The Imperative of Regulation: The Co-Creation of a Medical and Non-Medical US Opioid Crisis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Doctors and Pharmacists as Gatekeepers of Narcotic Drugs
3.2. Regulating New Addictive Wonders for the Doctor’s Bag
3.3. Unforeseen Consequences of a New Dosage Form: Slow Release
3.4. An Opioid Addiction Crisis in the Making: The First US Wave (1998–2010, see Figure 1 and Figure 2)
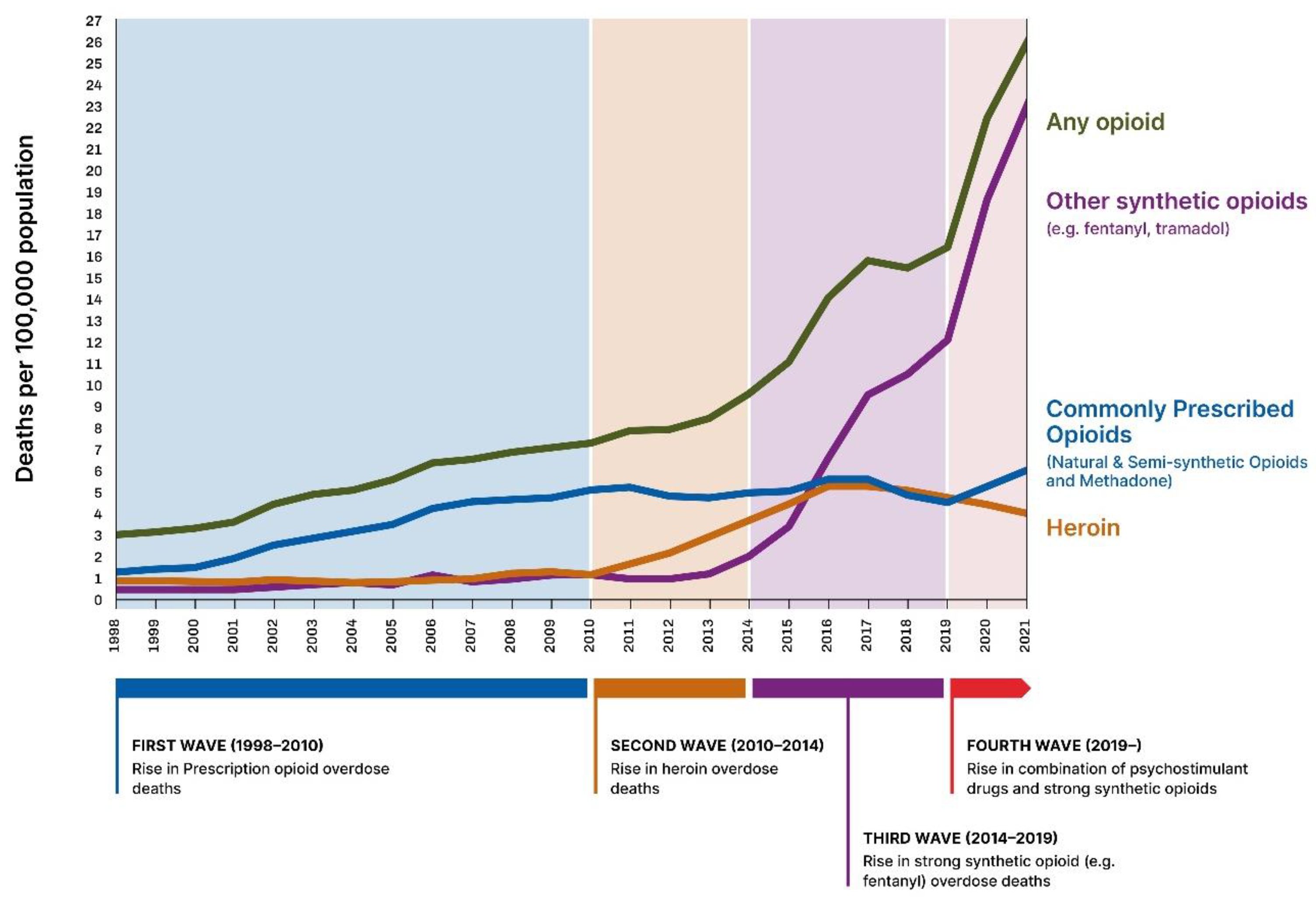
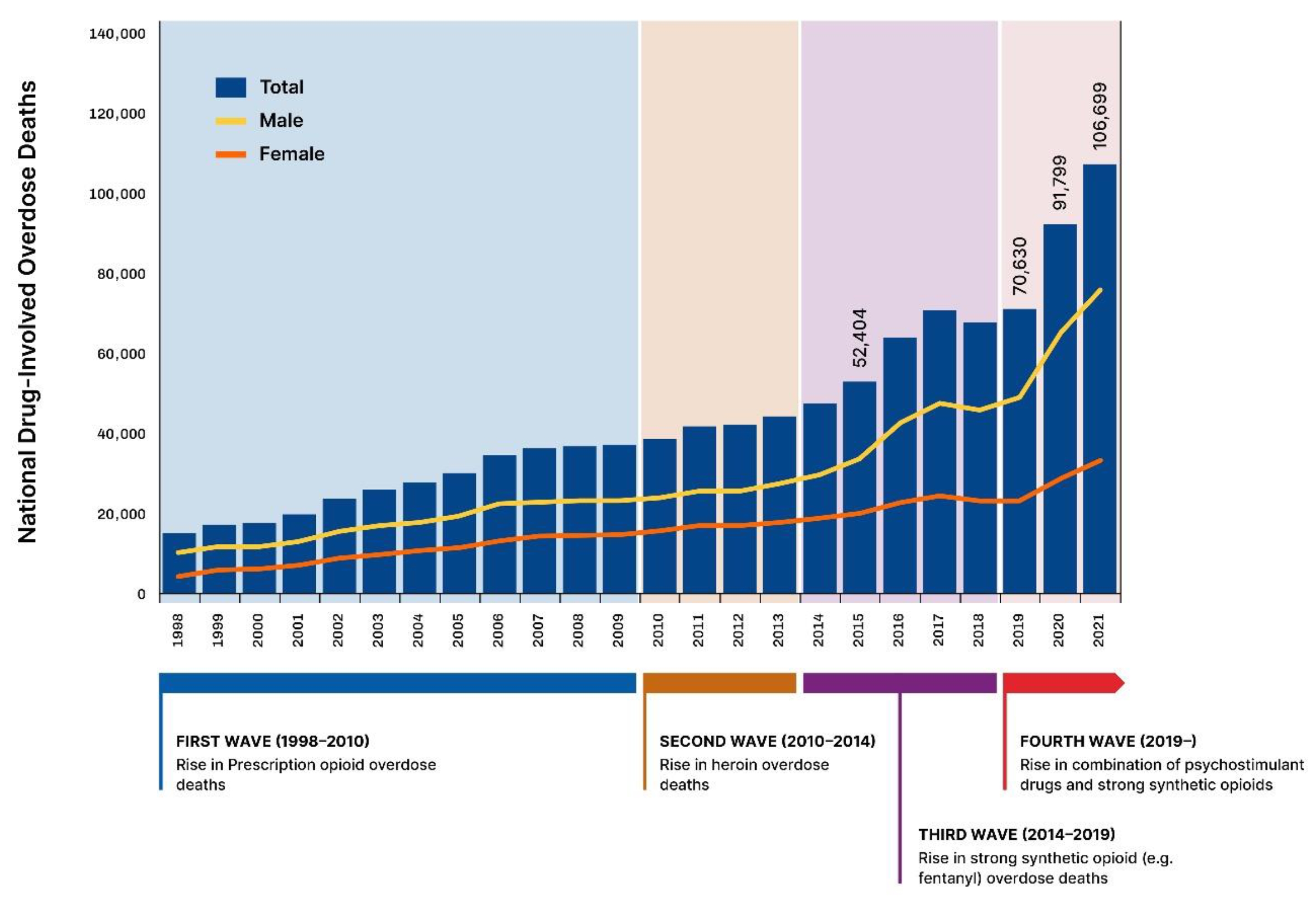
3.5. The Second (2010–2014), Third and Fourth Wave of the Opioid Crisis (See Figure 1 and Figure 2)
4. Discussion and Conclusions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Overdose Death Rates|National Institute on Drug Abuse (NIDA). 2021. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 19 August 2023).
- National Center for Health Statistics-Vital Statistics Rapid Release-Provisional Drug Overdose Data. Centers for Disease Control and Prevention: National Center for Health Statistics, Provisional Drug Overdose Death Counts. 2022. Available online: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm (accessed on 20 August 2023).
- World Health Organization. Opioid Overdose. 29 August 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/opioid-overdose (accessed on 10 September 2023).
- National Center for Health Statistics, All Injuries. 2022. Available online: https://www.cdc.gov/nchs/fastats/injury.htm (accessed on 10 September 2023).
- Chatterjee, R. Overdose Deaths Continued to Rise in 2021, Reaching Historic Highs. Health News from NPR. 2021. Available online: https://www.npr.org/sections/health-shots/2022/05/11/1098314220/ (accessed on 22 August 2023).
- Weiland, N.U.S. Recorded Nearly 110,000 Overdose Deaths in 2022. The Number Leveled off after Two Years of Sharp Increases, According to New Data from the Centers for Disease Control and Prevention. Available online: https://www.nytimes.com/2023/05/17/us/politics/drug-overdose-deaths.html (accessed on 22 August 2023).
