Abstract
Background: In this study, we compare the 12-month results of eyes that underwent ab externo, open-conjunctival XEN45 gel stent placement with mitomycin C (MMC) with and without the intraoperative addition of Ologen collagen matrix (XEN45 and XEN45 + Ologen groups, respectively). Methods: Intraocular pressure (IOP) measurements were recorded postoperation at 1 month, 3 months, 6 months, and 9 months, and 12 months, and IOP reduction, reduction in number of IOP-lowering medications, and success rate were compared between XEN45 and XEN45 + Ologen groups. A complete success was defined as a ≥20% drop from baseline IOP at 12 months without the use of medications and without any of the following: an additional procedure (e.g., needling), a recorded IOP ≥ 21 mm Hg at two consecutive visits, or the occurrence of catastrophic events (e.g., no light perception (NLP)). A qualified success was defined as an IOP reduction of ≥20% from baseline with the use of medications. We included 145 eyes with at least 1 month of follow-up data, 46 in the XEN45 group and 99 in the XEN45 + Ologen group. Of these, 113 eyes had 12 months of follow-up data comprising 41 XEN45 eyes and 72 XEN45 + Ologen eyes. Results: There were no significant differences in the IOP change from baseline between XEN45 and XEN45 + Ologen groups except at the 3-month postop timepoint (p < 0.05). At the 12-month follow-up, 41.5% (17/41) of XEN45 eyes and 52.8% (38/72) of XEN45 + Ologen eyes met complete or qualified success criteria. Conclusions: No significant differences in success rate and decrease in the number of IOP-lowering medications from baseline were identified between XEN45 and XEN45 + Ologen groups.
1. Introduction
Glaucoma is a leading cause of irreversible vision loss worldwide and is projected to affect more than 110 million people by 2040 [1]. Although trabeculectomy and tube shunts have been considered the standard of care for patients failing maximum medical therapy, these procedures have begun to fall out of favor due to their invasive nature and subsequent complications.
Minimally invasive glaucoma surgery (MIGS) has become widely acknowledged as a first-line surgical intervention for patients with uncontrolled intraocular pressure (IOP) despite maximally tolerated medical therapy. MIGS procedures either harness the eye’s natural aqueous humor drainage system or bypass the trabecular meshwork, similar to trabeculectomies. Among these latter MIGS procedures, the XEN45 Gel Stent (Allergan, Aliso Viejo, CA, USA) is a permanent hydrophilic gelatin implant that is placed via an ab interno or ab externo approach where it acts as a conduit for the outflow of aqueous humor from the anterior chamber into the subconjunctival space [2]. The stent lumen is 45 μm, such that the stent will not drain at physiologically normal IOP and hypotony is theoretically avoided [3].
Although the XEN45 Gel Stent has been an advancement in the treatment of primary open-angle glaucoma, post-surgical scarring of the filtering bleb remains a major challenge, with some studies describing rates of bleb needling as high as 30–40% [4,5,6,7]. To prevent this complication, mitomycin C (MMC) is commonly administered intraoperatively, inhibiting the proliferation of scar-forming cells [8].
The Ologen Collagen Matrix (Ologen CM, Aeon Astron, Leiden, The Netherlands) is a polymer matrix implant made up of porcine-derived collagen and glycosaminoglycans designed to prevent scarring following trabeculectomy or glaucoma drainage device (GDD) placement [9]. The Ologen matrix is placed between the subconjunctival and episcleral tissues, where it acts as a scaffold for wound healing and may maintain the patency of the subconjunctival space and protect against overfiltration [10]. Perez et al. found that use of the Ologen matrix was equally effective as MMC in reducing IOP at 2-year follow-up, and Yuan et al. also found that the use of Ologen following trabeculectomy had higher rates of surgical success at the 5-year follow-up [11,12]. Ologen’s reported success in minimizing scarring post-trabeculectomy has led to its use in other MIGS procedures, namely XEN Gel Stent implantation. However, whether Ologen similarly prevents bleb fibrosis in XEN procedures as it does for trabeculectomies has not been definitively established. Previous studies comparing surgical success rates of patients undergoing XEN implantation with and without Ologen found no statistically significant difference between these two groups. A 2022 study by Park et al. followed patients post-XEN implantation for 6 months and another case-control study followed patients for 12 months but included a smaller cohort of 39 eyes [13,14]. We present a study retrospectively following 145 eyes over 12 months, including a larger sample size for a longer follow-up period than the currently published literature, in order to provide additional input on whether Ologen is therapeutically beneficial in XEN45 stent placement.
2. Materials and Methods
This study was approved by the Institutional Review Board of our institution, with all aspects of the research protocol adhering to the principles outlined in the Declaration of Helsinki.
