Multiple Sclerosis-Associated Uveitis Therapy: Is Modern Better than Old Reliable?
Abstract
1. Introduction
2. Methods
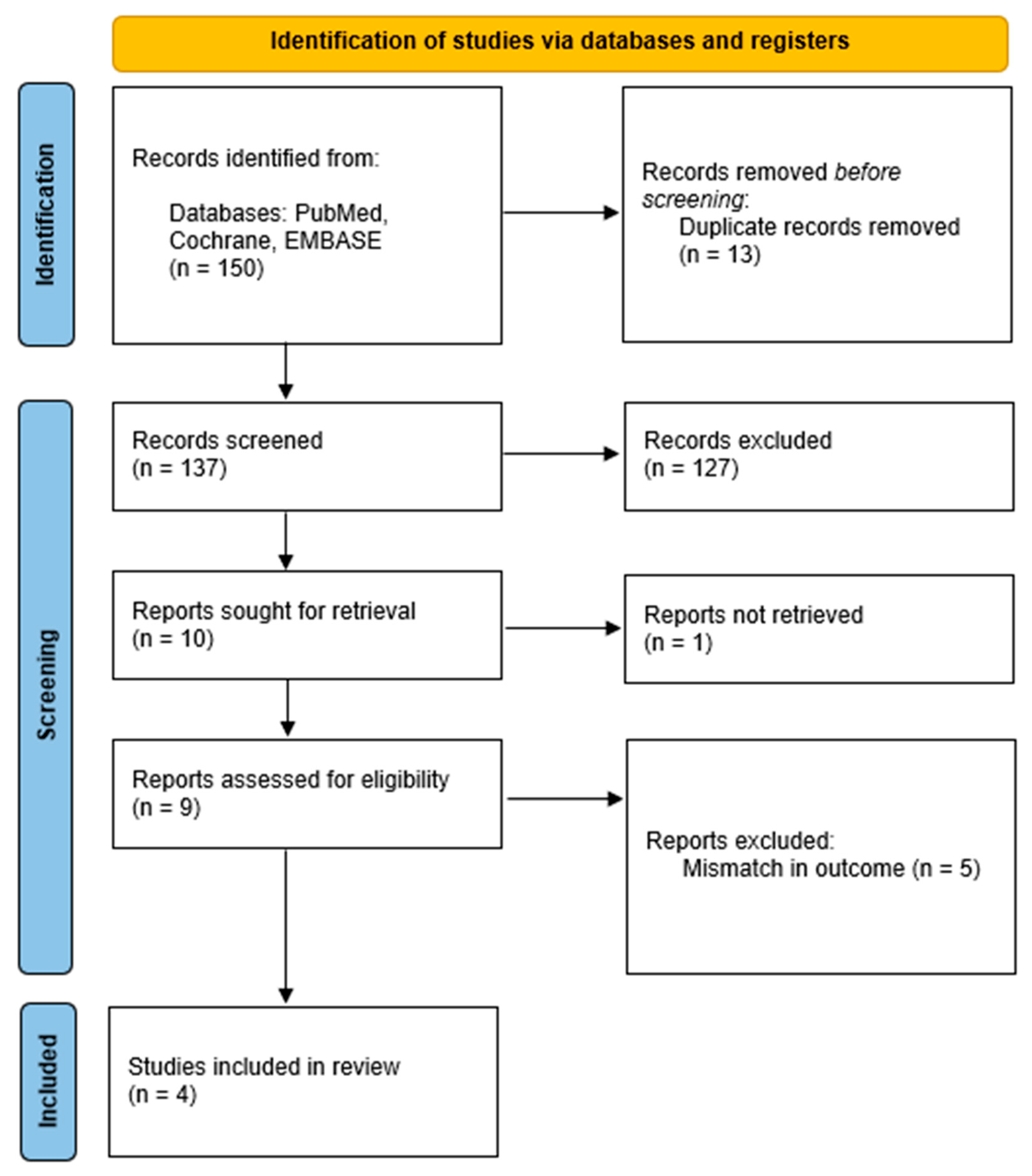
2.1. IFN-Βeta Literature Search Strategy
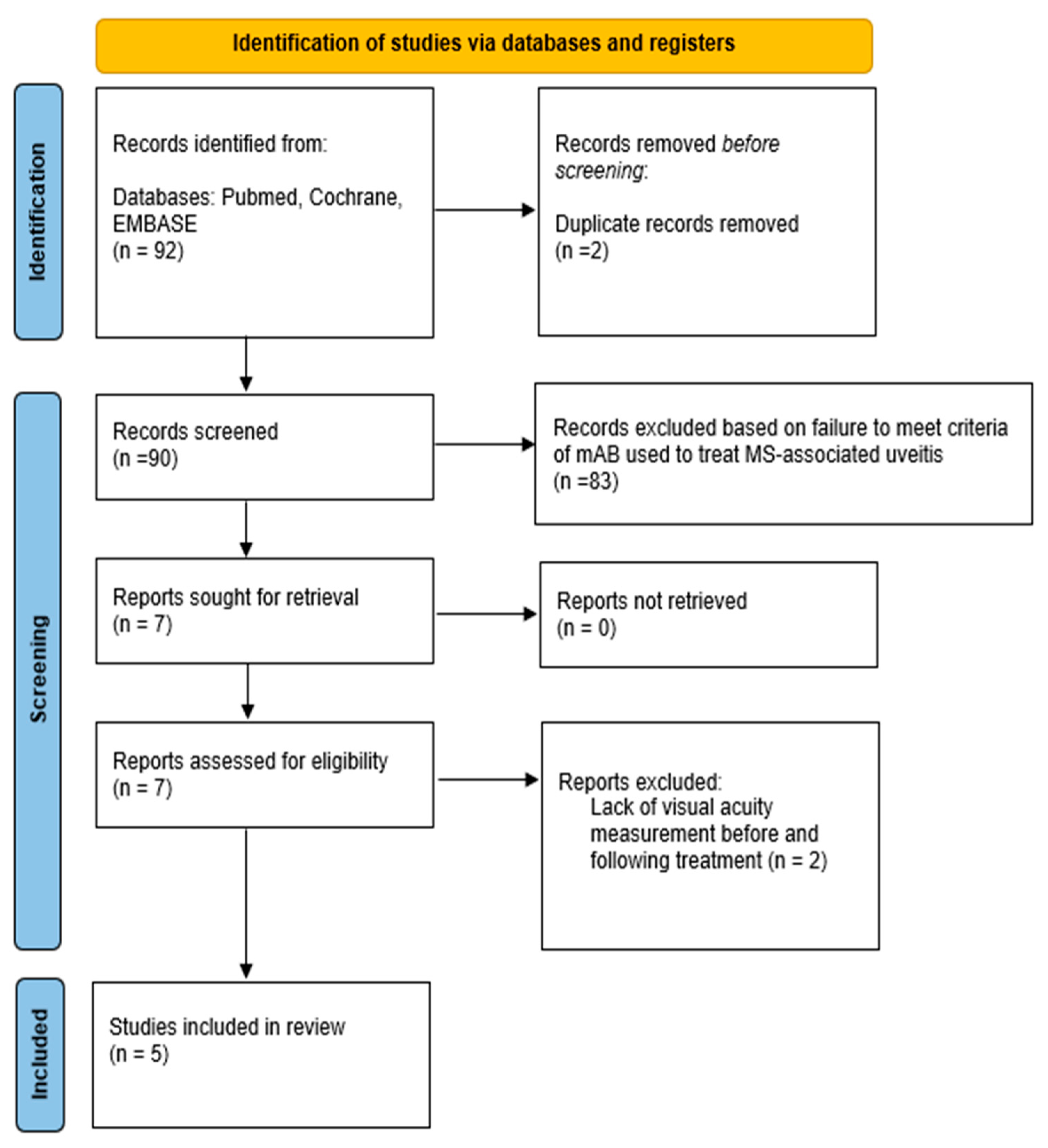
2.2. Monoclonal Antibodies Literature Search Strategy
2.3. Data Retrieval
2.4. Variables and Statistical Analysis
3. Results
3.1. IFN-Βeta Effect on MS-Uveitis
3.2. Effect of mAbs on MS-Uveitis
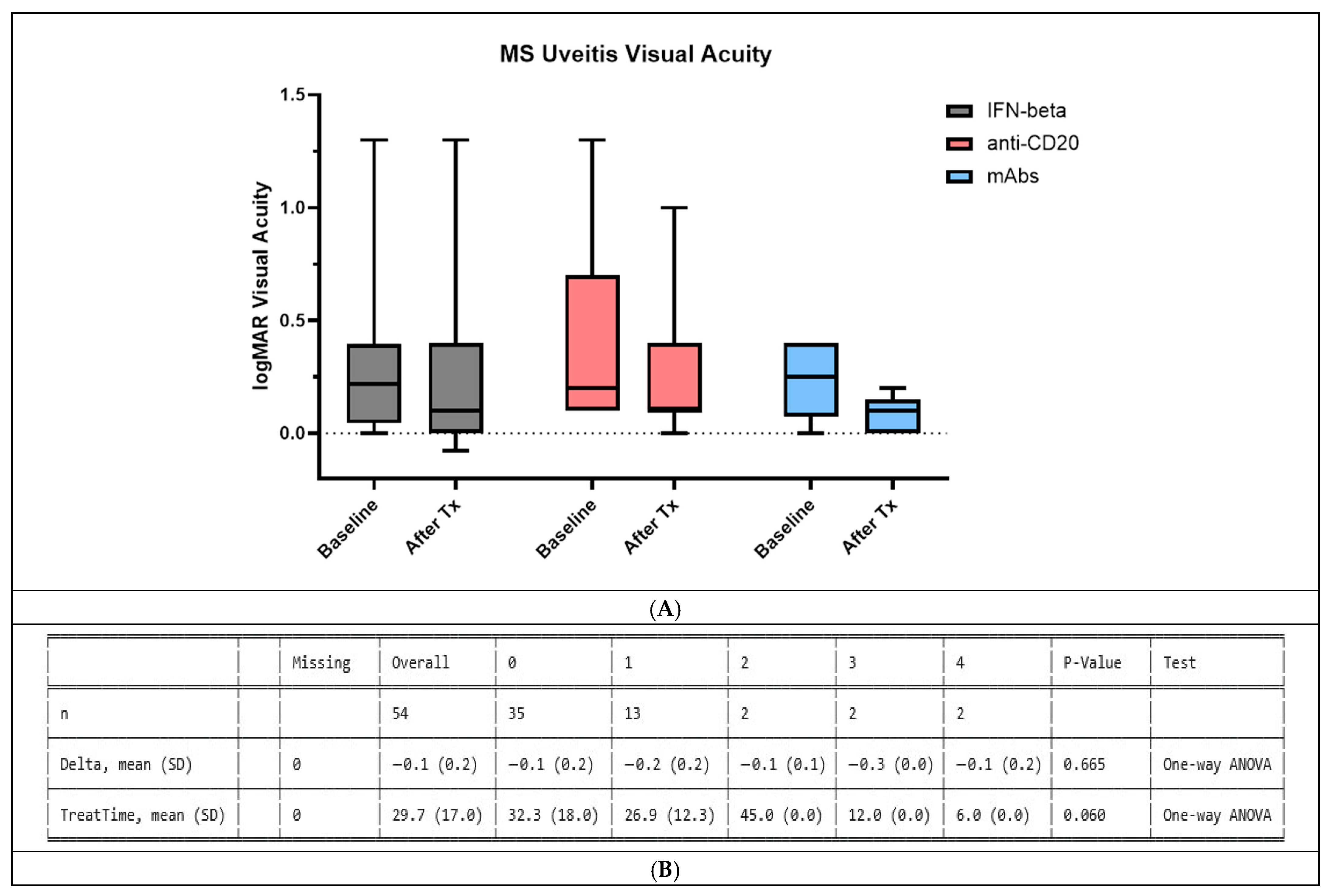
3.3. IFN-β Effect on MS-Associated Uveitis Is Comparable to mAbs
4. Discussion
4.1. Evaluation of the Effect of IFN-β and mAb Treatment of MS-AU
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar]
- Zein, G.; Berta, A.; Foster, S. Multiple-Sclerosis-Associated Uveitis. In Considerations for Treatment of This Unique Patient Population; Retinal Physician: Ambler, PA, USA, 2018. [Google Scholar]
- Allegri, P.; Rissotto, R.; Herbort, C.P.; Murialdo, U. CNS diseases and uveitis. J. Ophthalmic Vis. Res. 2011, 6, 284–308. [Google Scholar]
- Reekie, I.R.; Sharma, S.; Foers, A.; Sherlock, J.; Coles, M.C.; Dick, A.D.; Denniston, A.K.; Buckley, C.D. The cellular composition of the uveal immune environment. Front. Med. 2021, 8, 721953. [Google Scholar] [CrossRef]
- Casselman, P.; Cassiman, C.; Casteels, I.; Schauwvlieghe, P. Insights into multiple sclerosis-associated uveitis: A scoping review. Acta Ophthalmol. 2021, 99, 592–603. [Google Scholar] [CrossRef]
- Raskin, E.; Achiron, A.; Zloto, O.; Neuman, R.; Vishnevskia-Dai, V. Uveitis prior to clinical presentation of Multiple Sclerosis (MS) is associated with better MS prognosis. PLoS ONE 2022, 17, e0264918. [Google Scholar] [CrossRef]