- Abrahamson, A. Substance Use during the Pandemic: Opioid and Stimulant Use Is on the Rise—How Can Psychologists and Other Clinicians Help a Greater Number of Patients Struggling with Drug Use? 2021. Monitor on Psychology, Volume 52, Issue 2. Available online: https://www-apa-org.proxy.library.uu.nl/monitor/2021/03/substance-use-pandemic (accessed on 22 August 2023).
- Patterson Silver Wolf, D.A. Real-Time Data Are Essential for COVID—They’re Just as Important for the Opioid Overdose Crisis. 2020. STAT. Available online: https://www.statnews.com/2020/05/20/real-time-data-essential-for-opioid-overdose-crisis-as-for-covid-19/ (accessed on 20 August 2023).
- Manchikanti, L.; Vanaparthy, R.; Atluri, S.; Sachdeva, H.; Kaye, A.D.; Hirsch, J.A. COVID-19 and the Opioid Epidemic: Two Public Health Emergencies That Intersect with Chronic Pain. Pain Ther. 2021, 10, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Katzman, J.G.; Katzman, J.W. COVID-19 Has Provided 20/20 Vision Illuminating Our Nation’s Health Crises. Pain Med. 2020, 22, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Starr, P. The Social Transformation of American Medicine: The Rise of a Sovereign Profession & the Making of a Vast Industry, 2nd ed.; Basic Books: New York, NY, USA, 2017; pp. 465–493. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Drug Overdose Deaths. Available online: https://www.cdc.gov/drugoverdose/deaths/index.html (accessed on 10 September 2023).
- Kiang, M.V.; Basu, S.; Chen, J.; Alexander, M.J. Assessment of Changes in the Geographical Distribution of Opioid-Related Mortality Across the United States by Opioid Type, 1999–2016. JAMA Netw. Open 2019, 2, e190040. [Google Scholar] [CrossRef] [PubMed]
- UTSW Q&AP: Experts Talk about Opioid Abuse, Risks, Treatment. Available online: https://www.utsouthwestern.edu/newsroom/articles/year-2023/aug-q-a-opioid-abuse-risks-treatment.html (accessed on 20 August 2023).