2.1. Surgical
XEN45 gel stent placement was performed via ab externo technique. A superior corneal traction suture was placed and a paracentesis was created through which lidocaine with epinephrine, carbachol intraocular solution, and viscoelastic were injected into the anterior chamber. A fornix-based conjunctival peritomy was created near the future bleb site and MMC was added in the form of soaked sponges (0.2 mg/mL) placed posterior to the bleb site for 2 min or soaked sponges plus an injection of 0.15 mL to 0.4 mL. Calipers were used to mark 1–2 mm posterior to the limbus and the eye was rotated inferiorly with the corneal traction suture. The tip of the XEN45 injector entered the sclera at the mark and was visualized entering the anterior chamber. The XEN45 Gel Stent was then injected into the anterior chamber as the injector was withdrawn under the subconjunctival space. The intracameral portion was inspected with the gonioprism and, if needed, the XEN45 Gel Stent was manipulated so that around 1 mm of the stent was in the anterior chamber. If Ologen was used, a 12 × 1 mm Ologen collagen matrix circle was either cut in half, with one half positioned below and the other above the tip of the XEN45, or was placed whole over the XEN45 tip to prevent fibrosis and facilitate formation of a diffuse bleb. Due to manufacturer and distributor issues, Ologen was not available from approximately 30 December 2021 to 31 August 2022; therefore, only cases prior to 30 December 2021 received Ologen.
Next, the anterior chamber was irrigated, and the conjunctiva was closed with a notable bleb at the tip of the XEN45 Gel Stent. Intracameral moxifloxacin (0.1 mL) was injected for prophylaxis and the traction suture was removed. In most but not all cases, betamethasone (0.2 mL) was injected in the subconjunctival space around the future bleb site. At the end of the case, the wounds were examined to ensure that they were watertight. Surgical steps in this combination procedure are outlined in Figure 1. Standard postoperative procedure was followed after completion of the case. Surgeries were performed by surgeons C.C., A.N., R.S., B.S., M.T., and M.M. with no marked differences in surgical procedures between operators. There were variations the use of intraoperative betamethasone and amount of MMC, which are specified in Table 1.
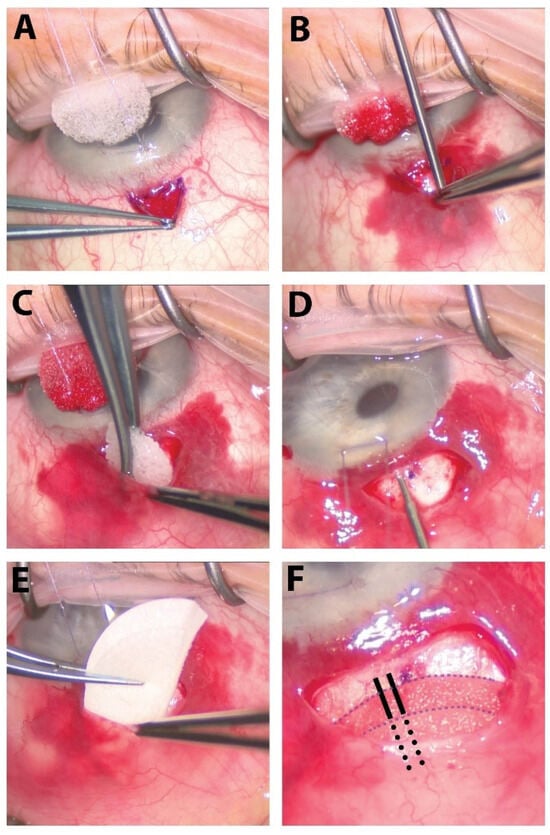
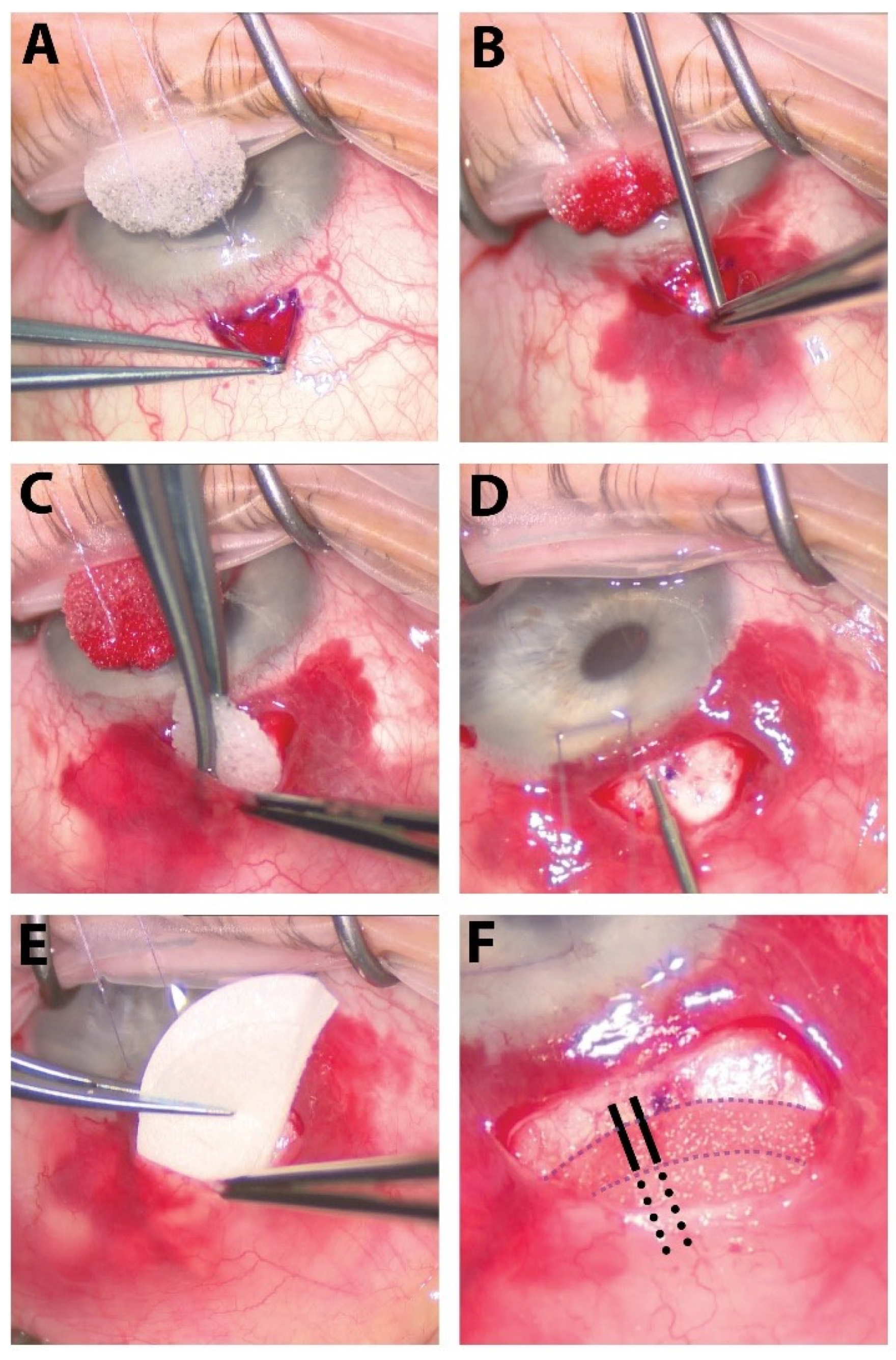
Figure 1.
The surgical steps of our procedure. (A) A small fornix-based peritomy is created superiorly. (B) Careful dissection of tenons capsule from the underlying sclera is performed with a Blumenthal conjunctival dissector. (C) Sponges soaked with 0.2 mg/mL of MMC are placed in the subTenon’s pocket in a diffuse manner for 2 min followed by copious irrigation of the sub-Tenon’s pocket with balanced salt solution. (D) The XEN45 stent is injected about 0.75–1.0 mm from the limbus, entering into the anterior chamber just above the pigmented trabecular meshwork parallel to the iris. (E) Ologen matrix is cut in half and placed