Ayahuasca, Pain, and Inflammation: A Systematic Review of Preclinical Studies
Abstract
1. Introduction
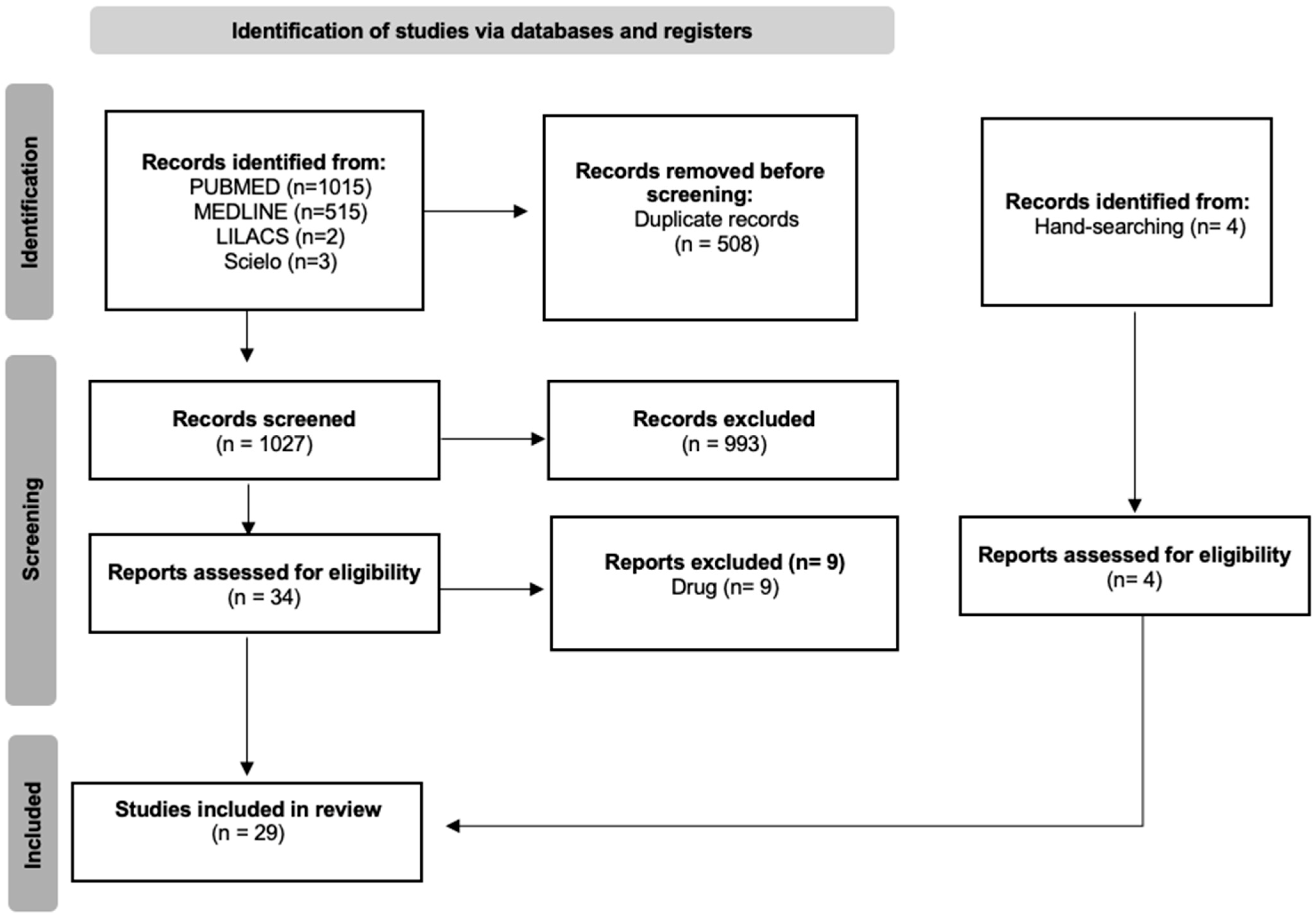
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria and Data Extraction
3. Results
3.1. Selected Studies
3.2. Antinociceptive Effect
| Reference | Sample | Group/Dose/Route | Model/Test | Result |
|---|---|---|---|---|
| Pires et al. (2018) [31] | Male Swiss mice | Control (saline i.p. or water.p.o.) Ayahuasca (120 and 1200 mg/kg i.p. or p.o.; Dimethyltriptamine 0.38 mg/mL, harmine 0.32 mg/mL, tetrahydroharmine 1.67 mg/mL, harmaline 0.12 mg/mL) Morphine (1 and 10 mg/kg i.p.) | Hot plate Acetic acid writhing (0.8%. i.p. of acetic acid) Formalin (20 μL) | Ayahuasca (1200 mg/kg) showed an antinociceptive effect in the acetic acid writhing test and the formalin test (but not in the hot plate test) and intensified the analgesic effect of morphine in the hot plate test and the acetic acid writhing test. |
| Alijanpour & Zarrindast (2020) [32] | NMRI male mice | Control (saline) Morphine sulfate (2 and 6 mg/kg i.p.) Naloxone hydrochloride (0.5 and 1 mg/kg i.p.) N-methyl-d-aspartate (NMDA) (0.06 and 0.1 μg/mouse; intra-vental tegmental area-VTA) Harmaline (1.25 and 5 mg/kg i.p.) dl-2-amino-5-phosphonoheptanoate (D-AP5) (NMDA receptor antagonist) (0.5 and 1 μg/mouse; intra-VTA) | Tail-flick: Pain threshold mu opioid receptor and analgesia Microinjection intra-VTA of NMDA and D-AP5 | No significant effects were observed at low doses of morphine (2 mg/kg) or harmaline (1.25 and 5 mg/kg) on pain threshold; Harmaline (5 mg/kg) intensified the analgesic effect of morphine; after a combination of morphine and harmaline at non-effective doses (2 and 5 mg/kg, respectively), pain threshold was increased and antinociception was induced. Microinjection of NMDA (0.06 and 0.1 μg) reduced the antinociceptive effect induced by that combination. In addition, analgesia induced by morphine and harmaline was inhibited by naloxone (0.5 and 1 mg). No effects were observed on pain threshold after microinjections intra-VTA of D-AP5 (0.5 and 1 μg) and NMDA (0.06 and 0.1 μg); However, D-AP5 increased pain threshold and intensified antinociception induced by morphine and harmaline. |
| Alijanpour et al. (2021) [33] | Male NMRI mice | Control (saline) Harmaline (0, 1.25, 2.5, 5, and 10 mg/kg i.p.) Morphine sulfate (0, 2, 4, and 6 mg/kg. i.p.) Muscimol (0, 200, and 300 ng/mice.i.ventral hippocampal route-vHIP) Bicuculline (1 μL/mouse.i.vHip route) | Tail-flick | Harmaline group showed an antinociception effect at 10 mg/kg. Harmaline (5 mg/kg) combined with morphine (2 mg/kg) produced an antinociceptive effect and enhanced morphine effects. Muscimol enhanced antinociceptive effects of harmaline combined with morphine. |
| Alijanpour et al. (2022) [34] | Male NMRI mice | Control (saline 10 mL/kg i.p.) Vehicle (0.6 μL/mouse. Microinjection into the basolateral amygdala (BLA)) Harmaline (4, 6, and 8 mg/kg i.p.) ACPA (0.5, 1, or 1.5 ng/mouse.micorinjection into the BLA) AM251 (0.1, 0.5, or 1 ng/mouse.microinjection into the BLA) | Tail-flick Hot plate | Harmaline (6 and 8 mg/kg) prolonged reaction time to thermal stimuli in tail-flick and hot plate tests. AM251 (0.5 and 1 ng/mouse) attenuated the antinociceptive activity of harmaline (8 ng/mouse) in both tests. |
| Kadyan & Singh (2024) [36] | Swiss albino mice | Control (vehicle 0.1% carboxymethyl cellulose in distilled water 10 mL/kg i.p.) Pathogenic control-vincristine and vehicle (0.1 mg/kg i.p.; once daily for 10 days) Positive control-vincristine (0.1 mg/kg i.p.) + pregabalin (10 mg/kg p.o.) Harmaline (5 mg/kg i.p.; for 14 days) Harmaline (10 mg/kg i.p.; for 14 days) Substance-P (10 μg/kg i.p.) + harmaline (10 mg/kg i.p) + vincristine (0.1 mg/kg i.p.); for 10 days | Vincristine-induced peripheral neuropathy Hot plate Cold plate Acetone drop Beam balance | Harmaline reduced licking and biting behavior and improved paw withdrawal latency in a dose-dependent manner on days 7 and 14 compared to the vincristine-treated group. It also decreased crossing time, with the 10 mg/kg dose showing a stronger effect than pregabalin. Harmaline (5 mg/kg) increased Nrf-2 levels, while 10 mg/kg reversed vincristine-induced Nrf-2 suppression. Additionally, harmaline countered vincristine-induced elevation of IL-1β, reducing its levels at both doses. |
| Lauria et al. (2024) [35] | Male Swiss and C57BL/6 mice | Control (water p.o.) Ayahuasca (24, 120, 600, and 3000 μL/kg p.o.) Harmine (0, 0.35, 3.5, and 35 mg/kg p.o.) Indomethacin (5 mg/kg p.o.) Morphine (5 mg/kg i.p.) Dexamethasone (2 mg/kg i.p.) Formalin (20 μL) Complete Freund’s Adjuvant (CFA) (20 μL) Gabapentin (70 mg/kg p.o.) Naloxone (5 mg/kg i.p.) Bicuculline (1 mg/kg i.p.) Methysergide (5 mg/kg i.p.) Rimonabant (10 mg/kg i.p.) Phaclofen (2 mg/kg i.p.) | Acute effect: Formalin test CFA Tail flick test Paw edema Acute and chronic effect: Partial sciatic nerve ligation model of neuropathic pain | Acute effect: Ayahuasca (120, 600, and 3000 μL/kg) and indomethacin (5 mg/kg) demonstrated antinociceptive effects at in the second phase of the formalin test. Morphine (5 mg/kg) was effective in inducing antinociceptive effects in both phases of the formalin test. Ayahuasca (600–3000 μL/kg) decreased CFA-induced allodynia but did not reduce paw edema. Allodynia and edema were both reduced by dexamethasone (2 mg/kg). In the tail flick test, ayahuasca did not show an antinociceptive effect, while morphine (5 mg/kg) caused antinoception. Ayahuasca (24–3000 μL/kg) and gabapentin (70 mg/kg) decreased allodynia in experimental neuropathy. The effects of ayahuasca (3000 μL/kg) lasted 10 h, while the effects of gabapentin (70 mg/kg) lasted only 3 h. At 3.5 mg/kg harmine produced an antinociceptive effect that lasted 10 h. Chronic effect: Ayahuasca (120–600 μL/kg twice a day for 14 days) and gabapentin (70 mg/kg daily for 14 days) induced antinoception in experimental neuropathy. Bicuculline (1 mg/kg) and methysergide (5 mg/kg) reverted the antinociceptive effect of ayahuasca (600 μL/kg), however, naloxone (5 mg/kg), phaclofen (2 mg/kg), and rimonabant (10 mg/kg) did not revert. At 3.5 mg/kg twice a day for 14 days, harmine produced an antinociceptive effect that lasted 10 h. |