- Dyer, O. US life expectancy falls for third year in a row. BMJ 2018, 363, k5118. [Google Scholar] [CrossRef]
- Woolf, S.H.; Schoomaker, H. Life Expectancy and Mortality Rates in the United States, 1959–2017. JAMA 2019, 322, 1996–2016. [Google Scholar] [CrossRef]
- Kariisa, M.; Seth, P.; Jones, C.M. Increases in Disparities in US Drug Overdose Deaths by Race and Ethnicity. JAMA 2022, 328, 421–422. [Google Scholar] [CrossRef]
- Lipari, R.; van Horn, S. Children Living with Parents Who Have a Substance Use Disorder. The CBHSQ Report. 2017. Available online: https://www.samhsa.gov/data/sites/default/files/report_3223/ShortReport-3223.html (accessed on 23 August 2023).
- Cosslet, R.L. Sesame Street Takes on Opioid Crisis as Muppet’s Mother Battles Addiction. Guardian. 9 October 2019. Available online: https://www.theguardian.com/tv-and-radio/2019/oct/09/sesame-street-karli-addiction-opioids-crisis (accessed on 23 August 2023).
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2020, 26, 218–233. [Google Scholar] [CrossRef]
- Friedman, S.R.; Krawczyk, N.; Perlman, D.C.; Mateu-Gelabert, P.; Ompad, D.C.; Hamilton, L.; Nikolopoulos, G.; Guarino, H.; Cerdá, M. The Opioid/Overdose Crisis as a Dialectics of Pain, Despair, and One-Sided Struggle. Front. Public Health 2020, 8, 540423. [Google Scholar] [CrossRef]
- Beletsky, L.; Ciccarone, D. Opioid Crisis: No Easy Fix to Its Social and Economic Determinants. Am. J. Public Health 2018, 108, 182–186. [Google Scholar] [CrossRef]
- Radden Keefe, P. Empire of Pain: The Secret History of the Sackler Dynasty; Picador: London, UK, 2021. [Google Scholar]
- Gluck, A.R.; Hall, A.; Curfman, G. Civil Litigation and the Opioid Epidemic: The Role of Courts in a National Health Crisis. J. Law Med. Ethic 2018, 46, 351–366. [Google Scholar] [CrossRef]
- Bewley-Taylor, D.; Jelsma, M. Regime change: Re-visiting the 1961 Single Convention on Narcotic Drugs. Regime change: Re-visiting the 1961 Single Convention on Narcotic Drugs. Int. J. Drug Policy 2012, 23, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Controlled Substances Act. 2022. Available online: https://en.wikipedia.org/wiki/Controlled_Substances_Act (accessed on 25 August 2023).
- Snelders, S. Drug Smuggler Nation: Narcotics and the Netherlands, 1920–1995; Manchester University Press: Manchester, UK, 2021. [Google Scholar]
- European Medicines Agency. European-Risk-Management-Strategy-Erms. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/european-risk-management-strategy-erms (accessed on 1 July 2023).
- US Food & Drug Administration. Risk Evaluation and Mitigation Strategies-Rems. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems (accessed on 1 July 2023).
- Carpenter, D. Reputation and Power Contested. In Reputation and Power: Organizational Image and Pharmaceutical Regulation at the FDA (Princeton Studies in American Politics: Historical, International, and Comparative Perspectives, 111); Princeton University Press: Princeton, NJ, USA, 2010; pp. 394–395. [Google Scholar]
- European Medicines Agency. Authorization of Medicines. Available online: https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines (accessed on 30 June 2023).
- US Food & Drug Administration (FDA). Development & Approval Process/Drugs. Available online: https://www.fda.gov/drugs/development-approval-process-drugs (accessed on 30 June 2023).
- FDA Drug Approval Process. 2023. Available online: https://www.drugwatch.com/fda/approval-process/#:~:text=FDA%20Drug-Approval%20Process,FDA%20post-market%20safety%20monitoring (accessed on 21 July 2023).
- Gaudillière, J.P. (Ed.) Drug trajectories. In Studies in the History and Philosophy of the Biological and Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2005; Volume 36. [Google Scholar]
- Snelders, S.; Kaplan, C.; Pieters, T. On Cannabis, Chloral Hydrate, and Career Cycles of Psychotrophic Drugs in Medicine. Bull. Hist. Med. 2006, 80, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Geels, F.W.; Pieters, T.; Snelders, S. Cultural Enthusiasm, Resistance and the Societal Embedding of New Technologies: Psychotropic Drugs in the 20th Century. Technol. Anal. Strat. Manag. 2007, 19, 145–165. [Google Scholar] [CrossRef]
- Van Der Gronde, T.; Groot, C.A.U.-D.; Pieters, T. Addressing the challenge of high-priced prescription drugs in the era of precision medicine: A systematic review of drug life cycles, therapeutic drug markets and regulatory frameworks. PLoS ONE 2017, 12, e0182613. [Google Scholar] [CrossRef]
- Smith, M. Small Comfort. In A History of the Minor Tranquilizers; Praeger: New York, NY, USA, 1985; pp. 4–5. [Google Scholar]
- Pieters, T.; Snelders, S. Psychotropic Drug Use: Between Healing and Enhancing the Mind. Neuroethics 2009, 2, 63–73. [Google Scholar] [CrossRef]
- Herzberg, D.; Guarino, H.; Mateu-Gelabert, P.; Bennett, A.S. Recurring Epidemics of Pharmaceutical Drug Abuse in America: Time for an All-Drug Strategy. Am. J. Public Health 2016, 106, 408–410. [Google Scholar] [CrossRef]
- Courtwright, D.T. Forces of Habit: Drugs and the Making of the Modern World; Harvard University Press: Cambridge, MA, USA, 2002; pp. 181–186. [Google Scholar]
- Berridge, V. Demons: Our Changing Attitudes to Alcohol, Tobacco, & Drugs; Oxford University Press: Oxford, UK, 2013; pp. 11–12. [Google Scholar]
- Courtwright, D.T. The Extent of Opiate Addiction: Dark Paradise: A History of Opiate Addiction in America; Harvard University Press: Cambridge, MA, USA, 2001; pp. 9–34. [Google Scholar]
- Berridge, V. Opium and the People; Free Association Books Ltd.: London, UK, 1999; pp. 241–243. [Google Scholar]
- Musto, D.F. The American Disease: Origins of Narcotic Control, 3rd ed.; Oxford University Press: Oxford, UK, 1999; p. 117. [Google Scholar]
- Courtwright, D. Addiction to Heroin. In Dark Paradise: A History of Opiate Addiction in America; Harvard University Press: Cambridge, MA, USA, 2001; pp. 89–109. [Google Scholar]
- Pieters, T.; Snelders, S. From King Kong Pills to Mother’s Little Helpers-Career Cycles of Two Families of Psychotropic Drugs: The Barbiturates and Benzodiazepines. Can. Bull. Med. Hist. 2007, 24, 93–112. [Google Scholar] [CrossRef][Green Version]
- Eddy, N.B. The History of the Development of Narcotics. Law Contemp. Probl. 1957, 22, 3–8. [Google Scholar] [CrossRef]
- Murphy, E. The Black Candle; Thomas Allen: Toronto, ON, Canada, 1922; p. 19. [Google Scholar]
- Weber, M.M. Oxycodon-A psychopharmacologic-historical note on an opium analgesic. Pharmacopsychiatry 2005, 38, A243. [Google Scholar] [CrossRef]
- Over slaap en slaappillen. Nieuwe Hoornsche Courant, 18 March 1935.