within the sub-Tenon’s pocket. (F) The external portion of the XEN45 stent is placed over the Ologen matrix (whole or bisected above and below stent, as pictured here). Solid black lines outline the portion of the XEN stent resting on top of the bottom layer of Ologen, and the dotted black lines outline the portion of the XEN stent lying between the bottom and top layers of the Ologen matrix. The peritomy is then closed using a buried, running horizontal mattress suture using 10-0 Vicryl on a VAS needle.

Table 1.
Descriptive summary of included patients and eyes. Asterisk next to variable (*) denotes p value < 0.01 between XEN45 and XEN45 + Ologen groups. Application of MMC was at a concentration of 0.2 mg/mL. “#” refers to number.
2.2. Chart Review and Inclusion/Exclusion Criteria
A chart review was conducted of all eyes that had undergone combined XEN45 + MMC from 2019 to August 2022. Inclusion criteria were patients aged 18 and older who underwent ab externo open conjunctival XEN45 implantation with MMC between 2019 and August 2022 and had a diagnosis of glaucoma or ocular hypertension. Patients were excluded if the XEN45 placement was not ab externo open conjunctival, if pre- or post-XEN45 IOP measurements were not recorded in their chart, and if they were younger than 18 years of age at the time of XEN45 placement. Baseline and demographic characteristics are summarized in Table 1.
2.3. Statistics
Descriptive statistics were calculated for each patient and eye in the study. The Mann–Whitney U test and chi-square test were used to compare continuous variables (age, HVF MD, average RNFL, mean preop IOP, and mean preop number of medications) and categorical variables (gender, phakic status, laterality, and surgical procedures) between procedure groups, respectively.
A linear mixed-effects model was used to estimate the expected change in IOP from baseline at each follow-up visit (1 month, 3 months, 6 months, 9 months, and 12 months) by group while controlling for the baseline IOP. As there were several patients that had two eyes undergo the procedure, a random eye (nested in person) intercept was included in the model to account for within-eye correlation due to repeated measures. In the model, we also adjusted for age as a fixed effect. For each model coefficient, we provide the estimate, 95% confidence interval (CI), and p-value. We also calculated the expected IOP at each time point and the 95% CI for the average aged patient by group. Post hoc comparisons of change in IOP from baseline between XEN45 and XEN45 + Ologen groups at each time point using a z-test were made with the glht function.
Success criteria were a ≥20% IOP reduction, no additional procedure, no catastrophic events (including no light perception (NLP) visual acuity, malignant glaucoma, and endophthalmitis), and no use of IOP-lowering medications. Qualified successes were successes that met the same requirements with the use of IOP-lowering medications. Success rates were compared between groups at each time point using chi-square tests.
