Psilocybin Use in an Intercollegiate Athlete with Persisting Symptoms After Concussion: A Case Report
Abstract
1. Introduction
2. Case Report
2.1. Acute Injury
2.2. Imaging Results
2.3. One Week Post-Concussion
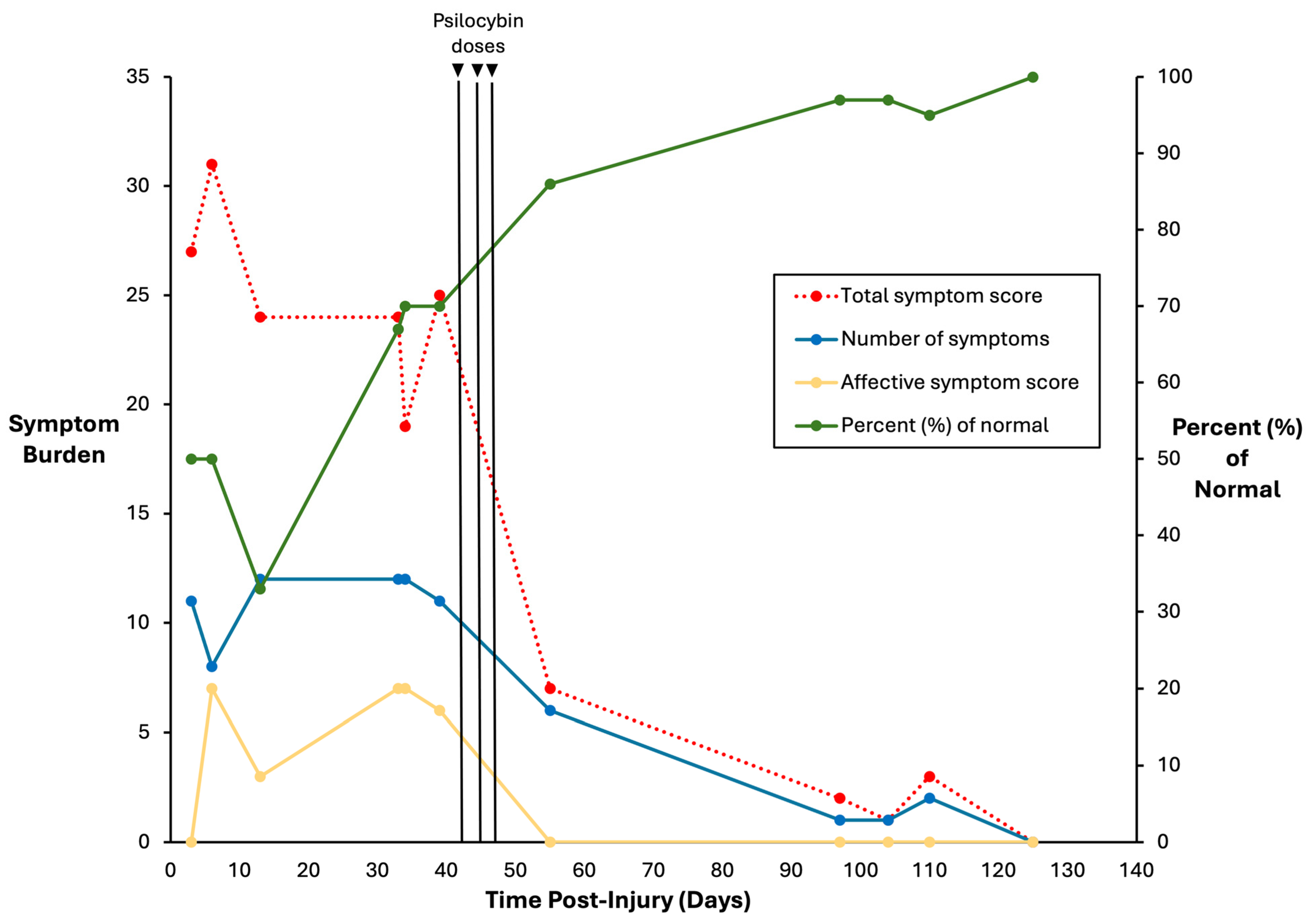
2.4. One Month Post-Concussion
2.5. Psilocybin Dosing
2.6. Concussion Clearance
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patricios, J.S.; Schneider, K.J.; Dvorak, J.; Ahmed, O.H.; Blauwet, C.; Cantu, R.C.; Davis, G.A.; Echemendia, R.J.; Makdissi, M.; McNamee, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport-Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The New Neurometabolic Cascade of Concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Barlow, K.M.; Iyer, K.; Yan, T.; Scurfield, A.; Carlson, H.; Wang, Y. Cerebral Blood Flow Predicts Recovery in Children with Persistent Post-Concussion Symptoms after Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Churchill, N.; Schweizer, T.A.; Rhind, S.G.; Richards, D.; Baker, A.J.; Hutchison, M.G. Blood biomarkers are associated with brain function and blood flow following sport concussion. J. Neuroimmunol. 2018, 319, 1–8. [Google Scholar] [CrossRef]
- Broshek, D.K.; Pardini, J.E.; Herring, S.A. Persisting symptoms after concussion: Time for a paradigm shift. PM R 2022, 14, 1509–1513. [Google Scholar] [CrossRef]
- Fordal, L.; Stenberg, J.; Iverson, G.L.; Saksvik, S.B.; Karaliute, M.; Vik, A.; Olsen, A.; Skandsen, T. Trajectories of Persistent Postconcussion Symptoms and Factors Associated with Symptom Reporting After Mild Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2022, 103, 313–322. [Google Scholar] [CrossRef]
- Hutchison, M.G.; Di Battista, A.P.; Lawrence, D.W.; Pyndiura, K.; Corallo, D.; Richards, D. Randomized controlled trial of early aerobic exercise following sport-related concussion: Progressive percentage of age-predicted maximal heart rate versus usual care. PLoS ONE 2022, 17, e0276336. [Google Scholar] [CrossRef]
- Lawrence, D.W.; Richards, D.; Comper, P.; Hutchison, M.G. Earlier time to aerobic exercise is associated with faster recovery following acute sport concussion. PLoS ONE 2018, 13, e0196062. [Google Scholar] [CrossRef]
- Comper, P.; Foster, E.; Chandra, T.; Langer, L.; Wiseman-Hakes, C.; Mochizuki, G.; Ruttan, L.; Lawrence, D.W.; Inness, E.L.; Gladstone, J.; et al. The Toronto Concussion Study: A prospective investigation of characteristics in a cohort of adults from the general population seeking care following acute concussion, 2016–2020. Front. Neurol. 2023, 14, 1152504. [Google Scholar] [CrossRef]
- Mayers, L. Return-to-play criteria after athletic concussion: A need for revision. Arch. Neurol. 2008, 65, 1158–1161. [Google Scholar] [CrossRef]
- Ellis, M.J.; Ritchie, L.J.; McDonald, P.J.; Cordingley, D.; Reimer, K.; Nijjar, S.; Koltek, M.; Hosain, S.; Johnston, J.; Mansouri, B.; et al. Multidisciplinary Management of Pediatric Sports-Related Concussion. Can. J. Neurol. Sci. 2017, 44, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Fried, E.; Balla, U.; Catalogna, M.; Kozer, E.; Oren-Amit, A.; Hadanny, A.; Efrati, S. Persistent post-concussive syndrome in children after mild traumatic brain injury is prevalent and vastly underdiagnosed. Sci. Rep. 2022, 12, 4364. [Google Scholar] [CrossRef]
