MIGS, Cataract Surgery, or Both? An Analysis of Clinical Trial Data to Compare Efficacy and Outcomes on Glaucoma Patients
Abstract
1. Introduction
2. Methods
2.1. Study Search and Selection
2.1.1. Inclusion Criteria
- MIGS clinical trials, cataract surgery clinical trials, or MIGS and cataract surgery clinical trials;
- Completed interventional studies;
- Publicly available studies with results;
- Institutional review board-approved studies;
- Outcome data including IOP and medication reduction.
2.1.2. Exclusion Criteria
- Follow-up shorter than 6 months;
- Glaucoma types other than POAG (e.g., NTG or ACG);
- Lack of data on medication usage or intraocular pressure (IOP);
- Studies still ongoing, open to accrual, or not published in English.
2.1.3. Interventions
- -
- Schlemm’s canal devices (e.g., iStent, Hydrus Microstent, trabectome, Kahook dual blade, and GATT);
- -
- Suprachoroidal space (e.g., CyPass);
- -
- Subconjunctival space (e.g., XEN Gel Stent, PRESERFLO MicroShunt);
- -
- Ciliary body (e.g., endocyclophotocoagulation) [21].
- -
- Cataract surgery/phacoemulsification;
- -
- iStent®;
- -
- CyPass® Micro-Stent;
- -
- Ex-PRESS®;
- -
- Hydrus®;
- -
- PRESERFLO™ MicroShunt (F.K.A., InnFocus, MIDI Arrow);
- -
- XEN® Gel Stent.
2.2. Data Synthesis and Analysis
3. Results
3.1. Study Selection
3.2. Baseline Characteristics
3.3. Outcomes Analysis
3.3.1. 6 Month Outcomes
3.3.2. 12 Month Outcomes
3.3.3. 24 Month Outcomes
4. Discussion
4.1. Outcomes
Stability of IOP Reduction and Glaucoma Medication Reduction
4.2. Safety Profile of Cataract Surgery and MIGS
4.3. Availability and Ease of Implementation of MIGS in Countries with High Glaucoma Burden
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Abdulhussein, D.; Abdul Hussein, M. WHO Vision 2020: Have We Done It? Ophthalmic Epidemiol. 2023, 30, 331–339. [Google Scholar] [CrossRef]
- Hashemi, H.; Pakzad, R.; Yekta, A.; Aghamirsalim, M.; Pakbin, M.; Ramin, S.; Khabazkhoob, M. Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 2020, 34, 1357–1370. [Google Scholar] [CrossRef]
- Allen, D.; Vasavada, A. Cataract and surgery for cataract. BMJ 2006, 333, 128–132. [Google Scholar] [CrossRef]
- Davis, G. The Evolution of Cataract Surgery. Mo. Med. 2016, 113, 58–62. [Google Scholar]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Dtsch. Arztebl. Int. 2020, 117, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sharaawy, T.; Bhartiya, S. Surgical management of glaucoma: Evolving paradigms. Indian J. Ophthalmol. 2011, 59 (Suppl. S1), S123–S130. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, A.C.; Dumitrescu, O.M.; Radu, M.; Rogoz, R.E. Adherence to Therapy in Glaucoma Treatment—A Review. J. Pers. Med. 2022, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.; Cate, H.; Broadway, D.C. Barriers to adherence with glaucoma medications: A qualitative research study. Eye 2009, 23, 924–932. [Google Scholar] [CrossRef]
- Tsai, J.C.; McClure, C.A.; Ramos, S.E.; Schlundt, D.G.; Pichert, J.W. Compliance barriers in glaucoma: A systematic classification. J. Glaucoma 2003, 12, 393–398. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Robin, A.L.; Blachley, T.; Farris, K.; Heisler, M.; Resnicow, K.; Lee, P.P. Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology 2015, 122, 1308–1316. [Google Scholar] [CrossRef]
- Chang, R.T.; Singh, K. Glaucoma Suspect: Diagnosis and Management. Asia-Pac. J. Ophthalmol. 2016, 5, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Binibrahim, I.H.; Bergström, A.K. The role of trabeculectomy in enhancing glaucoma patient’s quality of life. Oman J. Ophthalmol. 2017, 10, 150–154. [Google Scholar] [CrossRef]
- Gedde, S.J.; Herndon, L.W.; Brandt, J.D.; Budenz, D.L.; Feuer, W.J.; Schiffman, J.C. Postoperative Complications in the Tube Versus Trabeculectomy (TVT) Study During Five Years of Follow-up. Am. J. Ophthalmol. 2012, 153, 804–814.e1. [Google Scholar] [CrossRef]
- Birnbaum, F.A.; Neeson, C.; Solá-Del Valle, D. Microinvasive Glaucoma Surgery: An Evidence-Based Review. Semin. Ophthalmol. 2021, 36, 772–786. [Google Scholar] [CrossRef]
- Radcliffe, N. The case for standalone micro-invasive glaucoma surgery: Rethinking the role of surgery in the glaucoma treatment paradigm. Curr. Opin. Ophthalmol. 2023, 34, 138–145. [Google Scholar] [CrossRef]
- Yang, S.A.; Mitchell, W.; Hall, N.; Elze, T.; Lorch, A.C.; Miller, J.W.; Zebardast, N.; Pershing, S.; Hyman, L.; Haller, J.A.; et al. Trends and Usage Patterns of Minimally Invasive Glaucoma Surgery in the United States: IRIS® Registry Analysis 2013–2018. Ophthalmol. Glaucoma 2021, 4, 558–568. [Google Scholar] [CrossRef]
- Saheb, H.; Ahmed, I.I.K. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr. Opin. Ophthalmol. 2012, 23, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Otárola, F.; Pooley, F. Minimally invasive glaucoma surgery (MIGS) devices: Risks, benefits and suitability. Community Eye Health 2021, 34, 59–60. [Google Scholar] [PubMed]
- Gillmann, K.; Mansouri, K. Minimally Invasive Glaucoma Surgery: Where Is the Evidence? Asia Pac. J. Ophthalmol. 2020, 9, 203–214. [Google Scholar] [CrossRef]
- Cantor, L.; Lindfield, D.; Ghinelli, F.; Świder, A.W.; Torelli, F.; Steeds, C.; Jr, J.E.D.; Nguyen, D.Q. Systematic Literature Review of Clinical, Economic, and Humanistic Outcomes Following Minimally Invasive Glaucoma Surgery or Selective Laser Trabeculoplasty for the Treatment of Open-Angle Glaucoma with or Without Cataract Extraction. Clin. Ophthalmol. 2023, 17, 85–101. [Google Scholar] [CrossRef]
- Porter. Combined Cataract-Glaucoma Surgery and MIGS. American Academy of Ophthalmology. 25 May 2023. Available online: https://www.aao.org/eye-health/treatments/combined-cataract-glaucoma-surgery-facts (accessed on 15 January 2025).
- Berdahl, J.P. Cataract Surgery to Lower Intraocular Pressure. Middle East Afr. J. Ophthalmol. 2009, 16, 119–122. [Google Scholar] [CrossRef]
- Mansberger, S.L.; Gordon, M.O.; Jampel, H.; Bhorade, A.; Brandt, J.D.; Wilson, B.; Kass, M.A. Reduction in intraocular pressure after cataract extraction: The Ocular Hypertension Treatment Study. Ophthalmology 2012, 119, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Tarongoy, P.; Ho, C.L.; Walton, D.S. Angle-closure Glaucoma: The Role of the Lens in the Pathogenesis, Prevention, and Treatment. Surv. Ophthalmol. 2009, 54, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.Q.; Wang, J.J.; Rochtchina, E.; Maloof, A.; Mitchell, P. Systemic and ocular comorbidity of cataract surgical patients in a western Sydney public hospital. Clin. Exp. Ophthalmol. 2004, 32, 383–387. [Google Scholar] [CrossRef]
- PRISMA Statement. PRISMA 2020 Checklist. Available online: https://www.prisma-statement.org/prisma-2020-checklist (accessed on 14 March 2025).
- Ivantis, Inc. A Prospective, Multicenter, Randomized Comparison of the Hydrus Microstent to the iStent for Lowering Intraocular Pressure in Glaucoma Patients Undergoing Cataract Surgery. clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT02024464 (accessed on 2 March 2025).