3.3. Anti-Inflammatory Effect
3.3.1. Harmine
3.3.2. Harmaline
3.3.3. DMT
3.3.4. THH
3.3.5. Ayahuasca
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IASP—International Association for the Study of Pain. IASP Task Force on Taxonomy. In Classification of Chronic Pain; IASP: Malaga, Spain, 2017. [Google Scholar]
- Hylands-White, N.; Duarte, V.R.; Raphael, H.J. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Machado, A. Neuroanatomia Funcional, 4th ed.; Atheneu: Rio de Janeiro, Brazil, 2022. [Google Scholar]
- Kandel, E.R.; Schwartz, J.H.; Jessel, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Princípios De Neurociências, 5th ed.; AMGH: Porto Alegre, Brazil, 2014. [Google Scholar]
- Yang, S.; Chang, M.C. Chronic Pain: Structural and functional changes in brain structures and associated negative affective states. Int. J. Mol. Sci. 2019, 20, 3130. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.T.; Lu, P.L. Thalamus and pain. Acta Anesthesiol. Taiwanica 2013, 51, 73–80. [Google Scholar] [CrossRef]
- Abbas ALitchman, A.; Pillai, S. Imunologia Celular e Molecular, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, M.J.; Kettner, H.; Carhart-Harris, L.R. Positive effects of psychedelics on depression and wellbeing scores in individuals reporting an eating disorder. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2021, 26, 1265–1270. [Google Scholar] [CrossRef]
- De Gregorio, D.; Aguillar-Valles, A.; Preller, K.H.; Heifets, B.D.; Hibicke, M.; Mitchell, J.; Gobbi, G. Hallucinogens in mental health: Preclinical and clinical studies on LSD, psilocybin, MDMA, and ketamine. J. Neurosci. 2021, 41, 891–900. [Google Scholar] [CrossRef]
- Walsh, Z.; Mollaahmetoglu, O.M.; Rootman, J.; Golsof, S.; Keeler, J.; Marsh, B.; Nutt, D.J.; Morgan, C.J.A. Ketamine for the treatment of mental health and substance use disorders: Comprehensive systematic review. Br. J. Psychiatry 2022, 8, e19. [Google Scholar] [CrossRef]
- Keeler, L.J.; Treasure, J.; Juruena, M.F.; Kan, C.; Himmerich, H. Ketamine as a treatment for anorexia nervosa: A narrative review. Nutrients 2021, 13, 4158. [Google Scholar] [CrossRef]
- Castellanos, J.P.; Woolley, C.; Bruno, K.A.; Zeidan, F.; Halberstadt, A.; Furnish, T. Chronic pain and psychedelics: A review and proposed mechanism of action. Reg. Anesth. Pain Med. 2020, 45, 486–494. [Google Scholar] [CrossRef]
- Ramaekers, J.G.; Hutten, N.; Mason, N.L.; Dolder, P.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Feilding, A.; Kuypers, K.P. A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. J. Psychopharmacol. 2020, 35, 398–405. [Google Scholar] [CrossRef]
- Burmester, D.R.; Madsen, M.K.; Szabo, A.; Aripaka, S.S.; Stenbaek, D.S.; Frokjaer, V.G.; Elfving, B.; Mikkelsen, J.D.; Knudsen, G.M.; Fisher, P.M. Subacute effects of a single dose of psilocybin on biomarkers of inflammation in healthy humans: An open-label preliminary investigation. Compr. Psychoneuroendocrinol. 2022, 13, 100163. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.L.; Szabo, A.; Kuypers, K.P.C.; Mallaroni, P.A.; de la Torre Fornell, R.; Reckweg, J.T.; Tse, D.H.Y.; Hutten, N.R.P.W.; Feilding, A.; Ramaekers, J.G. Psilocybin induces acute and persisting alterations in immune status in healthy volunteers: An experimental, placebo-controlled study. Brain Behav. Immun. 2023, 114, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, A.; Loizaga-Velder, A.; Fletcher, K.J.; Renelli, M.; Files, N.; Tupper, K.W. Nourishing the spirit: Exploratory research on ayahuasca experiences along the continuum of recovery from eating disorders. J. Psychoact. Drugs 2017, 49, 427–435. [Google Scholar] [CrossRef]
- James, E.; Kepler, J.; Robertshaw, T.L.; Sessa, B. N, N-dimethyltryptamine and Amazonian ayahuasca plant medicine. Hum. Psychopharmacol. 2022, 37, e2835. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Osório, F.L.; Crippa, J.A.S.; Hallak, J.C. Antidepressive and Anxiolytic Effects of Ayahuasca: A Systematic Literature Review of Animal and Human Studies. Rev. Bras. De Psiquiatr. 2016, 38, 65–72. [Google Scholar] [CrossRef]
- Niznanska, L.; Niznanska, Z.; Kuruc, R.; Szoradova, A.; Sikuta, J.; Zummerova, A. Ayahuasca as a decoction applied to human: Analytical methods, pharmacology and potential toxic effects. J. Clin. Med. 2022, 11, 1147. [Google Scholar] [CrossRef]
- Kassik, H.; Souza, R.C.Z.; Zandonadi, F.S.; Tófoli, L.F.; Sussulini, A. Chemical composition of traditional and analog ayahuasca. J. Psychoact. Drugs 2021, 53, 65–75. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Osório, F.L.; Rocha, J.M.; Rossi, G.N.; Bouso, J.C.; Rodrigues, L.S.; Silveira, G.O.; Yonamine, M.; Hallak, J.E.C. Ayahuasca Improves Self-Perception of Speech Performance in Subjects With Social Anxiety Disorder: A Pilot, Proof-of-Concept, Randomized, Placebo-Controlled Trial. J. Clin. Psychopharmacol. 2021, 41, 540–550. [Google Scholar] [CrossRef]
- Almeida, C.A.F.; Pereira-Junior, A.A.; Rangel, J.G.; Pereira, B.P.; Costa, K.C.M.; Bruno, V.; Silveira, G.O.; Ceron, C.S.; Yonamine, M.; Camarini, R.; et al. Ayahuasca, a Psychedelic Beverage, Modulates Neuroplasticity Induced by Ethanol in Mice. Behav. Brain Res. 2022, 416, 113546. [Google Scholar] [CrossRef]