- Zemek, R.; Barrowman, N.; Freedman, S.B.; Gravel, J.; Gagnon, I.; McGahern, C.; Aglipay, M.; Sangha, G.; Boutis, K.; Beer, D.; et al. Clinical Risk Score for Persistent Postconcussion Symptoms Among Children with Acute Concussion in the ED. JAMA 2016, 315, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Sheldrake, E.; Deneault, A.A.; Wheeler, A.; Burke, M.; Scratch, S. Depressive Symptoms in Individuals with Persistent Postconcussion Symptoms: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2248453. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L.; Karr, J.E.; Maxwell, B.; Zafonte, R.; Berkner, P.D.; Cook, N.E. Examining Criteria for Defining Persistent Post-concussion Symptoms in Children and Adolescents. Front. Neurol. 2021, 12, 614648. [Google Scholar] [CrossRef]
- Moore, B.M.; Stark, R.K.; D’Angelo, E.C. Multidisciplinary care for patients with persistent symptoms following concussion: A systematic review. Disabil. Rehabil. 2024, 46, 1760–1775. [Google Scholar] [CrossRef]
- Ellis, M.J.; Leddy, J.; Willer, B. Multi-Disciplinary Management of Athletes with Post-Concussion Syndrome: An Evolving Pathophysiological Approach. Front. Neurol. 2016, 7, 136. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. New. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Rocha, J.M.; Rossi, G.N.; Osorio, F.L.; Ona, G.; Bouso, J.C.; Silveira, G.O.; Yonamine, M.; Marchioni, C.; Crevelin, E.J.; et al. Effects of ayahuasca on the endocannabinoid system of healthy volunteers and in volunteers with social anxiety disorder: Results from two pilot, proof-of-concept, randomized, placebo-controlled trials. Hum. Psychopharmacol. 2022, 37, e2834. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Kirchner, K.; Passie, T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: A qualitative study of acute and sustained subjective effects. J. Psychopharmacol. 2015, 29, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.B.; Pace, B.T.; Nicholas, C.R.; Raison, C.L.; Hutson, P.R. The experimental effects of psilocybin on symptoms of anxiety and depression: A meta-analysis. Psychiatry Res. 2020, 284, 112749. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Atli, M.; Bennett, J.C.; Croal, M.; DeBattista, C.; Dunlop, B.W.; Feifel, D.; Hellerstein, D.J.; et al. Single-dose psilocybin for a treatment-resistant episode of major depression: Impact on patient-reported depression severity, anxiety, function, and quality of life. J. Affect Disord. 2023, 327, 120–127. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Grob, C.S.; Danforth, A.L.; Chopra, G.S.; Hagerty, M.; McKay, C.R.; Halberstadt, A.L.; Greer, G.R. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry 2011, 68, 71–78. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef]
- Griffiths, R.; Richards, W.; Johnson, M.; McCann, U.; Jesse, R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J. Psychopharmacol. 2008, 22, 621–632. [Google Scholar] [CrossRef]
- Lawrence, D.W.; Carhart-Harris, R.; Griffiths, R.; Timmermann, C. Phenomenology and content of the inhaled N, N-dimethyltryptamine (N, N-DMT) experience. Sci. Rep. 2022, 12, 8562. [Google Scholar] [CrossRef]
- Allen, J.; Dames, S.S.; Foldi, C.J.; Shultz, S.R. Psychedelics for acquired brain injury: A review of molecular mechanisms and therapeutic potential. Mol. Psychiatry 2024, 29, 671–685. [Google Scholar] [CrossRef]
