (Range = 3–10)), and 180 min post-LSD (Median = 10 (Range = 6–10)).
Figure 2 visually represents these changes, showing means and standard errors of the mean for illustrative clarity.
4. Discussion
This pilot study presents, to our knowledge, the first characterization of salivary oxytocin dynamics following acute LSD administration in patients with major depressive disorder (MDD). It demonstrates that a non-invasive salivary sampling approach yields temporal information on oxytocin release comparable to that obtained via plasma sampling in earlier investigations. Further, we observed a significant modulation of salivary oxytocin levels over time (with a peak 90 min post-LSD intake). This finding aligns with previous observations in healthy participants [
5,
17,
32] and is consistent with rodent evidence suggesting potential 5-HT2A involvement in oxytocin release [
21]. While MDMA is more widely recognized for potent oxytocin release and distinct empathogenic properties [
18,
20], the shared capacity of these compounds to induce oxytocin release, albeit to differing extents, points to potentially convergent neuroendocrine circuits. On the other hand, psilocybin, also a serotonergic psychedelic, seems to produce less pronounced oxytocin release compared to LSD [
17].
Interestingly, the peak of oxytocin observed 90 min post-LSD intake coincides with the reported LSD plasmatic peak, as described by Dolder and colleagues (2015) [
29]. This is followed by a progressive decrease in both LSD plasma concentrations and, as observed in our study, salivary oxytocin levels by 180 min. Furthermore, Dolder and colleagues [
29] also describe parallel subjective effects that took longer to reach their peak and remained elevated even after plasmatic decrease. This pattern aligns with observations from our pilot study, where subjective effects reached a peak between 90 and 180 min after LSD intake, while we already observed a decrease in salivary oxytocin at 180 min.
The findings of this study contribute preliminary evidence supporting the consideration of oxytocin as a pharmacodynamic biomarker in the context of LSD-assisted psychotherapy for MDD. The observed modulation of salivary oxytocin levels as a function of acute LSD administration, which is consistent with previously reported patterns in both human plasma [
5,
6,
17,
18,
19,
20] and preclinical models [
21,
22], suggests that oxytocin reactivity serves as an indicator of the drug’s acute biological effects. This reproducible observation, obtained through a minimally invasive sampling method, underscores oxytocin’s potential utility for objectively assessing drug engagement and elucidating mechanistic insights. While the current pilot design and sample size preclude definitive conclusions regarding oxytocin’s role as a predictive biomarker, this study establishes the feasibility of characterizing oxytocin dynamics in MDD patients following LSD. Moving forward, larger, adequately powered clinical trials are warranted to conduct the complex correlational analyses necessary to determine if specific oxytocin profiles (e.g., baseline levels or acute changes) correlate with subsequent clinical improvements, such as reductions in depressive symptoms or enhanced mental flexibility, thereby informing patient stratification and personalized treatment approaches.
While the Linear Mixed Model revealed a significant main effect of time on oxytocin levels, the non-significant estimated variance for participant-specific random intercepts did not indicate significant individual differences in overall oxytocin levels within this pilot study. This could cautiously suggest a relatively consistent group-level change pattern rather than major inter-individual baseline variability. Similarly, the non-significant AR1 parameter indicated no significant temporal autocorrelation between adjacent oxytocin measurements, which might point to a more dynamic and less continuously correlated pattern of change in response to the substance. Given the pilot nature and small sample size, these interpretations regarding the absence of significant individual variability or autocorrelation are preliminary and should be made with caution, as the study may have been underpowered to detect subtle effects.
Despite these initial observations, the interpretation and generalizability of the findings are subject to several methodological limitations inherent to a pilot study design. The open-label nature of the investigation, the heterogeneity of symptoms of MDD patients, the heterogeneous medication status of participants, different LSD dosages across participants (100 µg or 150 µg), and the absence of a placebo or active control condition constrain the generalizability of the results. Future, larger clinical trials would ideally incorporate rigorous control conditions, such as a very low-dose psychedelic placebo (i.e., a sub-perceptual dose not producing psychedelic effects), association with Ketanserin or active controls like psilocybin or MDMA, as explored in previous studies with healthy volunteers [
5,
18]; however, implementing such designs may not always be feasible within established clinical routine. Regarding female participants, we did not control for menstrual cycle phase, hormonal contraception use, or menopausal status at this stage, though we acknowledge the critical importance of these variables. Given the well-established influence of sex hormones on the oxytocin system and associated physiological responses [
33], accounting for these factors is crucial for robust oxytocin research, particularly when investigating potential sex-specific effects or treatment outcomes in larger cohorts. Furthermore, consistent with the study’s integration within a habitual clinical routine, participants continued their prescribed antidepressant medication. As there are currently no recommendations to discontinue antidepressant medications that do not primarily act on 5-HT2A receptors during LSD-assisted psychotherapy, patients maintained their ongoing treatment according to standard clinical guidelines. However, the potential for chronic antidepressant administration to influence endogenous oxytocin secretion or reactivity warrants careful consideration (e.g., [
34]). Future investigations should therefore account for the impact of different classes of antidepressant medication on oxytocin dynamics. Additionally, different classes of antidepressants may interact differently with serotonergic psychedelics, either potentiating or attenuating their effects (Halman et al., 2023 [
35] for a detailed review). This should be carefully controlled and analyzed in further trials. Finally, this study did not include longitudinal oxytocin follow-up and was not powered to directly assess the relationship between oxytocin release and long-term treatment response (e.g., reduction of depressive symptoms) or changes in mental flexibility. Thus, future studies could incorporate longitudinal oxytocin follow-up, measuring both basal levels and acute reactivity to assess if acute oxytocin responses under LSD influence subsequent longer-term oxytocin dynamics or treatment response. Therefore, larger, rigorously controlled trials are essential to replicate these preliminary findings and to elucidate the mechanistic links between oxytocin dynamics and clinically meaningful outcomes.
In conclusion, this pilot study provides insights into the salivary oxytocin dynamics following LSD administration in patients with MDD, underscoring oxytocin’s potential as a pharmacodynamic biomarker. While acknowledging the study’s preliminary nature and limitations, these findings establish the foundation for future, larger-scale investigations aimed at clarifying the mechanistic role of oxytocin in psychedelic-assisted psychotherapy and its potential utility as a predictive biomarker for treatment outcomes in this population.