A paired sample Wilcoxon test was used to test significance between the mean number of IOP-lowering medications preoperatively and the number of medications postoperatively in the summed qualified and full successes at 12 months. Mann–Whitney U tests compared the number of drops and the reduction in drops at pre- and 12 months post-XEN timepoints between XEN45 and XEN45 + Ologen groups. p-values < 0.05 were considered statistically significant for all statistical tests. All analyses were conducted using IBM SPSS version 29.0.1.0 (SPSS Inc., Chicago, IL USA) and R version 4.3.1 [15].
3. Results
3.1. Study Population
A total of 258 adult eyes of 226 distinct patients who underwent XEN45 placement at Moran Eye Center were identified before August 2022. Forty-one (41) patients were excluded who had ab interno procedures, 28 patients had closed conjunctival procedures, 1 patient did not have a diagnosis of glaucoma or ocular hypertension, 21 patients did not have pre- or post-XEN IOP data in their medical chart, and 7 patients were under 18 years of age.
A total of 145 eyes from 128 patients were included in this study (Table 1). Glaucoma diagnoses varied, with primary open angle glaucoma being the most common, affecting 61.3% of eyes, followed by pseudoexfoliation (12.4% of eyes) and secondary glaucoma due to other causes (8.3%). Eighty-seven percent (87%) of eyes were pseudophakic at the time of the procedure.
Eight (8) eyes had previously undergone a XEN45 procedure that had failed, and the XEN45 + MMC procedure represented a revision of the prior surgery. All surgeries were conducted with the ab externo, open conjunctival technique. No intraoperative complications were reported. Of the total XEN45 cases, 17.2% were combined with cataract extraction and intraocular lens (CE + IOL) insertion; XEN45 placement was performed according to the previously described protocol during the latter half of surgery. Differing amounts of MMC at a concentration of 0.2 mg/mL were applied during surgery with soaked sponges only (77.2% of eyes) or with an additional injection of 0.1 mL to 0.4 mL (22.8% of eyes). Betamethasone was also injected intraoperatively in 77.9% of cases.
3.2. XEN45 and XEN45 + Ologen Success
Of the 46 included XEN45 group eyes and the 99 XEN45 + Ologen group eyes, 41 eyes and 72 eyes had 12-month follow-up data available, respectively, with 5 eyes and 27 eyes being lost to follow-up between postoperative months 1 and 12. Therefore, of the 145 included eyes, 113 had complete follow-up data available. In total, 48.7% (55/113) of eyes met success criteria at 12 months (Table 2). Of eyes in the XEN45 group, 45.7% (21/46), 44.4% (20/45), 44.4% (20/45), 41.5% (17/41), and 41.5% (17/41) met