- Messner, J. Űber neue wichtige Arzneimittel der letzten 4 Jahre. Z. Für Angew. Chem. 1919, 32, 381. [Google Scholar] [CrossRef][Green Version]
- Anonymous. Preparations and appliances. Br. Med. J. 1928, 3520, 1069. [Google Scholar]
- Anonymous. Zijn specialités te vervangen? Pharm. Weekbl. 1926, 63, 446–447. [Google Scholar]
- Wolff, P. Zur behandlung und bekämpfung der Alkaloidsuchten (Morphinismus, Kokainismus usw.) Auswertung einer Rundfrage. IV. Sucht mit Eukodal, Pantopon, Heroin, Trivalin, Dikodid, Dilaudid, Opium und anderen Opiumpräparaten. Dtsch. Med. Wochenschr. 1928, 54, 224–226. [Google Scholar] [CrossRef]
- Menninger-Lerchenthal, E. Eukodal-Vergitigung, chronische. (Eukodalismus). Samml. Von Vergift. 1932, 3, 173–174. [Google Scholar]
- Anonymous. Narcotic drugs in Germany. Br. Med. J. 1933, 3799, 789–790. [Google Scholar]
- de Ridder, M. Heroin: Vom Arzneimittel zur Droge; Campus Verlag: Frankfurt, Germany, 2000; pp. 122–126. [Google Scholar]
- Anonymous. Narcotica uit Britse Dump: Twee jaar geëist tegen smokkelaars. De Volkskrant, 30 September 1955. [Google Scholar]
- Courtwright, D. Heroin in Postwar America. In Dark Paradise: A History of Opiate Addiction in America; Harvard University Press: Cambridge, MA, USA, 2001; pp. 145–146,151. [Google Scholar]
- Vranken, M.J.; Mantel-Teeuwisse, A.K.; Jünger, S.; Radbruch, L.; Lisman, J.; Scholten, W.; Payne, S.; Lynch, T.; Schutjens, M.-H.D. Legal Barriers in Accessing Opioid Medicines: Results of the ATOME Quick Scan of National Legislation of Eastern European Countries. J. Pain Symptom Manag. 2014, 48, 1135–1144. [Google Scholar] [CrossRef]
- Vranken, M. Appropriate Access to Opioid Medicines: A Legal & Policy Perspective. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2019; pp. 23–33. [Google Scholar]
- Herzberg, D. Happy Pills in America: From Miltown to Prozac; The Johns Hopkins University Press: Baltimore, MD, USA, 2009. [Google Scholar]
- Courtwright, D. The Drug Wars. In Dark Paradise: A History of Opiate Addiction in America; Harvard University Press: Cambridge, MA, USA, 2001; p. 162. [Google Scholar]
- Bloomquist, E.R. The addiction potential of Oxycodone (Percodan®). Calif. Med. 1963, 99, 127–130. [Google Scholar]
- U.S. Food &Drug. Drug Approvals and Databases. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases (accessed on 30 August 2023).
- DiStefanon, J.N. The Opioid Wash Cycle: Pharma cos., gov’t Repeat a Lethal Dance. The Philadelphia Inquirer, 25 September 2017. [Google Scholar]
- Morgan, J. American Opiophobia: Customary underutilization of opioid analgesics. Adv. Alcohol Subst. Abus. 1985, 5, 163–172. [Google Scholar] [CrossRef]
- Tennant, F. Overcoming Opiaphobia & Doing Opioids Right. Pain Treatment Topics. 7 May 2007. Available online: https://www.endthepain.org/learning/documents/OvercomingOpiophobia.pdf (accessed on 19 July 2023).