- Ona, G.; Troncoso, S. Long-lasting analgesic effect of the psychedelic drug changa: A case report. J. Psychedelic Stud. 2019, 3, 7–13. [Google Scholar] [CrossRef]
- Jin, S.J.; Song, Y.; Park, H.S.; Park, K.W.; Lee, S.G.; Kang, H. Harmine Inhibits Multiple TLR-Induced Inflammatory Expression through Modulation of NF-κB p65, JNK, and STAT1. Life 2022, 12, 2022. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.W.L.; Moreira, D.C.; Borges, T.K.D.S.; Caldas, E.D. Components of Banisteriopsis caapi, a Plant Used in the Preparation of the Psychoactive Ayahuasca, Induce Anti-Inflammatory Effects in Microglial Cells. Molecules 2022, 27, 2500. [Google Scholar] [CrossRef]
- Villarinho, J.G.; Pinheiro Kde, V.; Pinheiro Fde, V.; Oliveira, S.M.; Machado, P.; Martins, M.A.P.; Bonacorso, H.G.; Zanatta, N.; Fachinetto, R.; Ferreira, J. The antinociceptive effect of reversible monoamine oxidase-A inhibitors in a mouse neuropathic pain model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 136–142. [Google Scholar] [CrossRef]
- Galvão-Coelho, N.; Galvão, A.C.M.; Almeida, R.N.; Palhano-Fontes, F.; Braga, I.C.; Soares, B.L.; Maia-de-Oliveira, J.P.; Perkins, D.; Sarris, J.; de Araujo, D.B. Changes in inflammatory biomarkers are related to the antidepressant effects of ayahuasca. J. Psychopharmacol. 2020, 10, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.X.B.; Ng, W.S.; Lim, E.S.Y.; Goh, B.H.; Kumari, Y. The immunomodulatory effects of classical psychedelics: A systematic review of preclinical studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 136, 111139. [Google Scholar] [CrossRef]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. A declaração PRISMA 2020: Diretriz atualizada para relatar revisões sistemáticas. Epidemiol. E Serviços Da Saúde 2022, 31, e112. [Google Scholar]
- Pires, J.M.; Mendes, F.R.; Pires, A.P.S.; Yonamine, M.; Amaral, J.L.G.; Carlini, E.A. Pre-clinical interaction of ayahuasca, a brew used in spiritual movements, with morphine and propofol. Braz. J. Pharm. Sci. 2018, 54, e17174. [Google Scholar] [CrossRef]
- Alijanpour, S.; Zarrindast, M.R. Potentiation of morphine-induced antinociception by harmaline: Involvement of μ-opioid and ventral tegmental area NMDA receptors. Psychopharmacology 2020, 237, 557–570. [Google Scholar] [CrossRef]
- Alijanpour, S.; Jafaripour, S.; Ghasemzadeh, Z.; Khakpai, F.; Zarrindast, M.R. Harmaline potentiates morphine-induced antinociception via affecting the ventral hippocampal GABA-A receptors in mice. Eur. J. Pharmacol. 2021, 893, 173806. [Google Scholar] [CrossRef]
- Alijanpour, S.; Ghasemzadeh, Z.; Ebrahimi-Ghiri, M.; Zarrindast, M.R. Basolateral amygadala cannabinoid CB1 receptors mediate the antinociceptive activity of harmaline in adolescent male mice. Physiol. Behav. 2022, 254, 113886. [Google Scholar] [CrossRef]
- Lauria, P.S.S.; Gomes, J.M.; Abreu, L.S.; Santana, R.C.; Nunes, V.L.C.; Couto, R.D.; Covalope, P.O.; da Silva, M.S.; Soares, M.B.P.; Villarreal, C.F. Ayahuasca and its major component harmine promote antinociceptive effects in mouse models of acute and chronic pain. J. Ethnopharmacol. 2024, 323, 117710. [Google Scholar] [CrossRef]
- Kadyan, P.; Singh, L. Harmaline attenuates chemotherapy-induced peripheral neuropathy: Modulation of Nrf-2 pathway and NK-1 receptor signaling. Neurosci. Lett. 2024, 842, 138003. [Google Scholar] [CrossRef] [PubMed]
- Hamsa, T.P.; Kuttan, G. Harmine inhibits tumour specific neo-vessel formation by regulating VEGF, MMP, TIMP and pro-inflammatory mediators both in vivo and in vitro. Eur. J. Pharmacol. 2010, 649, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Kawano, Y. Inhibitory Effects of Herbal Alkaloids on the Tumor Necrosis Factor-α and Nitric Oxide Production in Lipopolysaccharide-Stimulated RAW264 Macrophages. Chem. Pharm. Bull. 2011, 59, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, W.; Yao, Q.; Liu, Y.; Yu, J.; Zang, L.; Wang, S.; Zhou, L.; Wen, S.; Luo, Y.; et al. Harmine ameliorates CCl4-induced acute liver injury through suppression of autophagy and inflammation. Int. Immunopharmacol. 2024, 129, 111538. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Wang, W.; Ge, G.; Xu, N.; Zheng, D.; Jiang, S.; Zhao, G.; Xu, Y.; Zhu, R.; et al. Harmine Alleviates Titanium Particle-Induced Inflammatory Bone Destruction by Immunomodulatory Effect on the Macrophage Polarization and Subsequent Osteogenic Differentiation. Front. Immunol. 2021, 12, 657687. [Google Scholar] [CrossRef]
- Ghandari, A.; Jalili, C.; Salahshoor, M.R.; Javanmardy, S.; Ravankhah, S.; Akhshi, N. Harmine mitigates cisplatin-induced renal injury in male mice through antioxidant, anti-inflammatorym and anti-apoptosis effects. Pharm. Sci. 2022, 17, 417–427. [Google Scholar]
- Liu, X.; Li, M.; Tan, S.; Wang, C.; Fan, S.; Huang, C. Harmine is an inflammatory inhibitor through the suppression of NF-κB signaling. Biochem. Biophys. Res. Commun. 2017, 489, 332–338. [Google Scholar] [CrossRef]
- Niu, X.; Yao, Q.; Li, W.; Zang, L.; Li, W.; Zhao, J.; Liu, F.; Zhi, W. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3 inflammasome signalling pathway in mice. Eur. J. Pharmacol. 2019, 849, 160–169. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, T.; Zhang, L.; Cheng, X.; Wang, C. The Transdermal Performance, Pharmacokinetics and Anti-inflammatory Pharmacodynamics Evaluation of Harmine-loaded Ethosomes. Drug Dev. Ind. Pharm. 2020, 46, 101–108. [Google Scholar] [CrossRef]