- Saboya-Galindo, P.; Mejía-Salgado, G.; Cifuentes-González, C.; Rodríguez-Rodríguez, C.A.; Boada-Robayo, L.; Méndez-Marulanda, R.; Varela, J.S.; Riveros-Sierra, L.; Gaviria-Carrillo, M.; De-La-Torre, A. Uveitis characteristics and multiple sclerosis phenotype of patients with multiple sclerosis-associated uveitis: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0307455. [Google Scholar] [CrossRef]
- Olsen, T.G.; Frederiksen, J. The association between multiple sclerosis and uveitis. Surv. Ophthalmol. 2017, 62, 89–95. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [PubMed]
- Frohman, E.M.; Frohman, T.C.; Zee, D.S.; McColl, R.; Galetta, S. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol. 2005, 4, 111–121. [Google Scholar] [CrossRef]
- Gordon, L.K.; Goldstein, D.A. Gender and uveitis in patients with multiple sclerosis. J. Ophthalmol. 2014, 2014, 565262. [Google Scholar] [CrossRef]
- Multicenter Uveitis Steroid Treatment Trial Research Group. Benefits of Systemic Anti-inflammatory Therapy versus Fluocinolone Acetonide Intraocular Implant for Intermediate Uveitis, Posterior Uveitis, and Panuveitis: Fifty-four-Month Results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. Ophthalmology 2015, 122, 1967–1975. [Google Scholar]
- Yang, H.; Duchesneau, E.; Foster, R.; Guerin, A.; Ma, E.; Thomas, N.P. Cost-effectiveness analysis of ocrelizumab versus subcutaneous interferon beta-1a for the treatment of relapsing multiple sclerosis. J. Med. Econ. 2017, 20, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Canadian Agency for Drugs and Technologies in Health. Comparative Clinical and Cost-Effectiveness of Drug Therapies for Relapsing-Remitting Multiple Sclerosis; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2013. [Google Scholar]
- Ceniza, A.; Burrow, W.; Cervantes, J.L. American Federation for Medical Research 2025 Southeastern Regional Meeting. J. Investig. Med. 2025, 73, NP1–NP41. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019.