- Khan, M.; Carter, G.T.; Aggarwal, S.K.; Holland, J. Psychedelics for Brain Injury: A Mini-Review. Front. Neurol. 2021, 12, 685085. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Wu, K.J.; Wang, Y.S.; Bae, E.; Chianelli, F.; Bambakidis, N.; Wang, Y. Neuroprotective effects of psilocybin in a rat model of stroke. BMC Neurosci. 2024, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Kovacs, A.; Frecska, E.; Rajnavolgyi, E. Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS ONE 2014, 9, e106533. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The Endogenous Hallucinogen and Trace Amine N,N-Dimethyltryptamine (DMT) Displays Potent Protective Effects against Hypoxia via Sigma-1 Receptor Activation in Human Primary iPSC-Derived Cortical Neurons and Microglia-Like Immune Cells. Front. Neurosci. 2016, 10, 423. [Google Scholar] [CrossRef]
- Szabo, I.; Varga, V.E.; Dvoracsko, S.; Farkas, A.E.; Kormoczi, T.; Berkecz, R.; Kecskes, S.; Menyhart, A.; Frank, R.; Hantosi, D.; et al. N,N-Dimethyltryptamine attenuates spreading depolarization and restrains neurodegeneration by sigma-1 receptor activation in the ischemic rat brain. Neuropharmacology 2021, 192, 108612. [Google Scholar] [CrossRef]
- Nardai, S.; Laszlo, M.; Szabo, A.; Alpar, A.; Hanics, J.; Zahola, P.; Merkely, B.; Frecska, E.; Nagy, Z. N,N-dimethyltryptamine reduces infarct size and improves functional recovery following transient focal brain ischemia in rats. Exp. Neurol. 2020, 327, 113245. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Beug, M.W.; Bigwood, J. Psilocybin and psilocin levels in twenty species from seven genera of wild mushrooms in the Pacific Northwest, U.S.A. J. Ethnopharmacol. 1982, 5, 271–285. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Lo, L.A.; Pistawka, C.A.; Deol, J.K. Therapeutic use of psilocybin: Practical considerations for dosing and administration. Front. Psychiatry 2022, 13, 1040217. [Google Scholar] [CrossRef]
- Simon, M.; Maerlender, A.; Metzger, K.; Decoster, L.; Hollingworth, A.; McLeod, T.V. Reliability and Concurrent Validity of Select C3 Logix Test Components. Dev. Neuropsychol. 2017, 42, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Chrisman, S.P.D.; Whelan, B.M.; Zatzick, D.F.; Hilt, R.J.; Wang, J.; Marcynyszyn, L.A.; Rivara, F.P.; McCarty, C.A. Prevalence and risk factors for depression, anxiety and suicidal ideation in youth with persistent post-concussive symptoms (PPCS). Brain Inj. 2021, 35, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Cuff, S.; Maki, A.; Feiss, R.; Young, J.; Shi, J.; Hautmann, A.; Yang, J. Risk factors for prolonged recovery from concussion in young patients. Br. J. Sports Med. 2022, 56, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Langer, L.K.; Alavinia, S.M.; Lawrence, D.W.; Munce, S.E.P.; Kam, A.; Tam, A.; Ruttan, L.; Comper, P.; Bayley, M.T. Prediction of risk of prolonged post-concussion symptoms: Derivation and validation of the TRICORDRR (Toronto Rehabilitation Institute Concussion Outcome Determination and Rehab Recommendations) score. PLoS Med. 2021, 18, e1003652. [Google Scholar] [CrossRef]
- Sheldrake, E.; Al-Hakeem, H.; Lam, B.; Goldstein, B.I.; Wheeler, A.L.; Burke, M.; Dunkley, B.T.; Reed, N.; Scratch, S.E. Mental Health Outcomes Across the Lifespan in Individuals with Persistent Post-Concussion Symptoms: A Scoping Review. Front. Neurol. 2022, 13, 850590. [Google Scholar] [CrossRef]