- Ju, C.; Wang, Y.; Zang, C.; Liu, H.; Yuan, F.; Ning, J.; Shang, M.; Ma, J.; Li, G.; Yang, Y.; et al. Inhibition of Dyrk1A Attenuates LPS-Induced Neuroinflammation via the TLR4/NF-κB P65 Signaling Pathway. Inflammation 2022, 45, 2375–2387. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Lin, X.C.; Lu, Y.; Cao, S.R.; Liu, X.K.; Lin, D.; Yang, F.H.; Zhang, Y.B.; Tu, J.L.; Pan, B.X.; et al. Harmine exerts anxiolytic effects by regulating neuroinflammation and neuronal plasticity in the basolateral amygdala. Int. Immunopharmacol. 2023, 119, 110208. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, W.; Dang, R.; Hu, X.; Cai, F.; Xiang, Z.; Zhao, X.; Chang, X.; Wang, C. Effects and mechanisms of harmine on ameliorating ethanol-induced memory impairment. J. Ethnopharmacol. 2025, 337, 118789. [Google Scholar] [CrossRef]
- Zheng, D.; Zuo, Y.; Li, L.; McDowell, A.; Cao, Y.; Ye, X.; Zhou, H.; Peng, C.; Deng, Y.; Lu, J.; et al. Natural harmaline acts as novel fluorescent probe for hypochlorous acid and promising therapeutic candidate for rheumatoid arthritis. J. Photochem. Biol. B Biol. 2024, 258, 1011–1344. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N, N-dimethyltryptamine and 5-methoxy-N, N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef]
- Szilágyi, A.; Takács, B.; Szekeres, R.; Tarjányi, V.; Bombicz, M.; Priksz, D.; Kovacs, A.; Juhasz, B.; Frecska, E.; Szilvassy, Z.; et al. Therapeutic Properties of Ayahuasca Components in Ischemia/Reperfusion Injury of the Eye. Biomedicines 2022, 10, 997. [Google Scholar] [CrossRef]
- Nardai, S.; László, M.; Szabó, A.; Alpár, A.; Hanics, J.; Zahola, P.; Merkely, B.; Frecska, E.; Nagy, Z. N, N-dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp. Neurol. 2020, 327, 113245. [Google Scholar] [CrossRef]
- Kelley, D.P.; Venable, K.; Destouni, A.; Billac, G.; Ebenezer, P.; Stadler, K.; Nichols, C.; Barker, S.; Francis, J. Pharmahuasca and DMT rescue ROS production and differentially expressed genes observed after predator and psychosocial stress: Relevance to human PTSD. ACS Chem. Neurosci. 2022, 13, 257–274. [Google Scholar] [CrossRef]
- Borbély, E.; Varga, V.; Szogi, T.; Schuster, I.; Bozso, Z.; Penke, B.; Fulop, L. Impact of two neuronal sigma-1 receptor modulators, PRE084 and DMT, on neurogenesis and neuroinflammation in an AB1-42-injected, wild-type mouse model of AD. Int. J. Mol. Sci. 2022, 23, 2514. [Google Scholar] [CrossRef]
- Gonçalves, J.; Luís, Â.; Gradillas, A.; García, A.; Restolho, J.; Fernandez, N.; Domingues, F.; Gallardo, E.; Duarte, A.P.D. Ayahuasca Beverages: Phytochemical Analysis and Biological Properties. Antibiotics 2020, 9, 731. [Google Scholar] [CrossRef]
- De Camargo, R.W.; Joaquim, L.; Machado, R.S.; Ramos, S.D.S.; da Rosa, L.R.; Junior, L.R.N.; Mathuas, K.; Maximiano, L.; Strickert, Y.R.; Nord, R.; et al. Ayahuasca pretreatment prevents sepsis-induced anxiety-like behavior, neuroinflammation, and oxidative stress and increases brain-derived neurotrophic factor. Mol. Neurobiol. 2024, 62, 5695–5719. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.; Gagnon, J.D.; Goel, G.; Roche, M.I.; Conway, K.L.; Tran, K.; Aldrich, L.N.; Sundberg, T.B.; Paterson, A.M.; Mordecai, S.; et al. The kinases DYRK1A reciprocally regulates the differentiation of Th 17 and regulatory T cells. eLife. 2015, 4, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Graça, S.C.; Bustelli, I.S.; dos Santos, E.V.; Fernandes, C.G.; LAnaro, R.; Stilhano, R.S.; Linardi, A.; Caetano, A.L. Banisteriopsis caapi extract: Implications for neuroinflammatory pathways in Locus coeruleus lesion rodent model. J. Ethnopharmacol. 2025, 337, n.p. [Google Scholar] [CrossRef]
- Monsef, H.R.; Ghobadi, A.; Iranshahi, M. Antinociceptive effects of Peganum harmala L. alkaloid extract on mouse formalin test. J. Pharm. Sci. 2004, 7, 65–69. [Google Scholar]
- Farouk, L.; Laroubi, A.; Aboufatima, R.; Benharref, A.; Chait, A. Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L.: Possible mechanisms involved. J. Ethnopharmacol. 2008, 115, 449–454. [Google Scholar] [CrossRef]
- Kuraishi, Y.; Harada, Y.; Aratani, S.; Satoh, M.; Takagi, H. Separate involvement of the spinal noradrenergic and serotoninergic systems in morphine analgesia: The differences in mechanical and thermal algesic tests. Brain Res. 1983, 273, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ilif, J.; Martinez, S.S.; Bornemann, J.; Leon, R.; Oskooee, N.; Chowdhury, A. Could psychedelics treat neuropathic chronic pain? Neurol. Sci. Neurosurg. 2023, 4, 1–13. [Google Scholar]
- Kooijman, N.I.; Willegers, T.; Reuser, A.; Mulleners, W.M.; Kramers, C.; Vissers, K.C.P.; Wal, S.E.I.V.D. Are psychedelics the answer to chronic pain? A review of current literature. Pain Pract. 2023, 23, 447–458. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Shen, L.; Zhou, J.; Lv, X.; Ma, H.; Li, N.; Wu, Q.; Duan, J. Anti-inflammatory and analgesic actions of bufotenine through inhibiting lipid metabolism pathway. Biomed. Pharmacother. 2021, 140, 111749. [Google Scholar] [CrossRef]
- Zanikov, T.; Gerasymchuk, M.; Gojani, E.G.; Robinson, G.I.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cameron, M. The effect of combined treatment of psilocybin and eugenol on lipopolysaccharide-induced brain inflammation in mice. Molecules 2023, 28, 2624. [Google Scholar] [CrossRef]
- Robinson, G.I.; Li, D.; Rahman, T.; Gerasymchuk, M.; Hudson, D.; Kovalchuk, O.; Kovalchuk, I. Psilocybin and eugenol reduce inflammation in human 3D epiIntestinal tissue. Life 2023, 13, 2345. [Google Scholar] [CrossRef] [PubMed]
- Teksen, Y.; Gunduz, M.K.; Berikten, D.; Ozatik, F.Y.; Aydin, H.E. Peganum harmala L. seed extract attenuates anxiety and depression in rats by reducing neuroinflammation and restoring the BDNF/TrkB signaling pathway and monoamines after exposure to chronic unpredictable mild stress. Metab. Brain Disesase 2024, 8, 1523–1541. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Valle, M.; Bouso, J.C.; Nomdedeu, J.F.; Rodriguez-Espinosa, J.; Mcllhenny, E.H.; Barker, S.A.; Barbanoj, M.J.; Riba, J. Autonomic, neuroendocrine, and immunological effects of ayahuasca: A comparative study with d-amphetamine. J. Clin. Psychopharmacol. 2011, 31, 717–726. [Google Scholar] [CrossRef] [PubMed]