- Fan Gaskin, J.C.; Bigirimana, D.; Kong, G.Y.X.; McGuinness, M.B.; Atik, A.; Liu, L.; Brooks, A.M.; Ang, G.S. Prospective, Randomized Controlled Trial of Cataract Surgery vs Combined Cataract Surgery with Insertion of iStent Inject. Ophthalmol. Glaucoma 2024, 7, 326–334. [Google Scholar] [CrossRef]
- Fea, A.M. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma. J. Cataract. Refract. Surg. 2010, 36, 407–412. [Google Scholar] [CrossRef]
- Fernández-Barrientos, Y.; García-Feijoó, J.; Martínez-De-La-Casa, J.M.; Pablo, L.E.; Fernández-Pérez, C.; Sánchez, J.G. Fluorophotometric Study of the Effect of the Glaukos Trabecular Microbypass Stent on Aqueous Humor Dynamics. Investig. Opthalmology Vis. Sci. 2010, 51, 3327–3332. [Google Scholar] [CrossRef]
- Pfeiffer, N.; Garcia-Feijoo, J.; Martinez-De-La-Casa, J.M.; Larrosa, J.M.; Fea, A.; Lemij, H.; Gandolfi, S.; Schwenn, O.; Lorenz, K.; Samuelson, T.W. A Randomized Trial of a Schlemm’s Canal Microstent with Phacoemulsification for Reducing Intraocular Pressure in Open-Angle Glaucoma. Ophthalmology 2015, 122, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, T.W.; Katz, L.J.; Wells, J.M.; Duh, Y.-J.; Giamporcaro, J.E.; US iStent Study Group. Randomized Evaluation of the Trabecular Micro-Bypass Stent with Phacoemulsification in Patients with Glaucoma and Cataract. Ophthalmology 2011, 118, 459–467. [Google Scholar] [CrossRef]
- Samuelson, T.W.; Chang, D.F.; Marquis, R.; Flowers, B.; Lim, K.S.; Ahmed, I.I.K.; Jampel, H.D.; Aung, T.; Crandall, A.S.; Singh, K.; et al. A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract. Ophthalmology 2019, 126, 29–37. [Google Scholar] [CrossRef]
- Vold, S.; Ahmed, I.I.K.; Craven, E.R.; Mattox, C.; Stamper, R.; Packer, M.; Brown, R.H.; Ianchulev, T. Two-Year COMPASS Trial Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucoma and Cataracts. Ophthalmology 2016, 123, 2103–2112. [Google Scholar] [CrossRef]
- For The Apex Study Group; Reitsamer, H.; Sng, C.; Vera, V.; Lenzhofer, M.; Barton, K.; Stalmans, I. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 983–996. [Google Scholar] [CrossRef]
- Beckers, H.J.; Aptel, F.; Webers, C.A.; Bluwol, E.; Martínez-De-La-Casa, J.M.; García-Feijoó, J.; Lachkar, Y.; Méndez-Hernández, C.D.; Riss, I.; Shao, H.; et al. Safety and Effectiveness of the PRESERFLO® MicroShunt in Primary Open-Angle Glaucoma. Ophthalmol. Glaucoma 2022, 5, 195–209. [Google Scholar] [CrossRef]
- de Jong, L.A.M.S. The Ex-PRESS glaucoma shunt versus trabeculectomy in open-angle glaucoma: A prospective randomized study. Adv. Ther. 2009, 26, 336–345. [Google Scholar] [CrossRef]
- Denis, P.; Hirneiß, C.; Durr, G.M.; Reddy, K.P.; Kamarthy, A.; Calvo, E.; Hussain, Z.; Ahmed, I.K. Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial. Br. J. Ophthalmol. 2020, 106, 65–70. [Google Scholar] [CrossRef]
- Garcìa-Feijoo, J.; Höh, H.; Uzunov, R.; Dickerson, J.E., Jr. Supraciliary Microstent in Refractory Open-Angle Glaucoma: Two-Year Outcomes from the DUETTE Trial. J. Ocul. Pharmacol. Ther. 2018, 34, 538–542. [Google Scholar] [CrossRef]
- Grover, D.S.; Flynn, W.J.; Bashford, K.P.; Lewis, R.A.; Duh, Y.-J.; Nangia, R.S.; Niksch, B. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Arch. Ophthalmol. 2017, 183, 25–36. [Google Scholar] [CrossRef]
- Hoeh, H.; Vold, S.D.; Ahmed, I.K.; Anton, A.; Rau, M.; Singh, K.; Chang, D.F.; Shingleton, B.J.; Ianchulev, T. Initial Clinical Experience With the CyPass Micro-Stent. Eur. J. Gastroenterol. Hepatol. 2016, 25, 106–112. [Google Scholar] [CrossRef]
- Riss, I. A 2-Year, Single-Center Study to Assess the Safety and Effectiveness of the MicroShunt in Primary Open-Angle Glaucoma. Ophthalmic Res. 2022, 66, 206–217. [Google Scholar] [CrossRef]
- Ahmed, I.I.K.; Fea, A.; Au, L.; Ang, R.E.; Harasymowycz, P.; Jampel, H.D.; Samuelson, T.W.; Chang, D.F.; Rhee, D.J. A Prospective Randomized Trial Comparing Hydrus and iStent Microinvasive Glaucoma Surgery Implants for Standalone Treatment of Open-Angle Glaucoma. Ophthalmology 2020, 127, 52–61. [Google Scholar] [CrossRef]