- Kinyas, S.; Esgin, H. Peripheral Vasculitis, Intermediate Uveitis and Interferon Use in Multiple Sclerosis. Turk. J. Ophthalmol. 2016, 46, 41–43. [Google Scholar] [CrossRef]
- Gur Gungor, S.; Akova, Y.A.; Akar, E. Uveitis Associated with Multiple Sclerosis. Turk. J. Ophthalmol. 2012, 42, 462–465. [Google Scholar]
- Becker, M.D.; Heiligenhaus, A.; Hudde, T.; Storch-Hagenlocher, B.; Wildemann, B.; Barisani-Asenbauer, T.; Thimm, C.; Stübiger, N.; Trieschmann, M.; Fiehn, C. Interferon as a treatment for uveitis associated with multiple sclerosis. Br. J. Ophthalmol. 2005, 89, 1254–1257. [Google Scholar] [CrossRef]
- Llorenc, V.; Rey, A.; Mesquida, M.; Pelegrín, L.; Adán, A. Central nervous system demyelinating disease-associated uveitis. Arch. Soc. Esp. Oftalmol. 2012, 87, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kieseier, B.C. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs 2011, 25, 491–502. [Google Scholar] [CrossRef]
- Mackensen, F.; Jakob, E.; Springer, C.; Dobner, B.C.; Wiehler, U.; Weimer, P.; Rohrschneider, K.; Fiehn, C.; Max, R.; Storch-Hagenlocher, B.; et al. Interferon versus methotrexate in intermediate uveitis with macular edema: Results of a randomized controlled clinical trial. Am. J. Ophthalmol. 2013, 156, 478–486.e1. [Google Scholar] [CrossRef]
- Henriques, C.; da Ana, R.; Krambeck, K.; Miguel, S.; Santini, A.; Zielińska, A.; Souto, E.B. Monoclonal Antibodies for the Treatment of Ocular Diseases. J. Clin. Med. 2024, 13, 5815. [Google Scholar] [CrossRef]
- Voge, N.V.; Alvarez, E. Monoclonal Antibodies in Multiple Sclerosis: Present and Future. Biomedicines 2019, 7, 20. [Google Scholar] [CrossRef]
- Keehn, C.C.; Yazdian, A.; Hunt, P.J.; Davila-Siliezar, P.; Laylani, N.A.; Lee, A.G. Monoclonal antibodies in neuro-ophthalmology. Saudi J. Ophthalmol. 2024, 38, 13–24. [Google Scholar] [CrossRef]