| Cytokines | ||||
|---|---|---|---|---|
| Pro-Inflammatory | Anti-Inflammatory | |||
| Reference | Substance | IL-1β IL-6 IL-7 IL-17 IL-12 IFN-γ TNF-α | IL-2 IL- 4 IL-10 | |
| [36] | Harmaline | ↓ | ||
| [37] | Harmine | ↓ | ↑ | |
| [25] | Harmine | ↓ | ||
| [38] | Harmine | ↓ | ||
| [39] | Harmine | ↓ | ||
| [40] | Harmine | ↓ | ↑ | |
| [42] | Harmine | ↓ | ||
| [43] | Harmine | ↓ | ||
| [44] | Harmine | ↓ | ||
| [45] | Harmine | ↓ | ||
| [46] | Harmaline | ↓ | ||
| [47] | Harmine | ↓ | ||
| [49] | DMT | ↓ | ↑ | |
| [51] | DMT | ↓ | ↑ | |
| [52] | Harmaline + DMT | ↓ | ↓ | |
| [53] | DMT | ↓ | ||
| [26] | Harmine, harmaline and THH | ↓ | ↓ | |
| [55] | Ayahuasca | ↑ | ||
| [56] | Harmine | ↓ | ||
| [57] | Harmine, harmaline and THH | ↓ |
| Reference | Sample | Group/Dose/Route | Model/Test | Result |
|---|---|---|---|---|
| Hamsa & Kuttan (2010) [37] | Male C57BL/6 mice | Harmine (10 mg/kg i.p.) TNP-470 (reference compound) (30 mg/kg i.p.) | Melanoma cell-induced inflammatory processes (intradermally) | Harmine reduced serum levels of the pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and GM-CSF, of the pro-inflammatory transcription factor NF-κB, and of iNOS. Harmine also increased levels of the anti-inflammatory/anti-tumor cytokine IL-2. |
| Yamazaki & Kawano (2010 [38] | Mouse RAW264 macrophage cells/human THP-1 cells | Harmine (0.1–10 μM) LPS (100 μg/mL) | Lipopolysaccharide (LPS)-stimulated | The production of IL-6 and TNF-α in RAW264 cells were suppressed by harmine. Harmine also suppressed the production of TNF-α and NO in LPS-stimulated immune cells. |
| Szabo et al. (2014) [49] | Human primary monocyte-derived dendritic cell (moDCs) coats | N, N-dimethyltryptamine (DMT) (100 μM) 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) (100 μM) PRE-084 (high affinity sigma-1 agonist) (100 μM) | Induction of inflammatory responses induced by LPS, polyI:C or pathogen-derived stimuli (influenza virus and Escherichia coli) | Pre-treatment of LPS- and polyI:C-stimulated moDCs with DMT, 5-MeO-DMT, and PRE- reduced the mRNA expression and secreted levels of the pro-inflammatory cytokines IL-1b, IL-6, TNF-α, and also of the chemokine IL-8/CXCL8 (only with LPS). DMT increased the production of the anti-inflammatory cytokine IL-10. No significant differences between DMT and 5-MeO-DMT were observed. DMT and 5-MeO also reduced the CD4+ helper T cell-priming capacity of moDCs [increased expression of IFNϒ (Th1) and IL-17(Th17)] induced by influenza virus and Escherichia coli. Sigmar-1 gene expression silencing significantly reduced these effects. |
| Khor et al. (2015) [56] | Murines: Balb/c, C57Bl/6, Cd45.1+/+, Rag1−/−, Foxp3IRES-GFP, Il17IRES-GFP, NOD-scid and NOD-BDC2.5 Cells: CD4 + CD62L + naive T cells (murine) CD4 + CD45RA + naive T cells (human) | Harmine HCl (i.n.) 1 mg for 5 days | Immune homeostasis via Th17/T-regulatory cell balance (T-regulatory suppression assay, TD1, CD45RB colitis, and airway inflammation models) | It was shown through diverse experiments that harmine prevents inflammation through modulation and restoring of Th17/T-regulatory cell proportion. This is achieved specifically via inhibition of Dyrk1a activity, which in turn enhances T-regulatory cell differentiation. |
| Liu et al. (2017) [42] | ICR male mice, mouse RAW 264.7 macrophage cells, HEK-293T cells | Control LPS (10 mg/kg i.p.) + vehicle LPS (10 mg/kg i.p.) + dexamethason (3 mg/kg) LPS (10 mg/kg i.p.) + harmine (30 mg/kg) | LPS-induced inflammatory processes | Harmine has an anti-inflammatory effect by reducing NF-kB transcription activity in HEK-293T cells, decreasing TNF-α, IL-6, and IL-1β levels in RAW 264.7 cells, and reducing the production of NO in RAW 264.7 cells. Harmine (and dexamethason) also reduced the protein levels of TNF-α, IL-1β, and IL-6 and suppressed pulmonary congestion, alveolar wall and inflammatory cell infiltration in an acute lung injury mouse model (LPS-induced inflammation). |
| Pires et al. (2018) [31] | Male mice | Control (saline i.p. or water p.o.) Ayahuasca (120 and 1200 mg/kg i.p. or p.o; DMT 0.38 mg/mL, harmine 0.32 mg/mL, tetrahydroharmine 1.67 mg/mL, harmaline 0.12 mg/mL) Morphine (1 and 10 mg/kg i.p.) | Hot plate Acetic acid writhing (0.8% i.p. of acetic acid) Formalin (20 μL) | Ayahuasca (1200 mg/kg) showed an antinociceptive effect in the acetic acid writhing test and the formalin test (but not in the hot plate test) and intensified the analgesic effect of morphine in the hot plate test and the acetic acid writhing test. |
| Alijanpour & Zarrindast (2020) [32] | NMRI male mice | Control (saline) Morphine sulfate (2 and 6 mg/kg i.p.) Naloxone hydrochloride (0.5 and 1 mg/kg i.p.) N-methyl-d-aspartate (NMDA) (0.06 and 0.1 μg/mouse; intra-vental tegmental area-VTA) Harmaline (1.25 and 5 mg/kg i.p.) dl-2-amino-5-phosphonoheptanoate (D-AP5) (NMDA receptor antagonist) (0.5 and 1 μg/mouse; intra-VTA) | Tail-flick: Pain threshold mu opioid receptor and analgesia Microinjection intra-VTA of NMDA and D-AP5 | No significant effects were observed at low doses of morphine (2 mg/kg) or harmaline (1.25 and 5 mg/kg) on pain threshold; harmaline (5 mg/kg) intensified the analgesic effect of morphine; after a combination of morphine and harmaline at non-effective doses (2 and 5 mg/kg, respectively), pain threshold was increased and antinociception was induced. Microinjection of NMDA (0.06 and 0.1 μg) reduced the antinociceptive effect induced by that combination. In addition, analgesia induced by morphine and harmaline was inhibited by naloxone (0.5 and 1 mg). No effects were observed on pain threshold after microinjections intra-VTA of D-AP5 (0.5 and 1 μg) and NMDA (0.06 and 0.1 μg); however, D-AP5 increased pain threshold and intensified antinociception induced by morphine and harmaline. |