- Transcend Medical, Inc. Randomized, Prospective Clinical Evaluation of the Safety and Effectiveness of Visco-Assisted CyPass® Implantation in Patients with Open Angle Glaucoma. clinicaltrials.gov. 2019. Available online: https://clinicaltrials.gov/study/NCT02448875 (accessed on 1 March 2025).
- Baker, N.D.; Barnebey, H.S.; Moster, M.R.; Stiles, M.C.; Vold, S.D.; Khatana, A.K.; Flowers, B.E.; Grover, D.S.; Strouthidis, N.G.; Panarelli, J.F. Ab-Externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma. Ophthalmology 2021, 128, 1710–1721. [Google Scholar] [CrossRef]
- Netland, P.A.; Sarkisian, S.R., Jr.; Moster, M.R.; Ahmed, I.I.; Condon, G.; Salim, S.; Sherwood, M.B.; Siegfried, C.J. Randomized, prospective, comparative trial of EX-PRESS glaucoma filtration device versus trabeculectomy (XVT study). Am J Ophthalmol. 2014, 157, 433–440. [Google Scholar] [CrossRef]
- Wagschal, L.D.; Trope, G.E.; Jinapriya, D.; Jin, Y.P.; Buys, Y.M. Prospective Randomized Study Comparing Ex-PRESS to Trabeculectomy: 1-Year Results. J. Glaucoma 2015, 24, 624–629. [Google Scholar] [CrossRef]
- PROSPERO. Available online: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251028923 (accessed on 15 April 2025).
- UNSD—Methodology. Standard Country or Area Codes for Statistical Use (M49). Available online: https://unstats.un.org/unsd/methodology/m49 (accessed on 8 April 2025).
- Rowson, A.C.; Hogarty, D.T.; Maher, D.; Liu, L. Minimally Invasive Glaucoma Surgery: Safety of Individual Devices. J. Clin. Med. 2022, 11, 6833. [Google Scholar] [CrossRef]
- Fea, A.M.; Belda, J.I.; Rękas, M.; Junemann, A.; Chang, L.; Pablo, L.; Voskanyan, L.; Katz, L.J. Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin. Ophthalmol. 2014, 8, 875–882. [Google Scholar] [CrossRef]
- Ahmed, I.I.K.; Rhee, D.J.; Jones, J.; Singh, I.P.; Radcliffe, N.; Gazzard, G.; Samuelson, T.W.; Ong, J.; Singh, K. Three-Year Findings of the HORIZON Trial: A Schlemm Canal Microstent for Pressure Reduction in Primary Open-Angle Glaucoma and Cataract. Ophthalmology 2021, 128, 857–865. [Google Scholar] [CrossRef]
- Toneatto, G.; Zeppieri, M.; Papa, V.; Rizzi, L.; Salati, C.; Gabai, A.; Brusini, P. 360° Ab-Interno Schlemm’s Canal Viscodilation with OMNI Viscosurgical Systems for Open-Angle Glaucoma-Midterm Results. J. Clin. Med. 2022, 11, 259. [Google Scholar] [CrossRef]
- Hirsch, L.; Cotliar, J.; Vold, S.; Selvadurai, D.; Campbell, A.G.; Ferreira, G.; Aminlari, A.; Cho, A.; Heersink, S.; Hochman, M.; et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J. Cataract. Refract. Surg. 2021, 47, 907–915. [Google Scholar] [CrossRef]
- Silveira Seixas, R.C.; Balbino, M.; Basile Neto, A.; de Alcantara Almeida Costa, A.; Jordão, M.L.d.S.; Russ, H.H.A. Mid-Term Evaluation of iStent Inject® Trabecular Micro-Bypass Stent Implantation with or without Phacoemulsification: A Retrospective Study. Clin. Ophthalmol. 2020, 14, 4403–4413. [Google Scholar] [CrossRef]
- Briceno-Lopez, C.; Burguera-Giménez, N.; García-Domene, M.C.; Díez-Ajenjo, M.A.; Peris-Martínez, C.; Luque, M.J. Corneal Edema after Cataract Surgery. J. Clin. Med. 2023, 12, 6751. [Google Scholar] [CrossRef]
- Myers, J.S.; Masood, I.; Hornbeak, D.M.; Belda, J.I.; Auffarth, G.; Jünemann, A.; Giamporcaro, J.E.; Martinez-De-La-Casa, J.M.; Ahmed, I.I.K.; Voskanyan, L.; et al. Prospective Evaluation of Two iStent® Trabecular Stents, One iStent Supra® Suprachoroidal Stent, and Postoperative Prostaglandin in Refractory Glaucoma: 4-year Outcomes. Adv. Ther. 2018, 35, 395–407. [Google Scholar] [CrossRef]
- Li, R.; Liu, H.; Zhang, K.; Lu, Z.; Wang, N. Global tendency and research trends of minimally invasive surgery for glaucoma from 1992 to 2023: A visual bibliometric analysis. Heliyon 2024, 10, e36591. [Google Scholar] [CrossRef]
- Liu, H.; Li, R.; Zhang, Y.; Zhang, K.; Yusufu, M.; Liu, Y.; Mou, D.; Chen, X.; Tian, J.; Li, H.; et al. Economic evaluation of combined population-based screening for multiple blindness-causing eye diseases in China: A cost-effectiveness analysis. Lancet Glob. Health 2023, 11, e456–e465. [Google Scholar] [CrossRef]
- Sng, C.C.A.; Tham, C.C.; Budenz, D.L.; Healey, P.R.; Wang, N. Globalization of MIGS. In Minimally Invasive Glaucoma Surgery; Sng, C.C.A., Barton, K., Eds.; Springer: Singapore, 2021; pp. 147–156. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.G.; Greene, L. Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021, 4, e218348. [Google Scholar] [CrossRef]