success criteria at 1 month, 3 months, 6 months, 9 months, and 12 months postoperation, respectively. Of eyes in the XEN45 + Ologen group, 61.6% (61/99), 60.8% (59/97), 57.5% (50/87), 56.3% (45/80), and 52.8% (38/72) met success criteria at 1 month, 3 months, 6 months, 9 months, and 12 months postoperation, respectively. At 12 months, 7.3% (3/41) of eyes in XEN45 group and 12.5% (9/72) eyes in XEN45 + Ologen group met success criteria without the use of any medications. The Pearson chi-square test detected no significant association between Ologen use and success rate at all postoperative time periods.

Table 2.
Success rates for XEN45 and XEN45 + Ologen eyes at 1, 3, 6, 9, and 12 months post-XEN45 placement.
3.3. Change in Intraocular Pressure
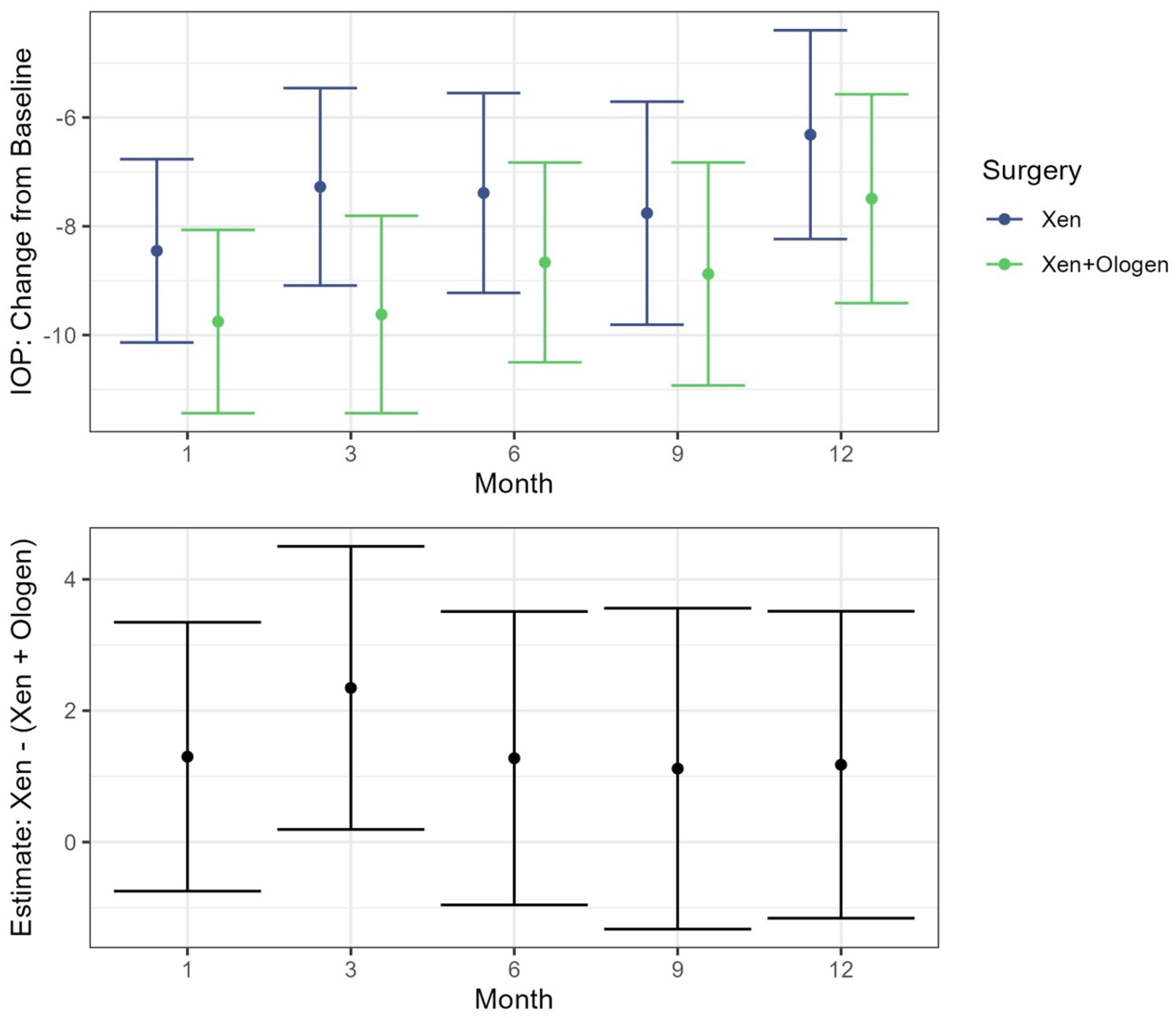
In both the XEN45 and XEN45 + Ologen groups, the IOP values at all postoperative follow-up periods were significantly reduced from baseline (p < 0.001) while controlling for age in the mixed effects model (Table 3). When comparing the IOP change from baseline between the XEN45 and XEN45 + Ologen groups, only at the 3-month timepoint was there a significant difference between the two groups, with a greater reduction seen in the XEN45 + Ologen group (−9.62, 95% CI −11.44, −7.81) than the XEN45 group (−7.27, 95% CI −9.09, −5.46) (p = 0.03) (Figure 2).

Table 3.
Left: Estimate, lower and upper bound of 95% CI average change in IOP from baseline after treatment of glaucoma, assuming a fixed age and accounting for baseline, using the linear mixed-effects model fit. Right: Estimate of difference between surgeries at each time point with p-value and lower and upper bound of 95% CI.
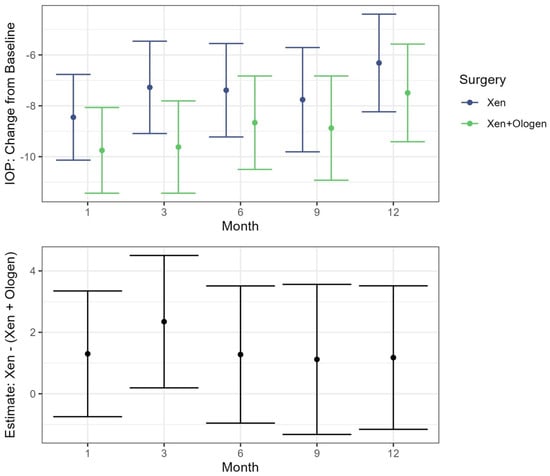
Figure 2.
Top: Estimates for change in IOP from the baseline visit following treatment with 95% CI by surgery type. Bottom: Estimated difference between the XEN and XEN + Ologen surgeries at each time point following baseline. Includes estimate and 95% CI.
3.4. Change in IOP-Lowering Medications
The average decrease in number of pressure-lowering medications between baseline and 12 months post-XEN did not differ significantly between groups, with an average decrease of 1.96 in the XEN45 group and of 2.31 in the XEN45 + Ologen group (p = 0.106) (Table 4). The mean number of medications was significantly lower in the XEN45 + Ologen group both at baseline (4.64 versus 5.87, p < 0.001) and at the 12-month post-XEN follow-up (2.33 versus 3.91, p < 0.001).

Table 4.
Number of pressure-lowering medications prescribed to patients before and after XEN45 placement for XEN45 and XEN45 + Ologen groups. p-values denote significance for Mann–Whitney U test with p < 0.05 being significant. “#” refers to number.