| Alijanpour et al. (2021) [33] | Male NMRI mice | Control (saline) Harmaline (0, 1.25, 2.5, 5, and 10 mg/kg i.p.) Morphine sulfate (0, 2, 4, and 6 mg/kg i.p.) Muscimol (0, 200, and 300 ng/mice.i.ventral hippocampal route-vHIP) Bicuculline (1 μL/mouse.i.vHip route) | Tail-flick | Harmaline group showed antinociception effect at 10 mg/kg. Harmaline (5 mg/kg) combined with morphine (2 mg/kg) produced antinociceptive effect and enhance morphine effects. Muscimol enhanced antinociceptive effects of harmaline combined with morphine. |
| Alijanpour et al. (2022) [34] | Male NMRI mice | Control (saline 10 mL/kg i.p.) Vehicle (0.6 μL/mouse. Microinjection into the basolateral amygdala (BLA)) Harmaline (4, 6, and 8 mg/kg i.p.) ACPA (0.5, 1, or 1.5 ng/mouse.micorinjection into the BLA) AM251 (0.1, 0.5, or 1 ng/mouse.microinjection into the BLA) | Tail-flick Hot plate | Harmaline (6 and 8 mg/kg) prolonged reaction time to thermal stimuli in tail-flick and hot plate tests. AM251 (0.5 and 1 ng/mouse) attenuated the antinociceptive activity of harmaline (8 ng/mouse) in both tests. |
| Jiang et al. (2019) [44] | Sprague-Dawley rats/pathogen free (SPF) Balb/c nude mice | Control (saline) Edema treatment: Carragenan without drug (1 g.s.i) Harmine solution gel-HSG (1 g.t.a) Harmine loaded ethosomes gel-HLEG (1 g.t.a) Positive control (dexamethasone acetate cream((CDXMC): 0.4 g.t.a) | Carragenan induced-paw edema | The levels of inflammation cytokines (IL-1β, NO, PGE-2, and TNF-α) were low after administration of HLEG. The degree of paw swelling was reduced after administration of HLEG, HSG, and CDXMC. HLE promotes prolonged therapeutic effect. |
| Niu et al. (2019) [43] | Male Kunming mice | Control (sodium carboxymethycellulose 0.5%) LPS (20 mg/kg i.p.) Dexamethasone + LPS (5 mg/kg of DEM) Harmine + LPS (25, 50 mg/kg of harmine i.g.) | LPS-induced kidney damage | At 50 mg/kg, harmine reduced the levels of TNF-α, IL-6, IL-1β and myeloperoxidase activity. Harmine also suppressed the expression of toll-like receptor 4 (TLR4), phosphorylation of nuclear factor-kappa B (NF-κB) p65, inhibitor of κBα (IκBα), NLRP3, caspase-1, and interleukin-1β (IL-1β). |
| Gonçalves et al. (2020) [54] | Vegetal samples: Commercial mixture (without composition information) Psychotria viridis Banisteriopsis caapi Mimosa hostilis Peganum harmala Decoctions: Psychotria viridis + Banisteriopsis caapi (ayahuasca) Psychotria viridis + Peganum harmala Mimosa hostilis + Banisteriopsis caapi Mimosa hostilis + Peganum harmala | 0.210 g of each vegetal sample | Protein denaturation | Higher anti-inflammatory activity: P. harmala, M. hostilis, P. harmala + M. hostilis, comercial mixture Lower anti-inflammatory activity: P. viridis, B. caapi, P. viridis + B. caapi (ayahuasca) |
| Nardai et al. (2020) [51] | Male Wistar rats | Control (vehicle bolus) N, N-dimethyltryptamine (DMT) (1 mg/kg/2 mg/kg bw/h. i.p.) 1-piperazine dihydrochloride (BD-1063) (2 mg/kg bw/24 h. i.p./1 mg/kg-body weight + DMT) | Ischemia-reperfusion injury/inflammation in the rat brain | DMT decreased ischemic lesion volume and downregulated mRNA expression of the pro-inflammatory citokines IL-1β and IL-6 bilaterally (contralateral and in peri-infarct cortex), while levels of TNF-α were reduced only in the contralateral cortex; mRNA expression of the anti-inflammatory citokine IL10 was upregulated in the peri-infarct cortex. Serum concentrations of IL-1β, IL-6 and TNF-α were reduced, and IL-10 increased. |
| Jin et al. (2021) [25] | Male Balb/c mice or C57BL/6 mice Mouse peritoneal macrophages | Control (saline i.p.) Harmine (30 mg/kg, i.p.) LPS (1 mg/kg i.p.) Poly(I:C) at 50 and 100 μg/mL for 24 h Lipoteichoic acid (TLR2 agonist) 10 μg/mL CpG DNA (TLR9 agonist) 2 μM for 6 h | LPS Polyinosinic:polycytidylic acid (Poly(I:C)) TLR agonists Endotoxemia model (in vivo) | Harmine inhibited IL-12 (IL-12 p70), TNF-, IL-6, COX-2, iNOS, JNK, and STAT1 levels and marker genes in a dose-dependent manner in macrophages (LPS, poly(I:C), TLR agonists). In the endotoxemia model, harmine also reduced liver inflammation by inhibiting the expression of COX-2 and iNOS genes (but not TNF-α). |
| Wang et al. (2021) [40] | Male C57BL/J6 mice/Murine macrophage RAW 264.7 cells | Sham operation Phosphate-buffered saline + titanium particles (30 mg) (vehicle) Titanium particles (30 mg) + harmine (5 mg/kg/day or 10 mg/kg/day) | Peri-prosthetic osteolysis (PPO) | Harmine presence significantly reduced the inflamation area, TNF-α, IL-1β (all on both concentrations) and Il-6 (high concentration). On the contrary, harmine increased levels of IL-4 and IL-10. These results also corroborate that harmine treatment diminishes inflammation in PPO by changing the polarization of macrophages from M1(phenotype pro-inflammatory) to M2 (phenotype anti-inflammatory). Lastly, Ti-particle-induced activation of JNK was suppressed by harmine. |
| Borbély et al. (2022) [53] | Male C57BL/6 wild-type mice | Control DMT (1 mg/kg i.p.) PRE-084 (1 mg/kg i.p.) | Amyloid-β-(Aβ-) induced neurotoxicity C28 | DMT reduced the effect of Aβ1-42 induced of astrocytes but not effects on microglial activation. DMT also reduced cytokines pro-IL-1β and TNF-α, however, only the change for TNF-α was significant and DMT negatively influenced neurogenesis. |
| Ju et al. (2022) [45] | Immortalized murine BV2 microglia lines/male ICR mice | Control (2 μL of saline) LPS challenge (1 μg/μL, 2 μL; injected into the left lateral ventricles) LPS + harmine (20 mg/kg i.p.) | LPS-stimulated BV2 cells | Harmine attenuated nitric oxide (NO), reactive oxygen species, tumor necrosis factor-α, cyclooxygenase 2 and inducible nitric synthase. The expression of inflammatory proteins (COX2 and iNOS) in the mice’s brains were reduced by harmine (20 mg/kg). Harmine at 3, 4, and 5 μM doses decreased the NO release in LPS- challenged BV2 cells. |