- Dhawale, K.K.; Tidake, P. A Comprehensive Review of Recent Advances in Minimally Invasive Glaucoma Surgery: Current Trends and Future Directions. Cureus 2024, 16, e65236. [Google Scholar] [CrossRef]
- Cheema, A.A.; Cheema, H.R. The Evolution and Current Landscape of Minimally Invasive Glaucoma Surgeries: A Review. Cureus 2024, 16, e52183. [Google Scholar] [CrossRef]
- Yim, C.K.; Teng, C.C.; Warren, J.L.; Tsai, J.C.; Chadha, N. Microinvasive Glaucoma Surgical Training in United States Ophthalmology Residency Programs. Clin. Ophthalmol. 2020, 14, 1785–1789. [Google Scholar] [CrossRef]

| Trial Type | Author, Year | Sponsor | Region | Follow Up (Months) | Treatment | Control |
|---|---|---|---|---|---|---|
| COMBINED VS. COMBINED | Unavailable, 2011 (NCT02024464) [29] | Ivantis, Inc. | Unknown | 12, 24 | HYDRUS COMBINED | ISTENT COMBINED |
| MIGS COMBINED VS. CATARACT SURGERY | Fan Gaskin, 2018 (NCT03106181) [30] | Glaukos Corporation; Centre for Eye Research Australia | Oceania | 24 | ISTENT COMBINED | CATARACT SURGERY |
| MIGS COMBINED VS. CATARACT SURGERY | Fea, 2008 (NCTABC) [31] | Glaukos Corporation; Ricerca Finalizzata della Regione Piemonte 2007 | Europe | 12, 15 | ISTENT COMBINED | CATARACT SURGERY |
| MIGS COMBINED VS. CATARACT SURGERY | Fernandez-Barrientos, 2005 (NCT00326066) [32] | Glaukos Corporation | Europe | 6, 12 | ISTENT COMBINED (TWO) | CATARACT SURGERY |
| MIGS COMBINED VS. CATARACT SURGERY | Pfeiffer, 2011 (NCT01818115) [33] | Ivantis, Inc. | Europe | 12, 24 | HYDRUS COMBINED | CATARACT SURGERY |
| MIGS COMBINED VS. CATARACT SURGERY | Samuelson, 2005 (NCT00323284) [34] | Glaukos Corporation | North America | 12, 24 | ISTENT COMBINED | CATARACT SURGERY |
| MIGS COMBINED VS. CATARACT SURGERY | Samuelson, 2012 (NCT01539239) [35] | Ivantis, Inc. | Multi-Regional | 12, 24 | HYDRUS | CATARACT SURGERY |
| MIGS COMBINED VS. CATARACT SURGERY | Vold, 2009 (NCT01085357, COMPASS) [36] | Transcend Medical, Inc. | Unknown | 12, 24 | CYPASS COMBINED | CATARACT SURGERY |
| MIGS COMBINED VS. MIGS | Reitsamer, 2013 (NCT02006693) [37] | AqueSys, Inc. | Multi-Regional | 12, 24 | XEN COMBINED | XEN |
| MIGS ONLY | Beckers, 2014 (NCT02177123) [38] | InnFocus Inc. | Europe | 12, 24 | PRESERFLO MICROSHUNT | NONE |
| MIGS ONLY | De Jong, 2003 (NCTXYZ) [39] | Amsterdam University Medical Center | Europe | 12, 24 | EXPRESS | NONE |
| MIGS ONLY | Denis, 2017 (NCT03193736, STAR-I) [40] | iSTAR Medical | Multi-Regional | 6, 12, 24 | MINIJECT | NONE |
| MIGS ONLY | Garcia-Feijoo, 2010 (NCT01166659, DUETTE) [41] | Transcend Medical, Inc. | Unknown | 12, 24 | CYPASS | NONE |
| MIGS ONLY | Grover, 2013 (NCT02036541) [42] | AqueSys, Inc. | North America | 6, 12 | XEN | NONE |
| MIGS ONLY | Hoeh, 2009 (NCT01097174) [43] | Transcend Medical, Inc. | Europe | 6, 12 | CYPASS | NONE |
| MIGS ONLY | Riss, 2011 (NCT01563237) [44] | InnFocus Inc. | Europe | 12, 24 | PRESERFLO MICROSHUNT | NONE |
| MIGS VS. MIGS | Ahmed, 2012 (NCT02023242, COMPARE) [45] | Ivantis, Inc. | North America | 12 | HYDRUS | ISTENT (TWO) |
| MIGS VS. MIGS | Unavailable, 2013 (NCT02448875, ViscoPass) [46] | Transcend Medical, Inc. | Multi-Regional | 12 | CYPASS | CYPASS |
| MIGS VS. TRABECULECTOMY | Baker, 2015 (NCT01881425, IMS) [47] | InnFocus Inc. | Multi-Regional | 12, 24 | PRESERFLO MICROSHUNT | TRABECULECTOMY |
| MIGS VS. TRABECULECTOMY | Netland, 2006 (NCT00444080) [48] | University of Virginia | North America | 6, 24 | EXPRESS | TRABECULECTOMY |
| MIGS VS. TRABECULECTOMY | Wagschal, 2013 (NCT01263561) [49] | University of Toronto | North America | 12, 24 | EXPRESS | TRABECULECTOMY |
| Participants N | 3330 |
| Female N (%) | 1785 (53.6) |
| Male N (%) | 1545 (46.4) |
| White N (%) | 2029 (76.1) |
| Black N (%) | 267 (10.0) |
| Asian N (%) | 157 (5.9) |
| Other N (%) | 212 (8.0) |
| Hispanic/Latino N (%) | 148 (9.6) |
| Not Hispanic/Latino N (%) | 1382 (89.2) |
| Not Reported or Unknown N (%) | 19 (1.2) |