3.5. XEN Stent Failure and Postoperative Complications
Table 5 shows the causes of XEN failure among both groups. We found that 17.34% and 17.17% of XEN45 and XEN45 + Ologen eyes, respectively, did not achieve IOP lowering of at least 20% from baseline and 15.2% and 10.1% of eyes experienced an increase in IOP from baseline at the 12-month follow-up. Of the eyes requiring postoperative intervention (17.4% of XEN45 eyes and 14.1% of XEN45 + Ologen eyes), the most common procedures were bleb needling/revision (9 cases total), MP laser diode (5 cases total), Ahmed valve placement (4 cases total), and another MIGS procedure, specifically Hydrus (2 cases) or AlloFlo (1 case). There was no significant difference in the incidence of additional surgery between the two groups. Post-XEN placement, the most common complication was transient hypotony (IOP < 6 mmHg), seen in 6 XEN45 cases and 2 XEN45 + Ologen cases, followed by hypotonous maculopathy (2 XEN45 + Ologen cases), choroidal effusion (2 XEN45 + Ologen cases), bleb leakage (1 case in each group), implant exposure (2 XEN45 + Ologen cases), and implant malposition (1 case in each group). Less common complications seen were one case of blebitis (resolved with antibiotics) and one case of persistent mydriasis. No other postoperative complications (including endophthalmitis, hyphema, NLP visual acuity, etc.) were recorded in this cohort.

Table 5.
Causes of XEN45 failure over the entire 12-month follow-up interval for XEN45 and XEN45 + Ologen eyes. If cause of failure was postoperative intervention, breakdown by type of postoperative intervention is below.
4. Discussion
This study compared the efficacy of the XEN45 gel stent implantation with and without the use of Ologen in an adult population using an open conjunctiva, ab externo approach with the application of MMC. Ologen has been thought to improve post-surgical outcomes in XEN45 stent placement due to its demonstrated impact in reducing postoperative fibrosis in trabeculectomy, providing a framework for the organization of proliferating fibroblasts and myofibroblasts and the deposition of extracellular matrix components (collagen), while shielding the trabeculectomy or XEN45 stent from this healing and contraction response [16]. However, in this study we found that Ologen did not significantly impact XEN45 success rates at the 12-month postoperative timepoint. There were no significant differences in the postoperative reduction of the number of pressure-lowering medications between XEN45 and XEN45 + Ologen groups and in IOP reduction from baseline at the 1-month, 6-month, 9-month, and 12-month timepoints. The one exception was the 3-month follow-up, at which point the XEN45 + Ologen group demonstrated an average reduction 2.35 mmHg greater than in the XEN45 group.
These results are consistent with findings published by Park et al. in their 2022 study comparing 6-month post-XEN surgical outcomes with and without Ologen (Oloxen and Xen groups), which also did not detect a significant difference in success rates defined by an IOP reduction of >20% [13]. In contrast to our study, Park et al. stratified cases with combined cataract surgery into two distinct experimental groups (Phaco-Oloxen and PhacoXen groups) and had more stringent inclusion criteria, such as best corrected visual acuity (BCVA) and refractive cutoffs. However, the 6-month reported success rates of Oloxen and Xen groups, at 56.7% and 43.3%, respectively, were nearly identical to ours at 52.8% and 41.5% at 12 months.
A case-control study of 62 eyes conducted by Navaro-Rodriguez et al. similarly did not identify significant differences between OLOXEN and XEN groups in terms of mean IOP reduction and mean pressure-lowering medication reduction, with higher reported success rates of 65.2% in the OLOXEN group and 64.9% in the XEN group [14]. Unlike our findings, which did not detect a significant difference in the rates of post-XEN surgical intervention between the two groups, this study reported higher rates of bleb fibrosis in the MMC alone group (46% vs. 4.3%), resulting in higher rates of bleb needling (23.1% vs. 13%) and 5-FU injections (38.5% vs. 13%). Other studies evaluating XEN45 success rates without Ologen have reported complete success rates (defined as ≥20% IOP reduction without medications) in the range of 28.6–60% and qualified success rates (≥20% IOP reduction with or without medications) in the range of 66.7–88% [16,17,18]. These studies included much smaller sample sizes (ranging from 13 to 23 eyes) in addition to different surgical approaches (ab interno and ab externo), which may account for the discrepancies from our study results. A recent meta-analysis of XEN efficacy and safety found a 2-year complete success rate of 21-70.8% and qualified success rate of 34–86% [19].
Despite the consistency of our XEN45 and XEN45 + Ologen success rates with a previous study, there are clinical nuances that impacted our results. Of the 17 patients who did not meet success criteria due to an increased IOP from baseline, two eyes were on topical steroids at the time of the 12-month follow-up visit. One of these eyes had met success criteria at 9 months, but developed HSV keratitis and required topical steroids, which likely contributed to the significant IOP elevation seen at the 12-month visit. Four patients passed away during the follow-up period, three of whom were last observed at the 3-month follow-up and had met success criteria at that time. For three cases, further surgery was scheduled shortly after the 12-month mark: one qualified success was being considered for a bleb revision at 1.5 years postop and two eyes with inadequate IOP reduction were scheduled for Ahmed valve and AlloFlo procedures at 13 months. Lastly, rates of follow-up during the study were likely impacted by the COVID-19 pandemic, particularly in the spring of 2020 and the winter of 2021, which may have resulted in fewer patients reaching the 12-month follow-up point.