| Ghandari et al. (2022) [41] | Balb/c male mice | Control (saline) Cisplatin (5.5 mg/kg i.p.) Harmine (5.10 and 15 mg/kg i.p.) Cisplatin (5.5 mg/kg) + harmine (5, 10 and 15 mg/kg) | Cisplatin-induced renal tissue toxicity | The cisplatin group showed an increase in the serum levels of blood urea nitrogen (BUN) and creatinine. No difference was observed between harmine and control groups when analyzing BUN and creatinine levels. In the cisplatin + harmine (5 mg/kg) group, the serum levels of BUN were high. The kidney damage was reduced at all doses of cisplatin with harmine. The groups who received harmine showed a decrease in malondialdehyde (MDA) levels compared to the cisplatin group. Cisplatin + harmine (5 mg/kg) and cisplatin + harmine (10 mg/kg) increased MDA levels. This parameter was also increased in the cisplatin groups. Total antioxidant capacity (TAC) level was reduced in the cisplatin group. Harmine (5, 10 and 15 mg/kg) and cisplatin + harmine (5, 10, and 15 mg/kg) groups demonstrated an increase in the TAC level compared to the cisplatin group. Cisplatin + harmine (5 mg/kg) and cisplatin + harmine (10 mg/kg) groups demonstrated a decrease in TAC levels compared to the control group. A reduction in the apoptosis cells was observed in the harmine and cisplatin + harmine (5, 10, and 15 mg/kg) groups compared to the cisplatin group. Harmine group (5, 10, and 15 m/kg) did not show effect on apoptotic cells. |
| Kelley et al. (2022) [52] | Male Sprague Dawley rats/Human embryonic kidney | Control Predator exposure/psychosocial stress (PE/PSS) PE/PSS + DMT (2 mg/kg i.p.) PE/PSS + DMT (4 mg/kg i.p.) PE/PSS + harmaline (1.5 mg/kg i.p.) PE/PSS + harmaline + DMT (Pharmahuasca) The administration occurred 30 days post stress every other day for five days | Traumatic stress (PE/PSS) | DMT and pharmahuasca decreased ROS production in the prefrontal cortex and in the hipoccampus, as well as reduced the transcription of inflammatory cytokines (TLR3, TLR4, TLR6, TLR7; IL-1R, IL-1,IL-10, IL-12, IL-13, IL-15, IL-17, IL-18, IL-21, IL-23, IL-33; NFkB2, and IFNGR1). Pharmahuasca downregulated NFkB2. |
| Santos et al. (2022) [26] | BV-2 microglial cells | Banisteriopsis caapi (6.917 mg/g harmine, 1.583 mg/g harmaline, and 3.084 mg/g Tetrahidroharmine-THH) extract (4 μL) Harmine (2.4; 9.4; 18.9; 37.7, and 75.5 μM) Harmaline (2.4; 9.3; 18.7; 37.4, and 74.8 μM) THH (2.3; 9.3; 18.5; 37.0, and 74.1 μM) | Cytokine production | Harmine at high doses (18.9 to 75.5 μM) reduced TNF and IL-2 (18.9 and 75.5 μM). Harmaline reduced IL-7A, IFNϒ and TNF levels at high doses (18.7; 37.4 and 74.8 μM). Harmaline (18.9 μM) reduced IL-10. THH (18.5 μM μg/mL) reduced IL-6, IFNϒ, and TNF, and increased IL-4 (2.3 μM). |
| Szilágyi et al. (2022 [50] | Male Sprague–Dawley rats | Control (vehicle-treated) N, N-dimethyltryptamine (DMT) (10 mg/kg/day; osmotic mini pum.i.s) + harmaline-treated (10 mg/kg/day.gavage) Harmaline-treated (10 mg/kg/day.gavage) | Retinal ischemia–reperfusion injuries | DMT + harmaline-treated group showed higher levels of NFkBp65protein compared to harmaline-treated and healthy untreated groups. No difference was observed between harmaline-treated and healthy untreated groups. |
| Zheng et al. (2023) [46] | Male C57BL/6J mice/BV-2 microglial cells | Control (Saline i.p.) LPS (0.83 mg/kg i.p.) Harmine (20 mg/kg i.p.) Positive control: Fluoxetine (10 mg/kg i.p.) | LPS-induced neuroinflammation | Harmine inhibited LPS-induced microglia neuroinflammation in basolateral amygdala and BV-2 microglial cells. |
| De Camargo et al. (2024) [55] | Male Wistar rats | Sham + saline Cecal ligation and puncture (CLP) + saline Sham + ayahuasca (1 mL/kg.gavage) Sham + ayahuasca (2 mL/kg.gavage) Sham + ayahuasca (4 mL/kg.gavage) CLP + ayahuasca (1 mL/kg.gavage) CLP + ayahuasca (2 mL/kg.gavage) CLP + ayahuasca (4 mL/kg.gavage) (3 days of treatment for all groups) (ayahuasca alkaloid concentration: 1.40 mg/mL DMT, 0.10 mg/mL harmaline, 2.43 mg/mL harmine, and 1.43 mg/mL THH) | Sepsis induction (CLP) or Sham procedure Elevated plus maze Open field test Object recognition test | Ayahuasca pretreatment (1 mL/kg) increased the levels of IL-4 in the prefrontal cortex and cortex and increased the levels of BDNF in the cortex. In general, the results show antioxidative and anti-inflammatory effects of ayahuasca in sepsis-induced damage. |
| Graça et al. (2024) [57] | Male Wistar rats | Banisteripsis caapi extract (380 μg/mL THH, 126 μg/mL harmaline, 650 μg/mL harmine; 1.5 mL/kg.gavage 3 times a week for 4 weeks) 6-hydroxydopamine (6-OHDA.injection into the Locus coeruleus) LC lesion group LC lesion group under the effect of the extract Vehicle group Vehicle group under the effect of the extract | Open field test Elevated plus maze Rotarod test Histology | B. caapi extract reduced the concentration of IL-10 and expression of BDNF in the hippocampus compared to the control group and the group only under the effect of the extract. The B. caapi extract also increased the number and activation of inflammatory cells (microglia) in the LC. |