- Jouve, L.; Benrabah, R.; Héron, E.; Bodaghi, B.; Le Hoang, P.; Touitou, V. Multiple Sclerosis-related Uveitis: Does MS Treatment Affect Uveitis Course? Ocul. Immunol. Inflamm. 2016, 25, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Stascheit, F.; Rübsam, A.; Otto, C.; Meisel, A.; Ruprecht, K.; Pleyer, U. Anti-CD20 therapy for multiple sclerosis-associated uveitis: A case series. Eur. J. Neurol. 2022, 29, 3028–3038. [Google Scholar] [CrossRef] [PubMed]
- Calma, A.D.; Young, S.; Sandbach, J.; Riminton, S.; Reddel, S.W.; Ramanathan, S. Targeting alpha-4 integrin with natalizumab for intermediate uveitis associated with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2024, 10, 20552173241301034. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Nicholson, L.; Dick, A.; Rice, C.; Atan, D. Intermediate uveitis associated with MS: Diagnosis, clinical features, pathogenic mechanisms, and recommendations for management. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e909. [Google Scholar] [CrossRef]
- Zong, Y.; Miyagaki, M.; Yang, M.; Zhang, J.; Zou, Y.; Ohno-Matsui, K.; Kamoi, K. Ophthalmic Use of Targeted Biologics in the Management of Intraocular Diseases: Current and Emerging Therapies. Antibodies 2024, 13, 86. [Google Scholar] [CrossRef]
- Wakefield, D.; McCluskey, P.; Wildner, G.; Thurau, S.; Carr, G.; Chee, S.-P.; Forrester, J.; Dick, A.; Hudson, B.; Lightman, S.; et al. Inflammatory eye disease: Pre-treatment assessment of patients prior to commencing immunosuppressive and biologic therapy: Recommendations from an expert committee. Autoimmun. Rev. 2017, 16, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Gil, W.; Lagrib, H.; Olagne, L.; Tilignac, C.; Perie, M.; Taithe, F.; Moisset, X.; Chiambaretta, F.; Clavelou, P.; Andre, M.; et al. Multiple Sclerosis-Associated Uveitis: A Case Report of Refractory Bilateral Chronic Granulomatous Panuveitis Successfully Treated with Tocilizumab. Ocul. Immunol. Inflamm. 