| Trial Type | IOP Baseline Type | Author, Year | Cohort | Intervention Type | Participants N | IOP-B | IOP-P | IOP-R | IOP-R (%) | MED-B | MED-P | MED-R (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Fernandez-Barrientos, 2005 (NCT00326066) [32] | Control | Cataract | 16 | 23.6 | 19.6 | 4 | 4.0 (16.9) | 1.2 | 0.5 | 0.7 (58.3) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Fernandez-Barrientos, 2005 (NCT00326066) [32] | Treatment | MIGS + Cataract | 17 | 24.2 | 15.6 | 8.6 | 8.6 (35.5) | 1.1 | 0.1 | 1.0 (90.9) |
| MIGS ONLY | Medicated | Denis, 2017 (NCT03193736) [40] | Treatment | MIGS | 26 | 23.2 | 14.2 | 9 | 9.0 (38.7) | 2 | 0.3 | 1.7 (85.0) |
| MIGS ONLY | Medicated | Grover, 2013 (NCT02036541) [42] | Treatment | MIGS | 65 | 25.1 | 17.8 | 7.3 | 7.3 (29.1) | NR | NR | NR |
| MIGS ONLY | Medicated | Hoeh, 2009 (NCT01097174) [43] | Control | MIGS + Cataract | 102 | 16.6 | 15.3 | 1.3 | 1.3 (7.8) | 2 | 0.4 | 1.6 (80.0) |
| MIGS ONLY | Medicated | Hoeh, 2009 (NCT01097174) [43] | Treatment | MIGS + Cataract | 65 | 25.9 | 17.3 | 8.6 | 8.6 (33.2) | 2.1 | 0.9 | 1.2 (57.1) |
| MIGS VS. TRABECULECTOMY | Unmedicated | Netland, 2006 (NCT00444080) [48] | Treatment | MIGS | 59 | 25.1 | 13.8 | 11.3 | 11.3 (45.0) | NR | NR | NR |
| Trial Type | IOP Baseline Type | Author, Year | Intervention | Intervention Type | Participants N | Cohort | IOP-B | IOP-P | IOP-R | IOP-R (%) | MED-B | MED-P | MED-R (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COMBINED VS. COMBINED | Unmedicated | Unavailable, 2011 (NCT02024464) [29] | Hydrus Microstent + Cataract Surgery | MIGS + Cataract | 154 | Treatment | 25.6 | 16.9 | 8.7 | 8.7 (34.0) | NR | NR | NR |
| COMBINED VS. COMBINED | Unmedicated | Unavailable, 2011 (NCT02024464) [29] | iStent + Cataract Surgery | MIGS + Cataract | 152 | Control | 26.1 | 18.1 | 8 | 8.0 (30.7) | NR | NR | NR |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Fea, 2008 (NCTABC) [31] | Phacoemulsification | Cataract | 24 | Control | 17.3 | 15.3 | 2 | 2.0 (11.6) | 1.9 | 1.3 | 0.6 (31.6) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Fea, 2008 (NCTABC) [31] | iStent + Cataract Surgery | MIGS + Cataract | 12 | Treatment | 17.9 | 14.9 | 3 | 3.0 (16.8) | 2 | 0.3 | 1.7 (85.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Pfeiffer, 2011 (NCT01818115) [33] | Hydrus + Cataract Surgery | MIGS + Cataract | 50 | Treatment | 18.9 | 16.6 | 2.3 | 2.3 (12.2) | 2 | 0.5 | 1.5 (75.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Pfeiffer, 2011 (NCT01818115) [33] | Phacoemulsification | Cataract | 50 | Control | 18.6 | 17.4 | 1.2 | 1.2 (6.5) | 2 | 0.8 | 1.2 (57.5) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2005 (NCT00323284) [34] | Cataract Surgery Only | Cataract | 116 | Control | 17.9 | 17 | 0.9 | 0.9 (5.0) | 1.5 | 0.4 | 1.0 (66.7) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2005 (NCT00323284) [34] | iStent + Cataract Surgery | MIGS + Cataract | 123 | Treatment | 18.6 | 17 | 1.6 | 1.6 (8.6) | 1.5 | 0.2 | 1.4 (93.3) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2012 (NCT01539239) [35] | Cataract Surgery Only | Cataract | 187 | Control | 18.1 | 19.1 | −1 | −1.0 (−5.5) | NR | NR | NR |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2012 (NCT01539239) [35] | Hydrus Aqueous Implant | MIGS | 369 | Treatment | 17.9 | 17 | 0.9 | 0.9 (5.0) | NR | NR | NR |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Fernandez-Barrientos, 2005 (NCT00326066) [32] | 2 iStent + Cataract Surgery | MIGS + Cataract | 17 | Treatment | 24.2 | 17.6 | 6.6 | 6.6 (27.3) | 1.1 | 0 | 1.1 (100.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Fernandez-Barrientos, 2005 (NCT00326066) [32] | Phacoemulsification | Cataract | 16 | Control | 23.6 | 19.8 | 3.8 | 3.8 (16.1) | 1.2 | 0.7 | 0.5 (41.7) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Pfeiffer, 2011 (NCT01818115) [33] | Hydrus + Cataract Surgery | MIGS + Cataract | 50 | Treatment | 26.3 | 16.6 | 9.7 | 9.7 (36.9) | 2 | 0.5 | 1.5 (75.