| Kadyan & Singh (2024) [36] | Swiss albino mice | Control (vehicle 0.1% carboxymethyl cellulose in distilled water 10 mL/kg i.p.) Pathogenic control-vincristine and vehicle (0.1 mg/kg i.p.; once daily for 10 days) Positive control-vincristine (0.1 mg/kg i.p.) + pregabalin (10 mg/kg p.o.) Harmaline (5 mg/kg i.p.; for 14 days) Harmaline (10 mg/kg i.p.; for 14 days) Substance-P (10 μg/kg i.p.) + harmaline (10 mg/kg i.p.) + vincristine (0.1 mg/kg i.p.); for 10 days | Vincristine- induced peripheral neuropathy Hot plate Cold plate Acetone drop Beam balance | Harmaline reduced licking and biting behavior and improved paw withdrawal latency in a dose-dependent manner on days 7 and 14 compared to the vincristine-treated group. It also decreased crossing time, with the 10 mg/kg dose showing a stronger effect than pregabalin. Harmaline (5 mg/kg) increased Nrf-2 levels, while 10 mg/kg reversed vincristine-induced Nrf-2 suppression. Additionally, harmaline countered vincristine-induced elevation of IL-1β, reducing its levels at both doses. |
| Lauria et al. (2024) [35] | Male Swiss and C57BL/6 mice | Control (water p.o.) Ayahuasca (24, 120, 600, and 3000 μL/kg p.o.) Harmine (0, 0.35, 3, 5, and 35 mg/kg p.o.) Indomethacin (5 mg/kg p.o.) Morphine (5 mg/kg i.p.) Dexamethasone (2 mg/kg i.p.) Formalin (20 μL) Complete Freund’s Adjuvant (CFA) (20uL) Gabapentin (70 mg/kg p.o.) Naloxone (5 mg/kg i.p.) Bicuculline (1 mg/kg i.p.) Methysergide (5 mg/kg i.p.) Rimonabant (10 mg/kg i.p.) Phaclofen (2 mg/kg i.p.) | Acute effect: Formalin test CFA Tail flick test Paw edema Acute and chronic effect: Partial sciatic nerve ligation model of neuropathic pain | Acute effect: Ayahuasca (120, 600, and 3000 μL/kg) and indomethacin (5 mg/kg) demonstrated antinociceptive effects at in the second phase of the formalin test. Morphine (5 mg/kg) was effective in inducing antinociceptive effects in both phases of the formalin test. Ayahuasca (600–3000 μL/kg) decreased CFA-induced allodynia, but did not reduce paw edema. Allodynia and edema were both reduced by dexamethasone (2 mg/kg). In the tail flick test, ayahuasca did not show an antinociceptive effect, while morphine (5 mg/kg) caused antinoception. Ayahuasca (24–3000 μL/kg) and gabapentin (70 mg/kg) decreased allodynia in experimental neuropathy. The effects of ayahuasca (3000 μL/kg) lasted 10 h, while the effects of gabapentin (70 mg/kg) lasted only 3 h. At 3.5 mg/kg, harmine produced an antinociceptive effect that lasted 10 h. Chronic effect: Ayahuasca (120–600 μL/kg twice a day for 14 days) and gabapentin (70 mg/kg daily for 14 days) induced antinoception in experimental neuropathy. Bicuculline (1 mg/kg) and methysergide (5 mg/kg) reverted the antinociceptive effect of ayahuasca (600 μL/kg), however, naloxone (5 mg/kg), phaclofen (2 mg/kg), and rimonabant (10 mg/kg) did not revert. At 3.5 mg/kg twice a day for 14 days, harmine produced an antinociceptive effect that lasted 10 h. |
| Ma et al. (2024) [39] | Male Kunming mice | Control Harmine (50 mg/kg p.o.) Model (0.2% CCl4, v/v, dissolved in rapeseed oil, 10 mL/kg body weight i.p.) Medium dose (harmine, 25 mg/kg; 0.2% CCl4 i.p.) High dose (harmine, 50 mg/kg; 0.2% CCl4 i.p.) Positive (bifendate, 200 mg/kg; 0.2% CCl4 p.o.) | Carbon tetrachloride (CCL4)-induced acute liver injury | Harmine reduced the serum levels of aspartate and alanine aminotransferase and the levels of TNF-α and IL-6. The levels of glutathione and superoxide dismutase were restored and the production of nitric oxide and malondialdehyde was suppressed by harmine. Harmine down-regulated LC3B II/I, p38 MAPK, TLR4, and NF-κB levels, while upregulating p62, Bcl-2, Beclin1, ULK1, and p-mTOR expression. |
| Xie et al. (2024) [47] | Male C57BL/6 J mice | Control (0.5% CMC-Na.gavage) Model (0.5% CMC-Na.gavage) Donepezil (5 mg/kg.gavage) Harmine (5 mg/kg; 10 mg/kg and 20 mg/kg.gavage) Ethanol (0.1 mL/10 g.gavage) | Morris water maze test Ethanol-induced memory impairment | Harmine reduced the levels of IL-6, IL-β and TNF-α while raising BDNF levels in plasma and hippocampus. From a cellular perspective, harmine promoted synaptic vesicle fusion and protected the myelin sheath, demonstrating beneficial effects against ethanol-induced memory impairment. |
| Zheng et al. (2024) [48] | Balb/c mice/RAW 264.7 cells | In vitro Control group Phosphate-buffered saline (PBS) (100 μL injection) Negative group 0.1% dexamethasone Positive group 25 and 50 μM dexamethasone Natural harmaline (HML) (25 and 50 μM) LPS 1 μg/mL (24 h after treatment) In vivo Test and positive control group (harmaline 10 mg/kg i.p. and physiological saline every 12 h for 3 days) λ-carrageenan (2.0 mg/mL, 100 µL) | Rheumatoid arthritis (RA) induction | Harmaline inhibited production of nitric oxide and the secretion of IL-1β and IL-6 in cells activated by LPS. Moreover, the thickness and extent of swelling of paw were reduced by HML. In cells activated by LPS, the expression of iNOS and COX-2 was decreased by HML, as well as the expression of p65 and p65 phosphorylation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanova, B.; Rossi, G.N.; Lopes Guerra, L.T.; Bouso, J.C.; Hallak, J.E.C.; dos Santos, R.G. Ayahuasca, Pain, and Inflammation: A Systematic Review of Preclinical Studies. Psychoactives 2025, 4, 24. https://doi.org/10.3390/psychoactives4030024
Villanova B, Rossi GN, Lopes Guerra LT, Bouso JC, Hallak JEC, dos Santos RG. Ayahuasca, Pain, and Inflammation: A Systematic Review of Preclinical Studies. Psychoactives. 2025; 4(3):24. https://doi.org/10.3390/psychoactives4030024
Chicago/Turabian StyleVillanova, Bianca, Giordano Novak Rossi, Lorena Terene Lopes Guerra, José Carlos Bouso, Jaime Eduardo Cecilio Hallak, and Rafael Guimarães dos Santos. 2025. "Ayahuasca, Pain, and Inflammation: A Systematic Review of Preclinical Studies" Psychoactives 4, no. 3: 24. https://doi.org/10.3390/psychoactives4030024
APA StyleVillanova, B., Rossi, G. N., Lopes Guerra, L. T., Bouso, J. C., Hallak, J. E. C., & dos Santos, R. G. (2025). Ayahuasca, Pain, and Inflammation: A Systematic Review of Preclinical Studies. Psychoactives, 4(3), 24. https://doi.org/10.3390/psychoactives4030024