There are several limitations of this study that are important to note. First, this is a broadly inclusive, retrospective study of all adult patients at Moran Eye Center undergoing XEN45 implantation with an open conjunctiva, ab externo approach. Patients were not excluded based on past ocular medical and surgical history and current ocular conditions such as cornea and retina pathology. Of the included eyes in the study, 6 received steroids at some point during the study course due to uveitis (4), corneal ulcer (1), or iridocyclitis (1).
Furthermore, there are several possible confounding variables that may have impacted the rates of postop complications and surgical success. Several surgeons (6) at Moran Eye Center performed the operations, most frequently CC (113 eyes) followed by AN (15 eyes), RS (13 eyes), BS (2 eyes), MT (1 eye), and MM (1 eye). Two XEN45 procedures were combined with IOL exchange and anterior vitrectomy. Other surgical variables that differed between cases are included in Table 1; namely, combination XEN45 implantation with CE+IOL, MMC application, injection of betamethasone intraoperatively, and the technique of Ologen application (either placed over the XEN as a whole or bisected with the XEN in between the two halves). Although the percentage of cases combined with cataract extraction and betamethasone injection did not significantly differ between the XEN45 and XEN45 + Ologen groups (p > 0.05), it may be significant to note that a much greater proportion of XEN45 + Ologen eyes received MMC intraoperatively only via soaked sponges (94.9% versus 40% of XEN45 eyes) rather than with an additional injection. This difference in MMC application is because XEN45 cases occurred more recently chronologically and recent studies have demonstrated equivalent or increased efficacy of intra-tenon MMC injection versus soaked sponge application [20,21]. Furthermore, of eyes receiving MMC with an injection, the volume of MMC varied from 0.1 mL to 0.4 mL. Based on the current literature, it is unclear whether the addition of MMC injection to soaked sponges improved postoperative XEN outcomes, and how the amount of MMC applied may have impacted these results.
Lastly, of note, our patient population was majority White and comprised a demographic makeup reflective of that of the state of Utah: 84.3% of all patients, 87.5% of patients in the XEN45 group, and 83.0% of patients in the XEN45 + Ologen group. This may elevate our reported success rates, since Black and Afro-Latino patients have demonstrated lower success rates following XEN45 insertion, possibly on account of increased pigment in the anterior chamber that can obstruct the XEN lumen [22]. This discrepancy in postoperative success is similar to that observed for trabeculectomy, with patients of African descent tending to have a greater incidence of bleb leaks, higher postoperative IOP, and higher need for additional medications and surgery than patients of European descent [23]. The presence of more fibroblasts and macrophages in the conjunctiva of Black patients is one physiologic explanation that can account for the greater incidence of scarring and need for bleb needling in this population [24].
5. Conclusions
We found that the addition of Ologen did not significantly improve post-XEN45 success rates at the 12-month postoperative timepoint in terms of IOP lowering and decrease in pressure-lowering medications. Ologen also did not appear to decrease rates of complications and the need for additional surgeries following XEN failure. Although potential confounders in this study may impact our findings, our conclusions are consistent with previously published research.
Author Contributions
Conceptualization, R.T.W. and C.J.C.; methodology, C.J., R.T.W., B.J.B., C.S., A.N., B.C.S., and C.J.C.; formal analysis, C.J., M.J., R.T.W., and B.J.B.; investigation, C.J., C.S., A.N., B.C.S., and C.J.C.; data curation, C.J., M.J., J.A.M., and R.T.W.; writing—original draft preparation, C.J., M.J., J.A.M., and R.T.W.; writing—review and editing, M.J., J.A.M., N.K., K.E., R.T.W., C.S., B.J.B., A.N., B.C.S., and C.J.C.; supervision, C.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This investigation was supported by TRIAD, with funding in part from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002538. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Supported in part by an unrestricted grant from Research to Prevent Blindness, New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by Institutional Review Board of University of Utah (protocol code 144457, approval date 3 September 2021).