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Pfeiffer, 2011 (NCT01818115) [33] | Phacoemulsification | Cataract | 50 | Control | 26.6 | 17.4 | 9.2 | 9.2 (34.6) | 2 | 0.8 | 1.2 (57.5) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2005 (NCT00323284) [34] | Cataract Surgery Only | Cataract | 116 | Control | 25.2 | 17 | 8.2 | 8.2 (32.5) | 1.5 | 0.4 | 1.0 (66.7) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2005 (NCT00323284) [34] | iStent + Cataract Surgery | MIGS + Cataract | 123 | Treatment | 25.4 | 17 | 8.4 | 8.4 (33.1) | 1.5 | 0.2 | 1.4 (93.3) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2012 (NCT01539239) [35] | Cataract Surgery Only | Cataract | 187 | Control | 25.4 | 19.1 | 6.3 | 6.3 (24.8) | NR | NR | NR |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2012 (NCT01539239) [35] | Hydrus Aqueous Implant | MIGS | 369 | Treatment | 25.5 | 17 | 8.5 | 8.5 (33.3) | NR | NR | NR |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Vold, 2009 (NCT01085357) [36] | Cataract Surgery Only | Cataract | 131 | Control | 24.5 | 18.3 | 6.2 | 6.2 (25.3) | 1.3 | 0.7 | 0.6 (46.2) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Vold, 2009 (NCT01085357) [36] | CyPass + Cataract Surgery | MIGS + Cataract | 374 | Treatment | 24.4 | 16.5 | 7.9 | 7.9 (32.4) | 1.4 | 0.2 | 1.2 (85.7) |
| MIGS COMBINED VS. MIGS | Medicated | Reitsamer, 2013 (NCT02006693) [37] | XEN Gel Stent | MIGS | 106 | Control | 21.7 | 15.1 | 6.6 | 6.6 (30.4) | 2.7 | 0.9 | 1.8 (66.7) |
| MIGS COMBINED VS. MIGS | Medicated | Reitsamer, 2013 (NCT02006693) [37] | XEN Gel Stent + Cataract Surgery | MIGS + Cataract | 79 | Treatment | 21 | 14.7 | 6.3 | 6.3 (30.0) | 2.5 | 0.9 | 1.6 (64.0) |
| MIGS ONLY | Medicated | Beckers, 2014 (NCT02177123) [38] | PreserFlo MicroShunt (InnFocus, MIDI Arrow) | MIGS | 81 | Treatment | 21.7 | 14.5 | 7.2 | 7.2 (33.2) | 2.1 | 0.5 | 1.6 (76.2) |
| MIGS ONLY | Medicated | De Jong, 2003 (NCTXYZ) [39] | Ex-PRESS | MIGS | 40 | Treatment | 22.8 | 12 | 10.8 | 10.8 (47.4) | 2.8 | 0.3 | 2.5 (89.3) |
| MIGS ONLY | Medicated | Denis, 2017 (NCT03193736) [40] | MINIject implant | MIGS | 26 | Treatment | 23.2 | 16 | 7.2 | 7.2 (31.0) | 2 | 0.4 | 1.6 (80.0) |
| MIGS ONLY | Medicated | Garcia-Feijoo, 2010 (NCT01166659) [41] | CyPass Micro-Stent | MIGS | 48 | Treatment | 24.5 | 16.4 | 8.1 | 8.1 (33.1) | 2.2 | 1.4 | 0.8 (36.4) |
| MIGS ONLY | Medicated | Grover, 2013 (NCT02036541) [42] | XEN 45 Gel Stent | MIGS | 65 | Treatment | 25.1 | 15.9 | 9.2 | 9.2 (36.7) | 3.5 | 1.7 | 1.8 (51.4) |
| MIGS ONLY | Medicated | Hoeh, 2009 (NCT01097174) [43] | CyPass + Cataract Surgery (IOP < 21) | MIGS + Cataract | 102 | Control | 16.6 | 15.7 | 0.9 | 0.9 (5.4) | 2 | 0.5 | 1.5 (75.0) |
| MIGS ONLY | Medicated | Hoeh, 2009 (NCT01097174) [43] | CyPass + Cataract Surgery (IOP >= 21) | MIGS + Cataract | 65 | Treatment | 25.9 | 16.3 | 9.6 | 9.6 (37.1) | 2.1 | 1.1 | 1.0 (47.6) |
| MIGS ONLY | Medicated | Riss, 2011 (NCT01563237) [44] | PreserFlo MicroShunt (InnFocus, MIDI Arrow) | MIGS | 61 | Treatment | 25.7 | 15.8 | 9.9 | 9.9 (38.5) | 2.9 | 0.6 | 2.3 (79.3) |
| MIGS VS. MIGS | Medicated | Ahmed, 2012 (NCT02023242) [45] | Hydrus Microstent | MIGS | 75 | Treatment | 19 | 17.3 | 1.7 | 1.7 (8.9) | 2.5 | 1.2 | 1.3 (50.8) |
| MIGS VS. MIGS | Medicated | Ahmed, 2012 (NCT02023242) [45] | Two iStents Trabecular Micro Bypass | MIGS | 77 | Control | 19.1 | 18.1 | 1 | 1.0 (5.2) | 2.7 | 1.9 | 0.8 (29.3) |
| MIGS VS. MIGS | Medicated | Unavailable, 2013 (NCT02448875) [46] | CyPass without Viscoelastic | MIGS | 60 | Control | 21.1 | 16.8 | 4.3 | 4.3 (20.4) | NR | NR | 0.8 |
| MIGS VS. MIGS | Medicated | Unavailable, 2013 (NCT02448875) [46] | CyPass30 | MIGS | 21 | Treatment | 22.2 | 18.2 | 4 | 4.0 (18.0) | NR | NR | 0.3 |
| MIGS VS. MIGS | Medicated | Unavailable, 2013 (NCT02448875) [46] | CyPass60 | MIGS | 61 | Treatment | 21.7 | 16.9 | 4.8 | 4.8 (22.2) | NR | NR | 0.8 |
| MIGS VS. MIGS | Unmedicated | Ahmed, 2012 (NCT02023242) [45] | Hydrus Microstent | MIGS | 75 | Treatment | 27.5 | 17.3 | 10.2 | 10.2 (37.1) | 2.5 | 1.2 | 1.3 (50.8) |
| MIGS VS. MIGS | Unmedicated | Ahmed, 2012 (NCT02023242) [45] | Two iStents Trabecular Micro Bypass | MIGS | 77 | Control | 27.3 | 18.1 | 9.2 | 9.2 (33.7) | 2.7 | 1.9 | 0.8 (29.3) |
| MIGS VS. TRABECULECTOMY | Medicated | Baker, 2015 (NCT01881425) [47] | PreserFlo MicroShunt (InnFocus, MIDI Arrow) | MIGS | 395 | Treatment | 21.1 | 14.3 | 6.8 | 6.8 (32.2) | 3.1 | 0.6 | 2.5 (80.6) |
| MIGS VS. TRABECULECTOMY | Medicated | Wagschal, 2013 (NCT01263561) [49] | ExPRESS shunt | MIGS | 33 | Treatment | 22.6 | 11.2 | 11.4 | 11.4 (50.4) | 3.5 | 0.4 | 3.1 (88.6) |