- Verloo, H.; Mpinga, E.K.; Ferreira, M.; Rapin, C.-H.; Chastonay, P. Morphinofobia: The situation among the general population and health care professionals in North-Eastern Portugal. BMC Palliat. Care 2010, 9, 15. [Google Scholar] [CrossRef]
- Vranken, M.J.; Schutjens, M.D.; Mantel-Teeuwisse, A.K. The double opioid crisis: A call for balance. Pharmacoepidemiol. Drug Saf. 2019, 28, 1–3. [Google Scholar] [CrossRef]
- Wailoo, K. Pain: A Political History; Johns Hopkins University Press: Baltimore, MA, USA, 2014; p. 135. [Google Scholar]
- Fishmann, S. The War on Pain: How Breakthroughs in the New Field of Pain Medicine Are Turning the Tide; P Harper Collins: New York, NY, USA, 2000; p. 81. [Google Scholar]
- Casper, M.J.; Baszanger, I. Inventing Pain Medicine: From the Laboratory to the Clinic. Contemp. Sociol. A J. Rev. 1999, 28, 229. [Google Scholar] [CrossRef]
- Baker, R. Controlled Release of Biological Agents; John Wiley & Sons: New York, NY, USA, 1987; pp. 5–20. [Google Scholar]
- Meier, B. Secrets of Dendur. In Pain Killer: An Empire of Deceit and the Origin of America’s Opioid Epidemic, 2nd ed.; Penguin: New York, NY, USA, 2018; pp. 48–52,59. [Google Scholar]
- Lavi, S. The Modern Art of Dying: A History of Euthanasia in the United States; Princeton University Press: Princeton, NJ, USA, 2005; pp. 137–139. [Google Scholar]
- Wailoo, K.A. Pain: Oxycontin Unleashed. A Political History; Johns Hopkins University Press: Montville, NJ, USA, 2014; pp. 168–169. [Google Scholar]
- Angell, M. Opioid Nation. New York Review of Books, 6 December 2018. [Google Scholar]
- Meier, B. The War Against Pain. In Pain Killer: An Empire of Deceit and the Origin of America’s Opioid Epidemic; Random House: New York, NY, USA, 2018; pp. 28–31. [Google Scholar]
- Portenoy, R.K. Cancer Pain, Epidemiology and syndromes. Cancer 1989, 63, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Foley, K.M. Chronic use of opioid analgesics in non-malignant pain: Report of 38 cases. Pain 1986, 25, 171–186. [Google Scholar] [CrossRef]
- McAuliffe, W.E.; Rohman, M.; Santangelo, S.; Feldman, B.; Magnuson, E.; Sobol, A.; Weissman, J. Psychoactive Drug Use among Practicing Physicians and Medical Students. N. Engl. J. Med. 1986, 315, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Horvatich, P.K.; Schnoll, S.H. Filling the knowledge gap: A continuing medical education course on prescribing drugs with abuse potential. N. Y. State J. Med. 1991, 91, 40S–42S. [Google Scholar]
- Keefe, P.R. The Ticking Clock. In Empire of Pain: The Secret History of the Sackler Dynasty; Doubleday Books: New York, NY, USA, 2021; pp. 180–183. [Google Scholar]
- Keefe, P.R. God of Dreams. In Empire of Pain: The Secret History of the Sackler Dynasty; Doubleday Books: New York, NY, USA, 2021; pp. 188–190. [Google Scholar]
- Meier, B. A Pot of Gold. In Pain Killer: An Empire of Deceit and the Origin of America’s Opioid Epidemic; Random House: New York, NY, USA, 2018; pp. 74–75. [Google Scholar]
- Anonymous. Oxycodon (Oxycontin), Opioïde Analgeticum. Gebu 2001, 35, 53–54. Available online: https://www.ge-bu.nl/artikel/oxycodon-oxycontin-opioide-analgeticum (accessed on 19 July 2023).
- Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010. Official Journal of the European Union L 348/74 (31 December, 2010). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:348:0074:0099:EN:PDF (accessed on 5 July 2023).
- Food and Drug Administration Safety and Innovation Act (FDASIA). Available online: https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/food-and-drug-administration-safety-and-innovation-act-fdasia (accessed on 5 July 2023).
- INCB, Narcotic Drugs Report Narcotic_Drugs_2001_full.pdf (incb.org). 2001. Available online: https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2001/narcotic_drugs_2001_full.pdf (accessed on 19 July 2023).
- OxyContin Retard 20 mg, Tabletten Met Verlengde Afgifte|Geneesmiddeleninformatiebank|College ter Beoordeling van Geneesmiddelen (cbg-meb.nl). Available online: https://db.cbg-meb.nl/ords/f?p=111:3::ATC:::P0_DOMAIN,P0_LANG,P3_RVG1:H,NL,22108 (accessed on 20 September 2023).
- Cherny, N.I.; Baselga, J.; de Conno, F.; Radbruch, L. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in Europe: A report from the ESMO/EAPC Opioid Policy Initiative. Ann. Oncol. 2010, 21, 615–626. [Google Scholar] [CrossRef]
- Meyer, A.; LeClair, C.; McDonald, J.V. Prescription Opioid Prescribing in Western Europe and the United States. Rhode Isl. Med. J. 2020, 103, 45–48. [Google Scholar]
- van Amsterdam, J.; Brink, W.V.D. The Misuse of Prescription Opioids: A Threat for Europe? Curr. Drug Abus. Rev. 2015, 8, 3–14. [Google Scholar] [CrossRef]
- Keefe, P.R. Med Man. In Empire of Pain: The Secret History of the Sackler Dynasty; Doubleday Books: New York, NY, USA, 2021; pp. 34–65. [Google Scholar]
- Pieters, T. Marketing medicines through randomised controlled trials: The case of interferon. BMJ 1998, 317, 1231–1233. [Google Scholar] [CrossRef][Green Version]
- Hawthorn, F. Inside the FDA: The Business and Politics behind the Drugs We Take and the Food We Eat; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Angell, M. The Truth about the Drug Companies: How They Deceive Is and What to Do about It; Random House: New York, NY, USA, 2004. [Google Scholar]
- Detrano, J. The Four-Sentenced Letter behind the Rise of Oxycontin. Rutgers Center of Alcohol & Substance Use Studies. 2016. Available online: https://alcoholstudies.rutgers.edu/the-four-sentence-letter-behind-the-rise-of-oxycontin/ (accessed on 5 July 2023).