- Melfi, C.A.; Chawla, A.J.; Croghan, T.W.; Hanna, M.P.; Kennedy, S.; Sredl, K. The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Arch. Gen. Psychiatry 1998, 55, 1128–1132. [Google Scholar] [CrossRef]
- Senthinathan, A.; Mainwaring, L.M.; Hutchison, M. Heart Rate Variability of Athletes Across Concussion Recovery Milestones: A Preliminary Study. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2017, 27, 288–295. [Google Scholar] [CrossRef]
- Lawrence, D.W. Heart rate (HR) and heart rate variability (HRV) response to inhaled N, N-dimethyltryptamine (N, N-DMT): A case report. J. Psychedelic Stud. 2022, 6, 63–68. [Google Scholar] [CrossRef]
- Rosas, F.E.; Mediano, P.A.M.; Timmermann, C.; Luppi, A.I.; Candia-Rivera, D.; Abbasi-Asl, R.; Gazzaley, A.; Kringelbach, M.L.; Muthukumaraswamy, S.; Bor, D.; et al. The entropic heart: Tracking the psychedelic state via heart rate dynamics. bioRxiv 2023. [Google Scholar] [CrossRef]
- Olbrich, S.; Preller, K.H.; Vollenweider, F.X. LSD and ketanserin and their impact on the human autonomic nervous system. Psychophysiology 2021, 58, e13822. [Google Scholar] [CrossRef]
- Sakashita, Y.; Abe, K.; Katagiri, N.; Kambe, T.; Saitoh, T.; Utsunomiya, I.; Horiguchi, Y.; Taguchi, K. Effect of psilocin on extracellular dopamine and serotonin levels in the mesoaccumbens and mesocortical pathway in awake rats. Biol. Pharm. Bull. 2015, 38, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, B.; Heifets, B.D. Expectancy Effects in Psychedelic Trials. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.D.; Forsyth, A.; Lumley, T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev. Clin. Pharmacol. 2021, 14, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Polich, G.; Iaccarino, M.A.; Kaptchuk, T.J.; Morales-Quezada, L.; Zafonte, R. Placebo Effects in Traumatic Brain Injury. J. Neurotrauma 2018, 35, 1205–1212. [Google Scholar] [CrossRef]
| Test Condition | Time Post-Concussion (Days) | Time Relative to First Psilocybin Dose (Days) | Trails A (s) | Trails B (s) | Processing Speed (Number Correct) | Simple RT (ms) | Choice RT (ms) |
|---|---|---|---|---|---|---|---|
| Pre-psilocybin | 39 | −3 | 25.8 | 57.6 | 60 | 247 | 358 |
| Post-psilocybin | 110 | +68 | 20.4 | 47.8 | 67 | 236 | 354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, D.W.; Di Battista, A.P.; Hutchison, M.G. Psilocybin Use in an Intercollegiate Athlete with Persisting Symptoms After Concussion: A Case Report. Psychoactives 2025, 4, 22. https://doi.org/10.3390/psychoactives4030022
Lawrence DW, Di Battista AP, Hutchison MG. Psilocybin Use in an Intercollegiate Athlete with Persisting Symptoms After Concussion: A Case Report. Psychoactives. 2025; 4(3):22. https://doi.org/10.3390/psychoactives4030022
Chicago/Turabian StyleLawrence, David W., Alex P. Di Battista, and Michael G. Hutchison. 2025. "Psilocybin Use in an Intercollegiate Athlete with Persisting Symptoms After Concussion: A Case Report" Psychoactives 4, no. 3: 22. https://doi.org/10.3390/psychoactives4030022
APA StyleLawrence, D. W., Di Battista, A. P., & Hutchison, M. G. (2025). Psilocybin Use in an Intercollegiate Athlete with Persisting Symptoms After Concussion: A Case Report. Psychoactives, 4(3), 22. https://doi.org/10.3390/psychoactives4030022