| Trial Type | IOP Baseline Type | Author, Year | Cohort | Intervention Type | Participants N | IOP-B | IOP-P | IOP-R | IOP-R (%) | MED-B | MED-P | MED-R (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COMBINED VS. COMBINED | Unmedicated | Unavailable, 2011 (NCT02024464) [29] | Control | MIGS + Cataract | 152 | 26.1 | 19.2 | 6.9 | 6.9 (26.4) | NR | NR | NR |
| COMBINED VS. COMBINED | Unmedicated | Unavailable, 2011 (NCT02024464) [29] | Treatment | MIGS + Cataract | 154 | 25.6 | 18.1 | 7.5 | 7.5 (29.3) | NR | NR | NR |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Fan Gaskin, 2018 (NCT03106181) [30] | Control | Cataract | 46 | 17.1 | 14.6 | 2.5 | 2.5 (14.6) | 1.8 | 1.5 | 0.3 (16.7) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Fan Gaskin, 2018 (NCT03106181) [30] | Treatment | MIGS + Cataract | 55 | 17.7 | 15.2 | 2.5 | 2.5 (14.1) | 1.7 | 0.7 | 1.0 (58.8) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Pfeiffer, 2011 (NCT01818115) [33] | Control | Cataract | 50 | 18.6 | 19.2 | −0.6 | −0.6 (−3.2) | 2 | 1 | 1.0 (50.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Pfeiffer, 2011 (NCT01818115) [33] | Treatment | MIGS + Cataract | 50 | 18.9 | 16.9 | 2 | 2.0 (10.6) | 2 | 0.5 | 1.5 (75.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2005 (NCT00323284) [34] | Control | Cataract | 116 | 17.9 | 17.8 | 0.1 | 0.1 (0.6) | 1.5 | 0.5 | 1.0 (66.7) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2005 (NCT00323284) [34] | Treatment | MIGS + Cataract | 123 | 18.6 | 17.1 | 1.5 | 1.5 (8.1) | 1.5 | 0.3 | 1.4 (93.3) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2012 (NCT01539239) [35] | Control | Cataract | 187 | 18.1 | 17.4 | 0.7 | 0.7 (3.9) | 1.7 | 0.7 | 1.0 (58.8) |
| MIGS COMBINED VS. CATARACT SURGERY | Medicated | Samuelson, 2012 (NCT01539239) [35] | Treatment | MIGS | 369 | 17.9 | 16.8 | 1.1 | 1.1 (6.1) | 1.7 | 0.3 | 1.4 (82.4) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Pfeiffer, 2011 (NCT01818115) [33] | Control | Cataract | 50 | 26.6 | 19.2 | 7.4 | 7.4 (27.8) | 2 | 1 | 1.0 (50.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Pfeiffer, 2011 (NCT01818115) [33] | Treatment | MIGS + Cataract | 50 | 26.3 | 16.9 | 9.4 | 9.4 (35.7) | 2 | 0.5 | 1.5 (75.0) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2005 (NCT00323284) [34] | Control | Cataract | 116 | 25.2 | 17.8 | 7.4 | 7.4 (29.4) | 1.5 | 0.5 | 1.0 (66.7) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2005 (NCT00323284) [34] | Treatment | MIGS + Cataract | 123 | 25.4 | 17.1 | 8.3 | 8.3 (32.7) | 1.5 | 0.3 | 1.4 (93.3) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2012 (NCT01539239) [35] | Control | Cataract | 187 | 25.4 | 19.2 | 6.2 | 6.2 (24.4) | 1.7 | 0.7 | 1.0 (58.8) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Samuelson, 2012 (NCT01539239) [35] | Treatment | MIGS | 369 | 25.5 | 17.4 | 8.1 | 8.1 (31.8) | 1.7 | 0.3 | 1.4 (82.4) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Vold, 2009 (NCT01085357) [36] | Control | Cataract | 131 | 24.5 | 19.3 | 5.2 | 5.2 (21.2) | 1.3 | 0.6 | 0.7 (53.8) |
| MIGS COMBINED VS. CATARACT SURGERY | Unmedicated | Vold, 2009 (NCT01085357) [36] | Treatment | MIGS + Cataract | 374 | 24.4 | 17 | 7.4 | 7.4 (30.3) | 1.4 | 0.2 | 1.2 (85.7) |
| MIGS COMBINED VS. MIGS | Medicated | Reitsamer, 2013 (NCT02006693) [37] | Control | MIGS | 106 | 21.7 | 15.4 | 6.3 | 6.3 (29.0) | 2.7 | 1.2 | 1.5 (55.6) |
| MIGS COMBINED VS. MIGS | Medicated | Reitsamer, 2013 (NCT02006693) [37] | Treatment | MIGS + Cataract | 79 | 21 | 14.9 | 6.1 | 6.1 (29.0) | 2.5 | 1 | 1.5 (60.0) |
| MIGS ONLY | Medicated | Beckers, 2014 (NCT02177123) [38] | Treatment | MIGS | 81 | 21.7 | 14.1 | 7.6 | 7.6 (35.0) | 2.1 | 0.5 | 1.6 (76.2) |
| MIGS ONLY | Medicated | De Jong, 2003 (NCTXYZ) [39] | Treatment | MIGS | 40 | 22.8 | 11.9 | 10.9 | 10.9 (47.8) | 2.8 | 0.5 | 2.3 (82.1) |
| MIGS ONLY | Medicated | Denis, 2017 (NCT03193736) [40] | Treatment | MIGS | 26 | 23.2 | 13.8 | 9.4 | 9.4 (40.7) | 2 | 1 | 1.0 (50.0) |
| MIGS ONLY | Medicated | Garcia-Feijoo, 2010 (NCT01166659) [41] | Treatment | MIGS | 48 | 24.5 | 16.8 | 7.7 | 7.7 (31.4) | 2.2 | 1.5 | 0.7 (31.8) |