- Van Zee, A. The Promotion and Marketing of OxyContin: Commercial Triumph, Public Health Tragedy. Am. J. Public Health 2009, 99, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Healy, D. Let Them Eat Prozac: The Unhealthy Relationship Between the Pharmaceutical Industry and Depression; New York University Press: New York, NY, USA, 2004. [Google Scholar]
- Wolfe, S. (Ed.) Worst Pills, Best Pills; Pocket Books: New York, NY, USA, 1988; pp. 279–287. [Google Scholar]
- Lembke, A. Big Pharma Joins Big Medicine. In Drug Dealer MD: How Doctors Were Duped, Patients Got Hooked and Why It’s so Hard to Stop; Johns Hopkins University Press: Baltimore, MA, USA, 2016; pp. 55–72. [Google Scholar]
- Abrahamson, J. Overdosed America. In The Broken Promise of American Medicine: How the Pharmaceutical Companies Distort Medical Knowledge, Mislead Doctors, and Compromise your Health; HaperCollins: New York, NY, USA, 2004. [Google Scholar]
- Marsa, L. Prescription for Profits: How the Pharmaceutical Industry Bankrolled the Unholy Marriage between Science and Business; Scribner: New York, NY, USA, 1997. [Google Scholar]
- Sismondo, S. Ghost-Managed Medicine: Big Pharma’s Invisible Hands; Mattering Press: Manchester, UK, 2018. [Google Scholar]
- McGreal, C. American Overdose: The Opioid Tragedy in Three Acts; Faber & Faber: London, UK, 2018; pp. 14–64. [Google Scholar]
- Macy, B. Dopesick: Dealers, Doctors and the Drug Company that Addicted America; Head of Zeus: London, UK, 2018; pp. 31–48. [Google Scholar]
- Schatman, M.E.; Shapiro, H. Chronic Pain Patient “Advocates” and Their Focus on Opiophilia: Barking Up the Wrong Tree? J. Pain Res. 2021, 14, 3627–3630. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.; Carr, D.B.; Cousins, M. Pain Management: A Fundamental Human Right. Anesth. Analg. 2007, 105, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, G.T.T.; Teunis, T.; Janssen, S.J.; Ring, D. An epidemic of the use, misuse and overdose of opioids and deaths due to overdose, in the United States and Canada. Bone Jt. J. 2017, 99-B, 856–864. [Google Scholar] [CrossRef]
- Zhang, S. The One-Paragraph Letter from 1980 that Fueled the Opioid Crisis. The Atlantic. 2 June 2017. Available online: https://www.theatlantic.com/health/archive/2017/06/nejm-letter-opioids/528840/ (accessed on 30 August 2023).
- Leung, P.T.; Macdonald, E.M.; Stanbrook, M.B.; Dhalla, I.A.; Juurlink, D.N. A 1980 Letter on the Risk of Opioid Addiction. N. Engl. J. Med. 2017, 376, 2194–2195. [Google Scholar] [CrossRef]
- Porter, J.; Jick, H. Addiction Rare in Patients Treated with Narcotics. N. Engl. J. Med. 1980, 302, 123. [Google Scholar] [CrossRef]
- Pieters, T. The Antidepressant Era Revisited: Towards Differentiation and Patient-empowerment in Diagnosis and Treatment. In The Routledge History of Madness and Mental Health; Eghigian., G., Ed.; Routledge: London, UK, 2017; pp. 379–390. [Google Scholar]
- Haddox, J.D.; Joranson, D.; Angarola, R.T.; Brady, A.; Carr, D.B. The use of opioids for the treatment of chronic pain: A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin. J. Pain 1997, 13, 6–8. [Google Scholar]
- Alexander, G.C.; Frattaroli, S.; Gielen, A.C. (Eds.) The Prescription Opioid Epidemic: An Evidence-Based Approach; Johns Hopkins Bloomberg School of Public Health: Baltimore, MA, USA, 2015; p. 25. [Google Scholar]
- Parker-Lue, S.; Santoro, M.; Koski, G. The ethics and economics of pharmaceutical pricing. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 191–206. [Google Scholar] [CrossRef]
- Perlis, R.H.; Perlis, C.S. Physician Payments from Industry Are Associated with Greater Medicare Part D Prescribing Costs. PLoS ONE 2016, 11, e0155474. [Google Scholar] [CrossRef]
- Kennedy-Hendricks, A.; Richey, M.; McGinty, E.E.; Stuart, E.A.; Barry, C.L.; Webster, D.W. Opioid Overdose Deaths and Florida’s Crackdown on Pill Mills. Am. J. Public Health 2016, 106, 291–297. [Google Scholar] [CrossRef]
- Gupta, A.; Daigle, S.; Mojica, J.; Hurley, R.W. Patient perception of pain care in hospitals in the United States. J. Pain Res. 2009, 2, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Understanding the Opioid Overdose Epidemic. Available online: https://www.cdc.gov/opioids/basics/epidemic.html (accessed on 15 August 2023).
- Oxycontin Advertising. Available online: https://www.google.nl/search?q=oxycontin+advertising&sxsrf=ACYBGNTplk3RimUyk7DBkECC8Gbj20RAUw:1579436975482&source=lnms&tbm=isch&sa=X&ved=2ahUKEwjlkvme1Y_nAhVLJFAKHdTWBmsQ_AUoAXoECAwQAw&biw=911&bih=445#imgrc=tnOVQDksUIhLlM (accessed on 18 July 2023).