Informed Consent Statement
Patient consent was waived due to this study protocol meeting exempt criteria.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Fea, A.M.; Durr, G.M.; Marolo, P.; Malinverni, L.; Economou, M.A.; Ahmed, I. XEN((R)) Gel Stent: Comprehensive Review on Its Use as a Treatment Option for Refractory Glaucoma. Clin. Ophthalmol. 2020, 14, 1805–1832. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, A.; Reitsamer, H.; Ahmed, I.I. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4789–4795. [Google Scholar] [CrossRef] [PubMed]
- Buffault, J.; Baudouin, C.; Labbe, A. XEN((R)) Gel Stent for management of chronic open angle glaucoma: A review of the literature. J. Fr. Ophtalmol. 2019, 42, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Rauchegger, T.; Angermann, R.; Willeit, P.; Schmid, E.; Teuchner, B. Two-year outcomes of minimally invasive XEN Gel Stent implantation in primary open-angle and pseudoexfoliation glaucoma. Acta Ophthalmol. 2021, 99, 369–375. [Google Scholar] [CrossRef]
- Smith, M.; Charles, R.; Abdel-Hay, A.; Shah, B.; Byles, D.; Lim, L.; Rossiter, J.; Kuo, C.; Chapman, P.; Robertson, S. 1-Year outcomes of the Xen45 glaucoma implant. Eye 2019, 33, 761–766. [Google Scholar] [CrossRef]
- Theilig, T.; Rehak, M.; Busch, C.; Bormann, C.; Schargus, M.; Unterlauft, J.D. Comparing the efficacy of trabeculectomy and XEN gel microstent implantation for the treatment of primary open-angle glaucoma: A retrospective monocentric comparative cohort study. Sci. Rep. 2020, 10, 19337. [Google Scholar] [CrossRef]
- Wilkins, M.; Indar, A.; Wormald, R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst. Rev. 2005, 4, CD002897. [Google Scholar]
- Stephens, J.D.; Sarkisian, S.R. The use of collagen matrix (Ologen) as a patch graft in glaucoma tube shunt surgery, a retrospective chart review. F1000Res 2016, 5, 1898. [Google Scholar] [CrossRef]
- Elwehidy, A.S.; Mokbel, T.; Abouelkheir, H.Y.; Samra, W.A.; Wagdy, F.M.; Abdelkader, A.M.E. Trabeculectomy with Ologen implant versus perfluoropropane gas bubble for open angle glaucoma in pseudophakic eyes. Int. J. Ophthalmol. 2021, 14, 510–516. [Google Scholar] [CrossRef]
- Perez, C.I.; Mellado, F.; Jones, A.; Colvin, R. Trabeculectomy combined with collagen matrix implant (Ologen). J. Glaucoma 2017, 26, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Li, L.; Chen, X.; Yan, X.; Wang, L. Biodegradable 3D-Porous Collagen Matrix (Ologen) Compared with Mitomycin C for Treatment of Primary Open-Angle Glaucoma: Results at 5 Years. J. Ophthalmol. 2015, 2015, 637537. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, J.W.; Sung, K.R. Comparison of surgical outcomes with and without Ologen collagen matrix augmentation during XEN gel stent implantation. BMC Ophthalmol. 2022, 22, 426. [Google Scholar] [CrossRef] [PubMed]
- Navero-Rodriguez, J.M.; Espinosa-Barberi, G.; Morilla-Grasa, A.; Anton, A. Efficacy of the Ologen collagen matrix in combination with the XEN gel stent implantation in the treatment of open-angle glaucoma: A case-control study. Clin. Exp. Ophthalmol. 2020, 48, 1003–1005. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 October 2023).
- Galal, A.; Bilgic, A.; Eltanamly, R.; Osman, A. XEN Glaucoma Implant with Mitomycin C 1-Year Follow-Up: Result and Complications. J. Ophthalmol. 2017, 2017, 5457246. [Google Scholar] [CrossRef]
- Ibanez-Munoz, A.; Soto-Biforcos, V.S.; Chacon-Gonzalez, M.; Rua-Galisteo, O.; Arreita-Los Ssantos, A.; Lizuain-Abadia, M.E.; Del Rio Mayor, J.L. One-year follow-up of the XEN(R) implant with mitomycin-C in pseudoexfoliative glaucoma patients. Eur. J. Ophthalmol. 2019, 29, 309–314. [Google Scholar] [CrossRef]
- Dangda, S.; Radell, J.E.; Mavrommatis, M.A.; Lee, R.; Do, A.; Sidoti, P.A.; Panarelli, J.F. Open Conjunctival Approach for Sub-Tenon’s Xen Gel Stent Placement and Bleb Morphology by Anterior Segment Optical Coherence Tomography. J. Glaucoma 2021, 30, 988–995. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Z.; Yang, K.; Lv, K.; Ma, Y.; Meng-yang, L.; Wu, H. The outcomes of XEN gel stent implantation: A systematic review and meta-analysis. Front. Med. 2022, 9, 804847. [Google Scholar] [CrossRef]
- Guimarães, M.E.; de Pádua Soares Bezerra, B.; de Miranda Cordeiro, F.; Carvalho, C.H.; Danif, D.N.; Dorairaj, S.K.; Kanadani, F.N. Glaucoma Surgery with Soaked Sponges with Mitomycin C vs Sub-Tenon Injection: Short-term Outcomes. J. Curr. Glaucoma Pract. 2019, 13, 50–54. [Google Scholar]
- Shih, E.J.; Chen, Y.Y. Two-stage intra-tenon injection versus sponge-applied mitomycin C-augmented trabeculectomy: A one-year study. Int. Ophthalmol. 2023, 43, 2593–2603. [Google Scholar] [CrossRef]
- Laroche, D.; Nkrumah, G.; Ng, C. Real-World Retrospective Consecutive Study of Ab Interno XEN 45 Gel Stent Implant with Mitomycin C in Black and Afro-Latino Patients with Glaucoma: 40% Required Secondary Glaucoma Surgery at 1 Year. Middle East. Afr. J. Ophthalmol. 2020, 26, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; Fatehi, N.; Romero, P.; Miraftabi, A.; Kim, E.; Morales, E.; Giaconi, J.; Coleman, A.L.; Law, S.K.; Caprioli, J.; et al. Observational Outcomes of Initial Trabeculectomy with Mitomycin C in Patients of African Descent vs Patients of European Descent: Five-Year Results. JAMA Ophthalmol. 2018, 136, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Broadway, D.; Grierson, I.; Hitchings, R. Racial differences in the results of glaucoma filtration surgery: Are racial differences in the conjunctival cell profile important? Br. J. Ophthalmol. 1994, 78, 466–475. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).