2024, 32, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Heppell, C.; Subramanian, A.; Adderley, N.J.; Nirantharakumar, K.; Denniston, A.K.; Pavesio, C.; Braithwaite, T. Comprehensive Update on Multiple Sclerosis-Associated Uveitis and New Epidemiological Insights from the United Kingdom. Ocul. Immunol. Inflamm. 2025, 33, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kaya, D.; Kaya, M.; Özakbaş, S.; Idiman, E. Uveitis associated with multiple sclerosis: Complications and visual prognosis. Int. J. Ophthalmol. 2014, 7, 1010–1013. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burrow, W.; Ceniza, A.; Kan, B.; Colwell, S.; Cervantes, J. Multiple Sclerosis-Associated Uveitis Therapy: Is Modern Better than Old Reliable? J. Clin. Transl. Ophthalmol. 2025, 3, 22. https://doi.org/10.3390/jcto3040022
Burrow W, Ceniza A, Kan B, Colwell S, Cervantes J. Multiple Sclerosis-Associated Uveitis Therapy: Is Modern Better than Old Reliable? Journal of Clinical & Translational Ophthalmology. 2025; 3(4):22. https://doi.org/10.3390/jcto3040022
Chicago/Turabian StyleBurrow, Wesley, Armand Ceniza, Brian Kan, Skyler Colwell, and Jorge Cervantes. 2025. "Multiple Sclerosis-Associated Uveitis Therapy: Is Modern Better than Old Reliable?" Journal of Clinical & Translational Ophthalmology 3, no. 4: 22. https://doi.org/10.3390/jcto3040022
APA StyleBurrow, W., Ceniza, A., Kan, B., Colwell, S., & Cervantes, J. (2025). Multiple Sclerosis-Associated Uveitis Therapy: Is Modern Better than Old Reliable? Journal of Clinical & Translational Ophthalmology, 3(4), 22. https://doi.org/10.3390/jcto3040022