- Sun, F. Rurality and opioid prescribing rates in U.S. counties from 2006 to 2018: A spatiotemporal investigation. Soc. Sci. Med. 2022, 296, 114788. [Google Scholar] [CrossRef] [PubMed]
- Bedene, A.; Lijfering, W.M.; Niesters, M.; Van Velzen, M.; Rosendaal, F.R.; Bouvy, M.L.; Dahan, A.; Van Dorp, E.L.A. Opioid Prescription Patterns and Risk Factors Associated with Opioid Use in the Netherlands. JAMA Netw. Open 2019, 2, e1910223. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, A.; Marsch, L.A.; Joseph, H.; Portenoy, R.K. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp. Clin. Psychopharmacol. 2008, 16, 405–416. [Google Scholar] [CrossRef]
- Millgate, A.G.; Pogson, B.J.; Wilson, I.W.; Kutchan, T.M.; Zenk, M.H.; Gerlach, W.L.; Fist, A.J.; Larkin, P.J. Morphine-pathway block in top1 poppies. Nature 2004, 431, 413–414. [Google Scholar] [CrossRef]
- Smith, P.A. How an Island in the Antipodes Became the World’s Leading Supplier of Licit Opioids. Pacific Standard. 24 July 2019. Available online: https://pulitzercenter.org/stories/how-island-antipodes-became-worlds-leading-supplier-licit-opioids (accessed on 21 September 2023).
- Bosman, H. The History of the Nederlandsche Cocaine Fabriek and Its Successors as Manufacturers of Narcotic Drugs, Analysed from An International Perspective. Ph.D. Thesis, Maastricht University, Maastricht, The Netherlands, 2012; pp. 323–329. [Google Scholar]
- Keefe, P.R. Warpath. In Empire of Pain: The Secret History of the Sackler Dynasty; Doubleday Books: New York, NY, USA, 2021; p. 363. [Google Scholar]
- DeWeerdt, S. Tracing the US opioid crisis to its roots. Nature 2019, 573, S10–S12. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Kuo, Y.-F.; Brown, D.; Juurlink, D.; Raji, M. Association of Chronic Opioid Use with Presidential Voting Patterns in US Counties in 2016. JAMA Netw. Open 2018, 1, e180450. [Google Scholar] [CrossRef]
- Yongbo, S. The Spillover Effects of Oxycontin’s Introduction on Crime. 5 August 2021. Available online: https://ssrn.com/abstract=3900229 (accessed on 21 September 2023). [CrossRef]
- Evans, W.N.; Lieber, E.M.J.; Power, P. How the Reformulation of OxyContin Ignited the Heroin Epidemic. Rev. Econ. Stat. 2019, 101, 1–15. [Google Scholar] [CrossRef]
- Anonymous. 2020 Drug Enforcement Administration, NDTÂ National Drug Threat Assessment. DEA. March 2021. Available online: https://www.dea.gov/sites/default/files/2021-02/DIR-008-21%202020%20National%20Drug%20Threat%20Assessment_WEB.pdf (accessed on 10 September 2023).
- Smith, B.T. The Dope: The Real History of the Mexican Drug Trade; Ebury Press: London, UK, 2021; pp. 331–359. [Google Scholar]
- Quinones, M. Dreamland: The True Tale of America’s Opiate Epidemic; Bloomsburry Press: New York, NY, USA, 2015; pp. 222–225. [Google Scholar]
- Lee, B.; Zhao, W.; Yang, K.C.; Ahn, Y.Y.; Perry, B.L. Systematic Evaluation of State Policy Interventions Targeting the US Opioid Epidemic, 2007–2018. JAMA Netw. Open 2021, 4, e2036687. [Google Scholar] [CrossRef]
- Ciccarone, D. The rise of illicit fentanyls, stimulants and the fourth wave of the opioid overdose crisis. Curr. Opin. Psychiatry 2021, 34, 344–350. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, V.M.; Staats, P.S.; Trescot, A.M.; Prunskis, J.; Knezevic, N.N.; Soin, A.; Kaye, A.D.; Atluri, S.; Boswell, M.V.; et al. Fourth Wave of Opioid (Illicit Drug) Overdose Deaths and Diminishing Access to Pre-scription Opioids and Interventional Techniques: Cause and Effect. Pain Physician 2022, 25, 97–124. [Google Scholar] [PubMed]
- Rangachari, P.; Govindarajan, A.; Mehta, R.; Seehusen, D.; Rethemeyer, R.K. The relationship between Social Determinants of Health (SDoH) and death from cardiovascular disease or opioid use in counties across the United States (2009–2018). BMC Public Health 2022, 22, 236. [Google Scholar] [CrossRef] [PubMed]
- Jalal, H.; Buchanich, J.M.; Sinclair, D.R.; Roberts, M.S.; Burke, D.S. Age and generational patterns of overdose death risk from opioids and other drugs. Nat. Med. 2020, 26, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Drucker, E. A Plague of Prisons: The Epidemiology of Mass Incarceration in America; The New Press: New York, NY, USA, 2013. [Google Scholar]
- National Opioids Settlement. 2022. Available online: https://nationalopioidsettlement.com/ (accessed on 11 September 2023).
- Mann, B. 4 U.S. Companies Will Pay $26 Billion to Settle Claims They Fueled the Opioid Crisis. NPR. 25 February 2022. Available online: https://www.npr.org/2022/02/25/1082901958/opioid-settlement-johnson-26-billion (accessed on 24 September 2023).
- Felbab-Brown, V. China and Synthetic Drugs Control: Fentanyl, Methamphetamines, and Precursors; Brookings Institution: Washington, DC, USA, 2022; p. 2. Available online: https://www.brookings.edu/research/china-and-synthetic-drugs-control-fentanyl-methamphetamines-and-precursors/ (accessed on 24 September 2023).
- Helmore, E. Blinken Will Seek China’s Cooperation in Curbing Fentanyl at High-Stakes Visit. Guardian. 18 June 2023. Available online: https://www.theguardian.com/us-news/2023/jun/18/antony-blinken-china-visit (accessed on 29 June 2023).