| MIGS ONLY | Medicated | Riss, 2011 (NCT01563237) [44] | Treatment | MIGS | 61 | 25.7 | 16.5 | 9.2 | 9.2 (35.8) | 2.9 | 1 | 1.9 (65.5) |
| MIGS VS. TRABECULECTOMY | Medicated | Baker, 2015 (NCT01881425) [47] | Treatment | MIGS | 395 | 21.1 | 14.6 | 6.6 | 6.6 (31.0) | NR | NR | NR |
| MIGS VS. TRABECULECTOMY | Medicated | Wagschal, 2013 (NCT01263561) [49] | Treatment | MIGS | 33 | 22.6 | 12.5 | 10.1 | 10.1 (44.7) | 3.5 | 0.6 | 2.9 (82.9) |
| MIGS VS. TRABECULECTOMY | Unmedicated | Netland, 2006 (NCT00444080) [48] | Treatment | MIGS | 59 | 25.1 | 14.7 | 10.4 | 10.4 (41.4) | 3.1 | 0.9 | 2.2 (71.0) |
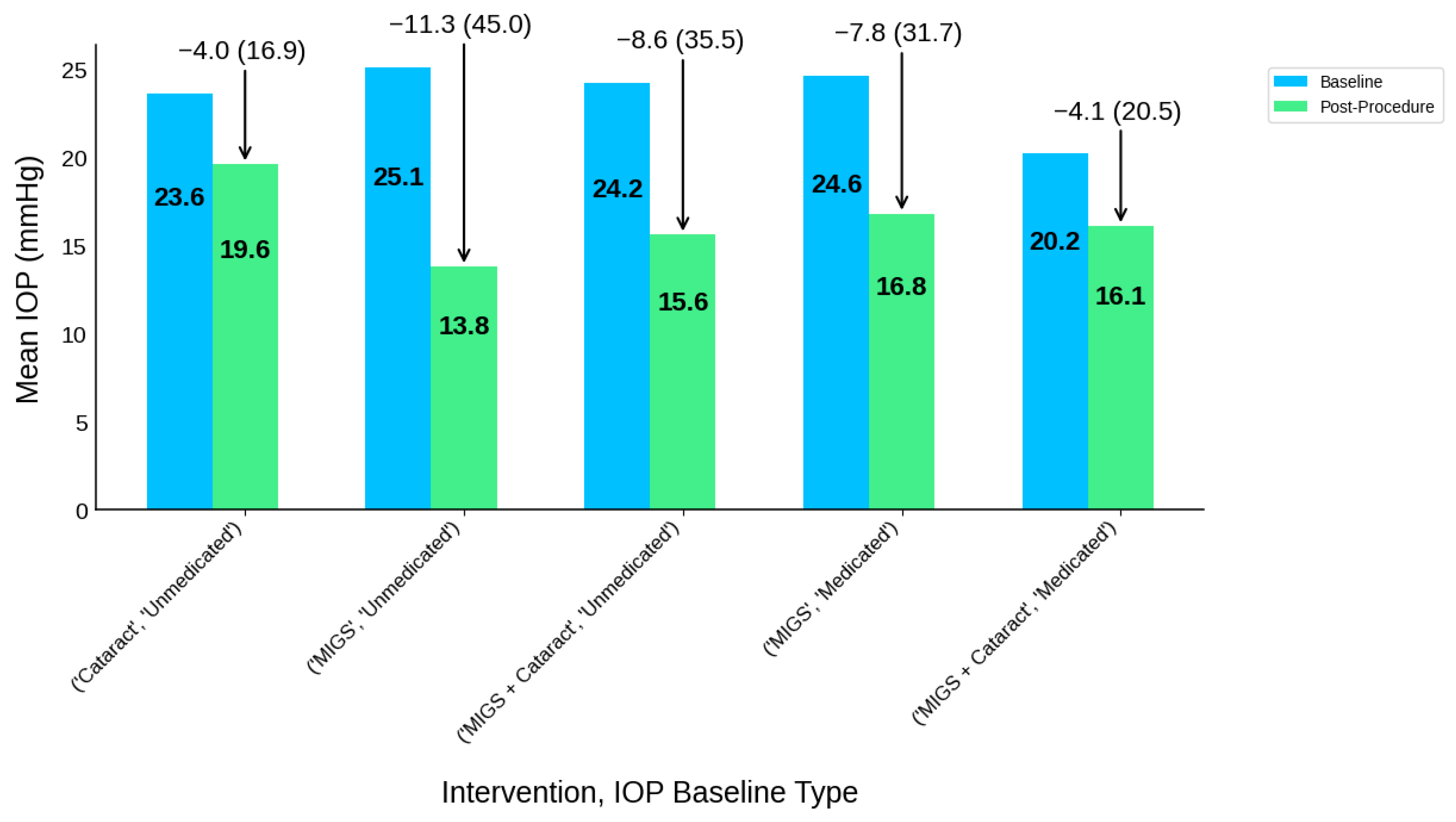
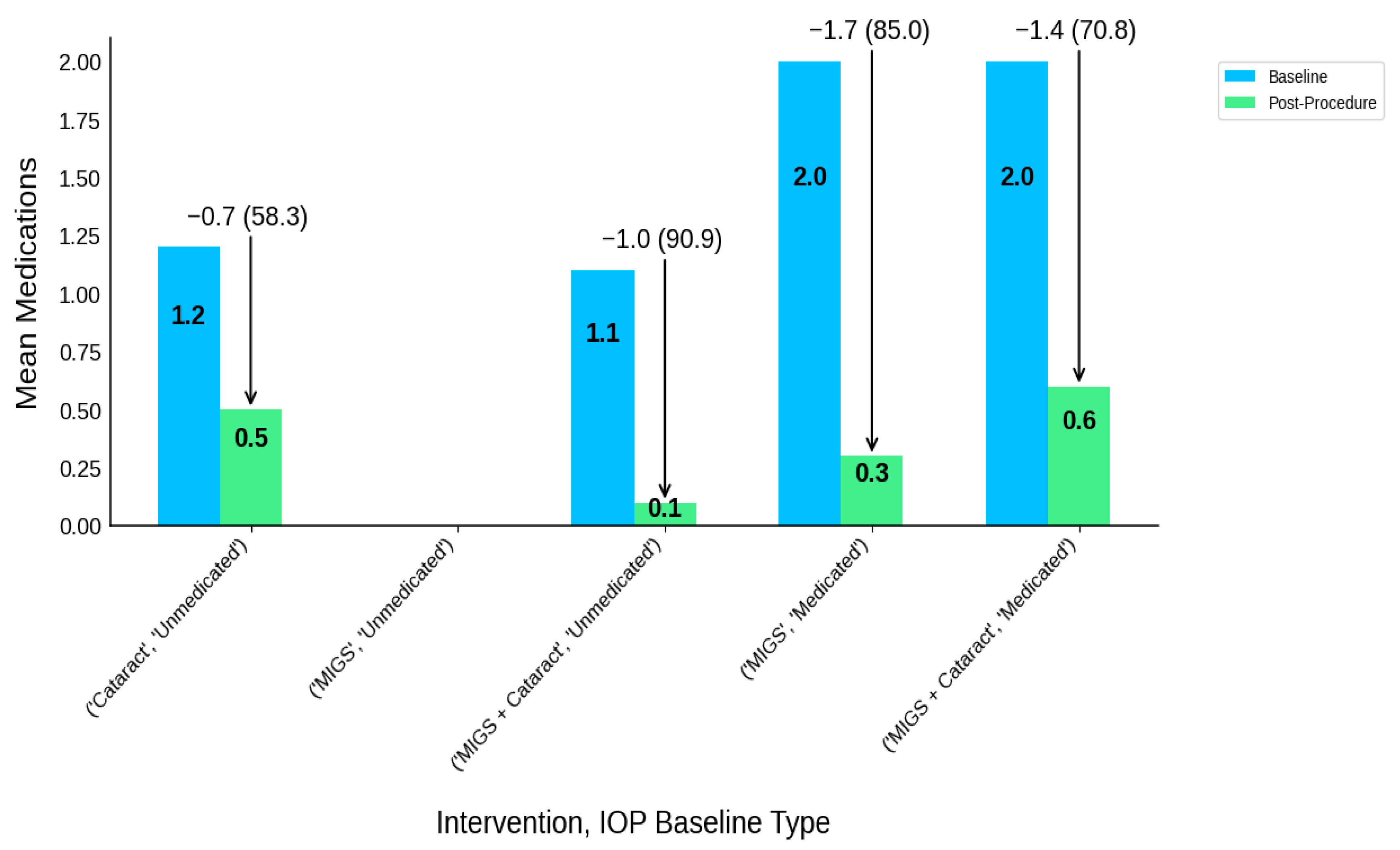
| Intervention Type | IOP Baseline Type | Participants N | IOP-B | IOP-P | IOP-R | IOP-R (%) | MED-B | MED-P | MED-R | MED-R (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cataract | Unmedicated | 16 | 23.6 | 19.6 | 4 | 4.0 (16.9) | 1.2 | 0.5 | 0.7 | 0.7 (58.3) |
| MIGS | Medicated | 91 | 24.6 | 16.8 | 7.8 | 7.8 (31.7) | 2 | 0.3 | 1.7 | 1.7 (85.0) |
| MIGS | Unmedicated | 59 | 25.1 | 13.8 | 11.3 | 11.3 (45.0) | NR | NR | NR | NR |
| MIGS + Cataract | Medicated | 167 | 20.2 | 16.1 | 4.1 | 4.1 (20.5) | 2 | 0.6 | 1.4 | 1.4 (70.8) |
| MIGS + Cataract | Unmedicated | 17 | 24.2 | 15.6 | 8.6 | 8.6 (35.5) | 1.1 | 0.1 | 1 | 1.0 (90.9) |
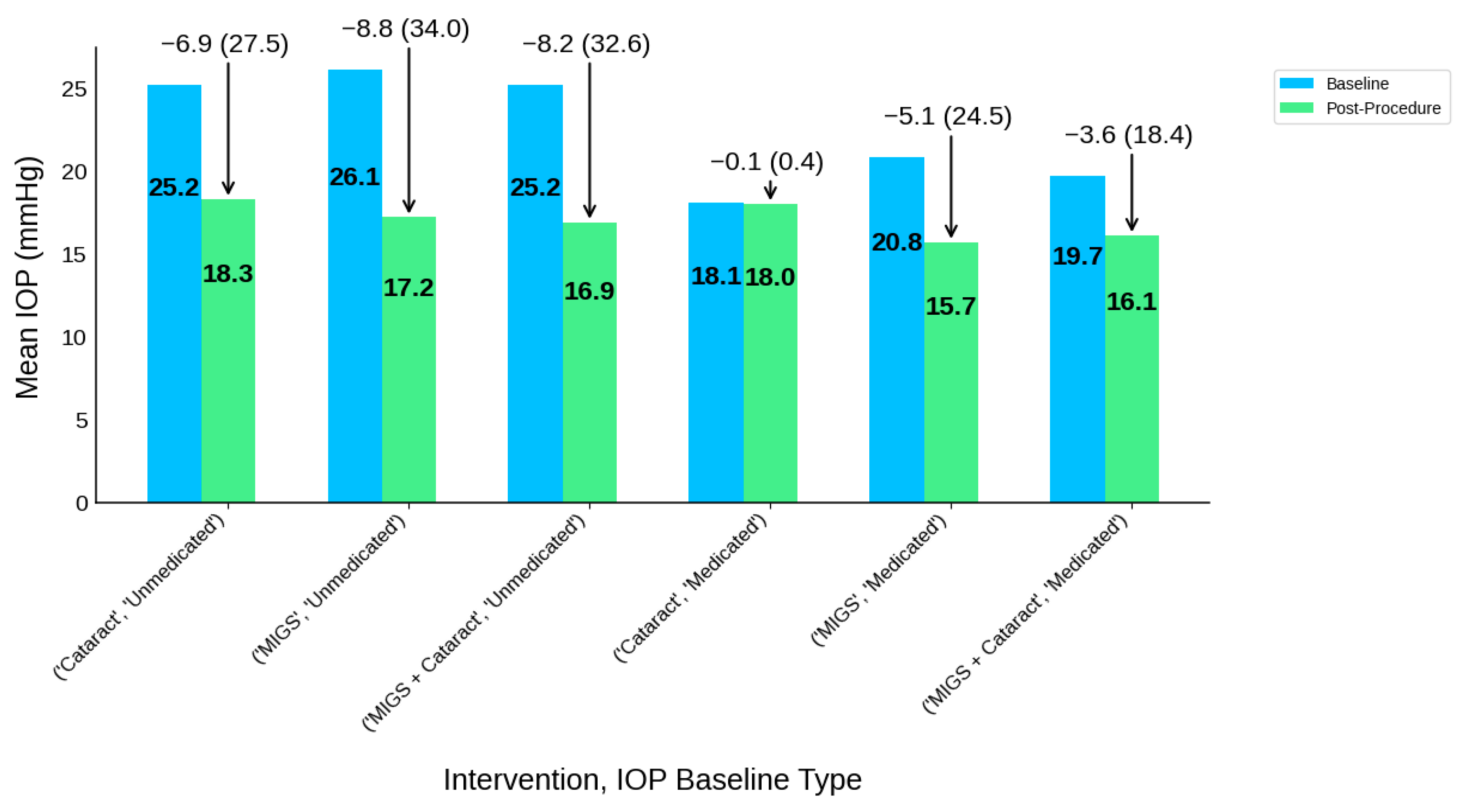
| Intervention Type | IOP Baseline Type | Participants N | IOP-B | IOP-P | IOP-R | IOP-R (%) | MED-B | MED-P | MED-R | MED-R (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cataract | Medicated | 377 | 18.1 | 18 | 0.1 | 0.1 (0.4) | 1.7 | 0.6 | 1 | 1.0 (58.8) |
| Cataract | Unmedicated | 500 | 25.2 | 18.3 | 6.9 | 6.9 (27.5) | 1.5 | 0.6 | 0.8 | 0.8 (56.1) |
| MIGS | Medicated | 1518 | 20.8 | 15.7 | 5.1 | 5.1 (24.5) | 2.8 | 0.9 | 1.8 | 1.8 (64.4) |
| MIGS | Unmedicated | 521 | 26.1 | 17.2 | 8.8 | 8.8 (34.0) | 2.6 | 1.6 | 1 | 1.0 (39.5) |
| MIGS + Cataract | Medicated | 431 | 19.7 | 16.1 | 3.6 | 3.6 (18.4) | 2 | 0.6 | 1.4 | 1.4 (72.3) |
| MIGS + Cataract | Unmedicated | 870 | 25.2 | 16.9 | 8.2 | 8.2 (32.6) | 1.5 | 0.2 | 1.3 | 1.3 (86.4) |
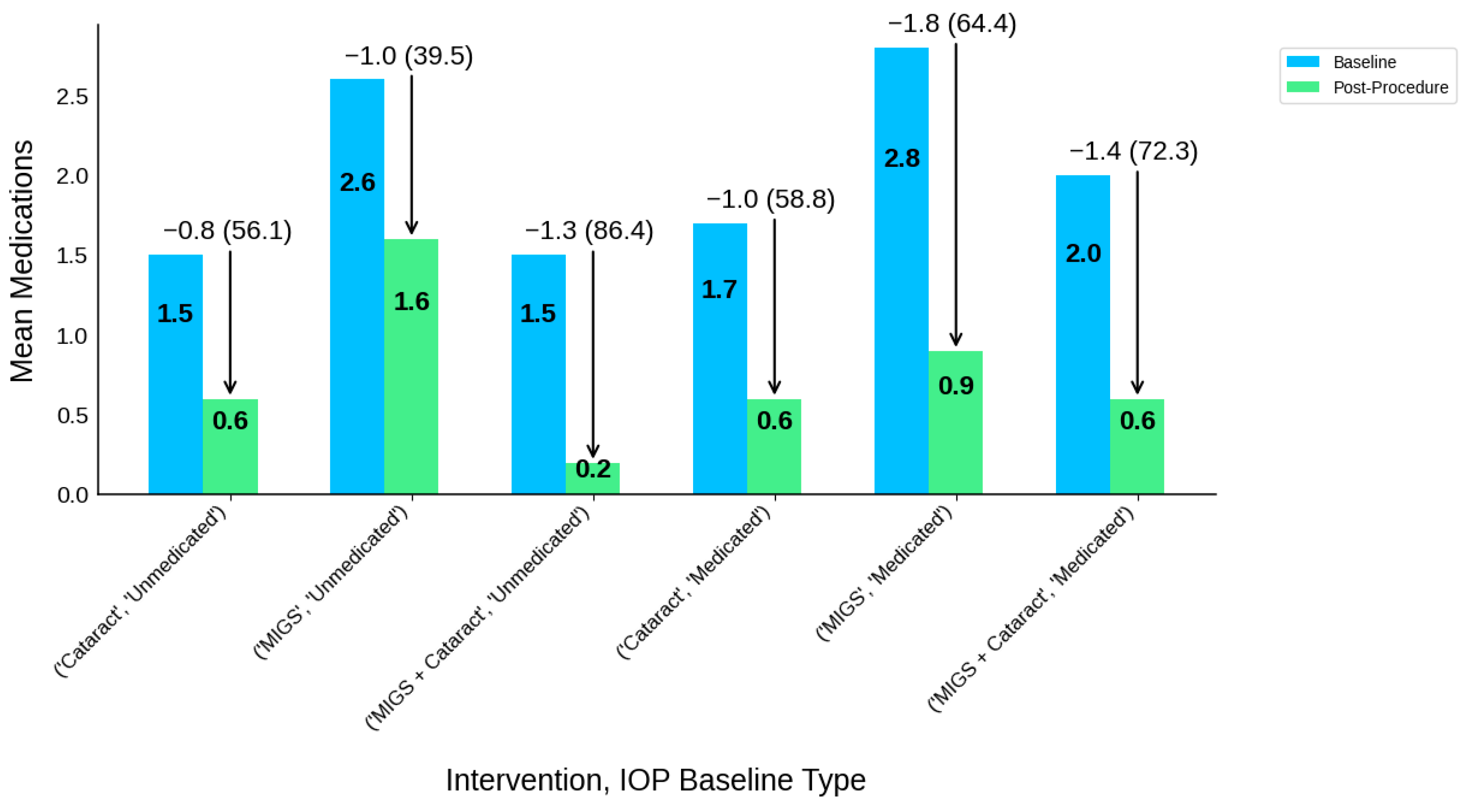
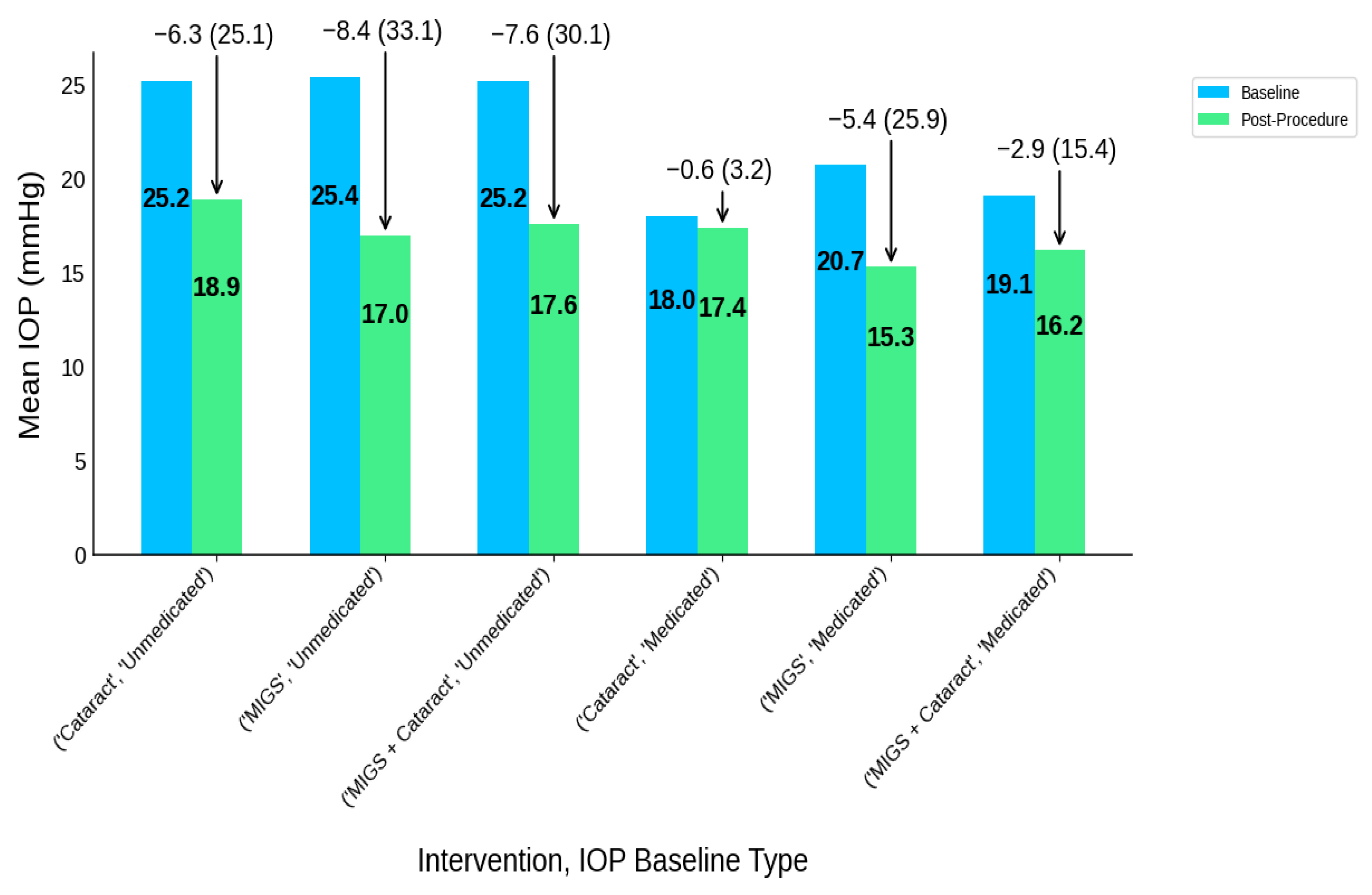
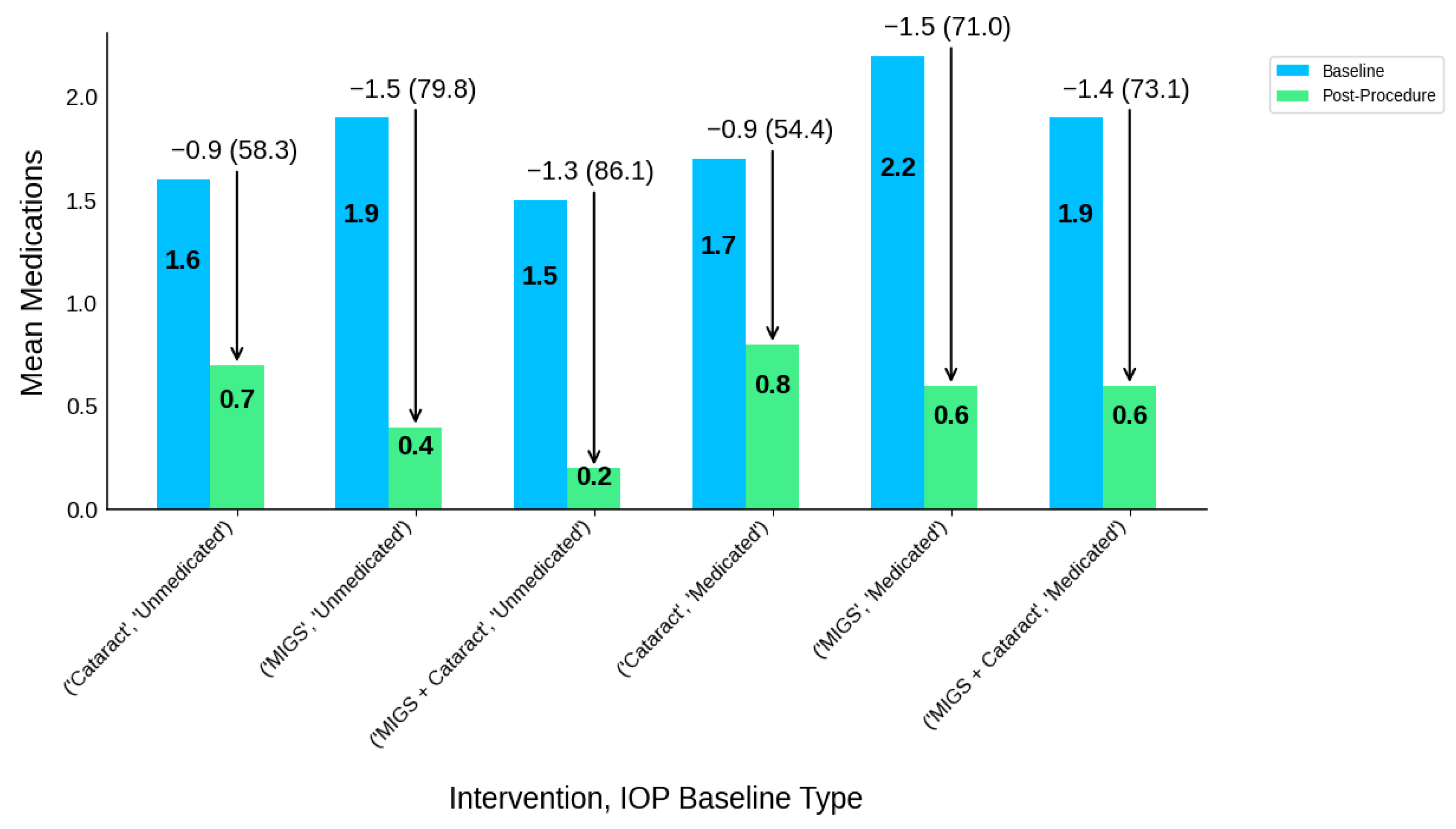
| Intervention Type | IOP Baseline Type | Participants N | IOP-B | IOP-P | IOP-R | IOP-R (%) | MED-B | MED-P | MED-R | MED-R (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cataract | Medicated | 399 | 18 | 17.4 | 0.6 | 0.6 (3.2) | 1.7 | 0.8 | 0.9 | 0.9 (54.4) |
| Cataract | Unmedicated | 484 | 25.2 | 18.9 | 6.3 | 6.3 (25.1) | 1.6 | 0.7 | 0.9 | 0.9 (58.3) |
| MIGS | Medicated | 1159 | 20.7 | 15.3 | 5.4 | 5.4 (25.9) | 2.2 | 0.6 | 1.5 | 1.5 (71.0) |
| MIGS | Unmedicated | 428 | 25.4 | 17 | 8.4 | 8.4 (33.1) | 1.9 | 0.4 | 1.5 | 1.5 (79.8) |
| MIGS + Cataract | Medicated | 307 | 19.1 | 16.2 | 2.9 | 2.9 (15.4) | 1.9 | 0.6 | 1.4 | 1.4 (73.1) |
| MIGS + Cataract | Unmedicated | 853 | 25.2 | 17.6 | 7.6 | 7.6 (30.1) | 1.5 | 0.2 | 1.3 | 1.3 (86.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appelbaum, J.; Virk, A.; Patel, D.; Allison, K. MIGS, Cataract Surgery, or Both? An Analysis of Clinical Trial Data to Compare Efficacy and Outcomes on Glaucoma Patients. J. Clin. Transl. Ophthalmol. 2025, 3, 20. https://doi.org/10.3390/jcto3040020
Appelbaum J, Virk A, Patel D, Allison K. MIGS, Cataract Surgery, or Both? An Analysis of Clinical Trial Data to Compare Efficacy and Outcomes on Glaucoma Patients. Journal of Clinical & Translational Ophthalmology. 2025; 3(4):20. https://doi.org/10.3390/jcto3040020
Chicago/Turabian StyleAppelbaum, Jeremy, Abdullah Virk, Deepkumar Patel, and Karen Allison. 2025. "MIGS, Cataract Surgery, or Both? An Analysis of Clinical Trial Data to Compare Efficacy and Outcomes on Glaucoma Patients" Journal of Clinical & Translational Ophthalmology 3, no. 4: 20. https://doi.org/10.3390/jcto3040020
APA StyleAppelbaum, J., Virk, A., Patel, D., & Allison, K. (2025). MIGS, Cataract Surgery, or Both? An Analysis of Clinical Trial Data to Compare Efficacy and Outcomes on Glaucoma Patients. Journal of Clinical & Translational Ophthalmology, 3(4), 20. https://doi.org/10.3390/jcto3040020