- Fischer, B.; Vojtila, L.; Rehm, J. The ‘fentanyl epidemic’ in Canada—Some cautionary observations focusing on opioid-related mortality. Prev. Med. 2018, 107, 109–113. [Google Scholar] [CrossRef]
- Belzak, L.; Halverson, J. Evidence synthesis—The opioid crisis in Canada: A national perspective. Health Promot. Chronic Dis. Prev. Can. 2018, 38, 224–233. [Google Scholar] [CrossRef]
- Kalkman, G.A.; Kramers, C.; van Dongen, R.T.; Brink, W.v.D.; Schellekens, A. Trends in use and misuse of opioids in the Netherlands: A retrospective, multi-source database study. Lancet Public Health 2019, 4, e498–e505. [Google Scholar] [CrossRef]
- Larjow, E.; Papavasiliou, E.; Payne, S.; Scholten, W.; Radbruch, L. A Systematic Content Analysis of Policy Barriers Impeding Access to Opioid Medication in Central and Eastern Europe: Results of ATOME. J. Pain Symptom Manag. 2016, 51, 99–107. [Google Scholar] [CrossRef]
- Vranken, M.J.M.; Mantel-Teeuwisse, A.K.; Jünger, S.; Radbruch, L.; Scholten, W.; Lisman, J.A.; Subataite, M.; Schutjens, M.D.B. Barriers to access to opioid medicines for patients with opioid dependence: A review of legislation and regulations in eleven central and eastern European countries. Addiction 2017, 112, 1069–1076. [Google Scholar] [CrossRef]
- Ciulla, M.; Marinelli, L.; Di Biase, G.; Cacciatore, I.; Santoleri, F.; Costantini, A.; Dimmito, M.P.; Di Stefano, A. Healthcare Systems across Europe and the US: The Managed Entry Agreements Experience. Healthcare 2023, 11, 447. [Google Scholar] [CrossRef]
- Bedene, A.; van Dorp, E.L.A.; Faquih, T.; Cannegieter, S.C.; Mook-Kanamori, D.O.; Niesters, M.; van Velzen, M.; Gademan, M.G.J.; Rosendaal, F.R.; Bouvy, M.L.; et al. Causes and consequences of the opioid epidemic in the Netherlands: A population-based cohort study. Sci. Rep. 2020, 10, 15309. [Google Scholar] [CrossRef] [PubMed]
- Ayoo, K.; Mikhaeil, J.; Huang, A.; Wąsowicz, M. The opioid crisis in North America: Facts and future lessons for Europe. Anaesthesiol. Intensiv. Ther. 2020, 52, 139–147. [Google Scholar] [CrossRef]
- di Gaudio, F.; Mortali, C.; Tini, A. Opioid epidemic spread from Northern and Eastern Europe to Mediterranean Area. Clin. Ter. 2021, 172, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Verney, S. Opioid Addiction Rising in India as US Drugmakers Push Painkillers. Available online: https://www.theguardian.com/world/2019/aug/28/india-opioids-addiction-us-drugmakers-push-painkillers (accessed on 23 September 2023).
- Synthetic Opioid Use Booms Worldwide Amid Africa ‘Crisis’, UN Says. Available online: https://www.theguardian.com/politics/2019/jun/26/synthetic-opioid-use-booms-worldwide-amid-africa-crisis-un-says (accessed on 23 September 2023).
- Furlan, A.D.; Harvey, A.M.; Chadha, R. Warning from Canada: Latin America, South Africa and India may face an opioid epidemic in the coming years. J. Glob. Health 2020, 10, 010324. [Google Scholar] [CrossRef] [PubMed]
- In Order to Understand the Brutality of American Capitalism, You Have to Start on the Plantation. Available online: https://www.nytimes.com/interactive/2019/08/14/magazine/slavery-capitalism.html (accessed on 22 September 2023).
- Payer, L. Disease Mongers: How Doctors, drug Companies and Insurers are Making You Feel Sick; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Urquhart, J. Comparative regulation of drug and aircraft development: Lessons for regulatory reform? Clin. Pharmacol. Ther. 1997, 62, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Kolodny, A.; Courtwright, D.T.; Hwang, C.S.; Kreiner, P.; Eadie, J.L.; Clark, T.W.; Alexander, G.C. The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction. Annu. Rev. Public Health 2015, 36, 559–574. [Google Scholar] [CrossRef]
- Madras, B.K. The Surge of Opioid Use, Addiction, and Overdoses. JAMA Psychiatry 2017, 74, 441–442. [Google Scholar] [CrossRef]
- McCoul, E.D.; Barnett, M.L.; Brenner, M.J. Reducing Opioid Prescribing and Consumption After Surgery—Keeping the Lock on Pandora’s Box. JAMA Otolaryngol. Neck Surg. 2021, 147, 819–821. [Google Scholar] [CrossRef]
- Anonymous. Not Allowed to Be Compassionate’ Chronic Pain, the Overdose Crisis, and Unintended Harms in the US. Human Rights Watch. 2018, pp. 3–4. Available online: https://www.hrw.org/report/2018/12/18/not-allowed-be-compassionate/chronic-pain-overdose-crisis-and-unintended-harms-us (accessed on 24 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieters, T. The Imperative of Regulation: The Co-Creation of a Medical and Non-Medical US Opioid Crisis. Psychoactives 2023, 2, 317-336. https://doi.org/10.3390/psychoactives2040020
Pieters T. The Imperative of Regulation: The Co-Creation of a Medical and Non-Medical US Opioid Crisis. Psychoactives. 2023; 2(4):317-336. https://doi.org/10.3390/psychoactives2040020
Chicago/Turabian StylePieters, Toine. 2023. "The Imperative of Regulation: The Co-Creation of a Medical and Non-Medical US Opioid Crisis" Psychoactives 2, no. 4: 317-336. https://doi.org/10.3390/psychoactives2040020
APA StylePieters, T. (2023). The Imperative of Regulation: The Co-Creation of a Medical and Non-Medical US Opioid Crisis. Psychoactives, 2(4), 317-336. https://doi.org/10.3390/psychoactives2040020





