Immunotherapy in Ophthalmic Oncology: Current Trends and Future Directions
Abstract
1. Introduction
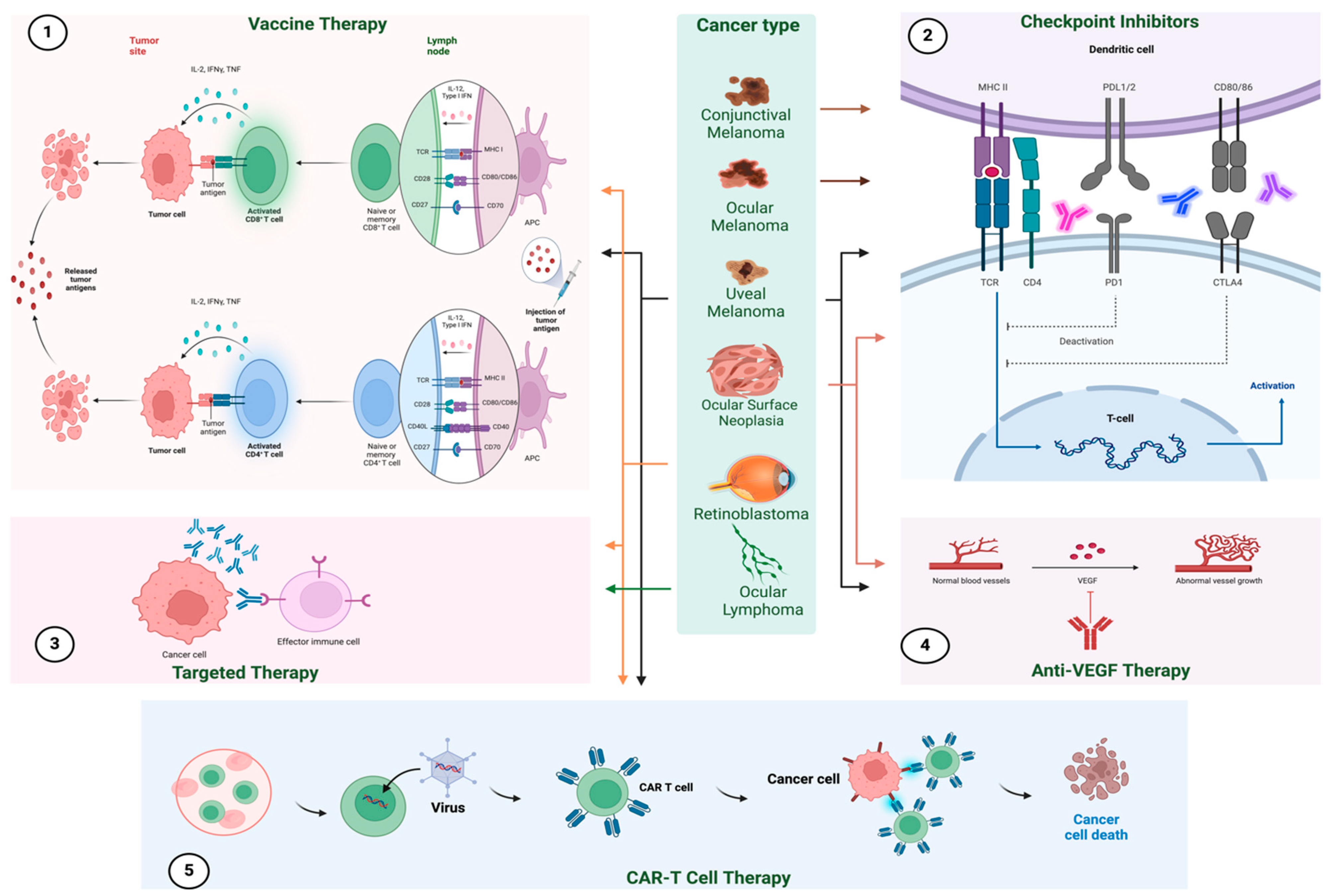
2. Checkpoint Inhibitors in Ocular Oncology
3. Clinical Trials
4. Vaccine Therapies
5. Adoptive Cell Transfer Therapies
6. Targeted Therapy
6.1. Bispecific T-Cell Engagers
6.2. Immunotherapy in Retinoblastoma
7. Immunotherapy in Ocular Lymphoma
8. Immunotherapy in Ocular Surface Neoplasia
9. Variations in the Immune Response of Ocular Tumors and Future Endeavors
10. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masalkhi, M.; Wahoud, N.; Moran, B.; Elhassadi, E. Impact of immune checkpoint inhibitors on vision and eye health. Eye 2024, 38, 2854–2856. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Virgili, G.; Parravano, M.; Gatta, G.; Capocaccia, R.; Mazzini, C.; Mallone, S.; Botta, L. Incidence and Survival of Patients With Conjunctival Melanoma in Europe. JAMA Ophthalmol. 2020, 138, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.S.; De Rosa, F.; Di Terlizzi, P.; Toneatto, G.; Gabai, A.; Finocchio, L.; Salati, C.; Spadea, L.; Zeppieri, M. Uveal melanoma: Recent advances in immunotherapy. World J. Clin. Oncol. 2024, 15, 23–31. [Google Scholar] [CrossRef]
- Ny, L.; Jespersen, H.; Karlsson, J.; Alsén, S.; Filges, S.; All-Eriksson, C.; Andersson, B.; Carneiro, A.; Helgadottir, H.; Levin, M.; et al. The PEMDAC phase 2 study of pembrolizumab and entinostat in patients with metastatic uveal melanoma. Nat. Commun. 2021, 12, 5155. [Google Scholar] [CrossRef]
- Wong, J.R.; Nanji, A.A.; Galor, A.; Karp, C.L. Management of conjunctival malignant melanoma: A review and update. Expert Rev. Ophthalmol. 2014, 9, 185–204. [Google Scholar] [CrossRef]
- Brouwer, N.J.; Verdijk, R.M.; Heegaard, S.; Marinkovic, M.; Esmaeli, B.; Jager, M.J. Conjunctival melanoma: New insights in tumour genetics and immunology, leading to new therapeutic options. Prog. Retin. Eye Res. 2022, 86, 100971. [Google Scholar] [CrossRef]
- Luke, J.J.; Callahan, M.K.; Postow, M.A.; Romano, E.; Ramaiya, N.; Bluth, M.; Giobbie-Hurder, A.; Lawrence, D.P.; Ibrahim, N.; Ott, P.A.; et al. Clinical activity of ipilimumab for metastatic uveal melanoma. Cancer 2013, 119, 3687–3695. [Google Scholar] [CrossRef]
- Lee, J.B.; Kim, H.R.; Ha, S.J. Immune Checkpoint Inhibitors in 10 Years: Contribution of Basic Research and Clinical Application in Cancer Immunotherapy. Immune Netw. 2022, 22, e2. [Google Scholar] [CrossRef]
- Tan, Y.; Lu, Y.; Chen, S.; Zou, C.; Qin, B. Immunotherapy for ocular melanoma: A bibliometric and visualization analysis from 1991 to 2022. Front. Oncol. 2023, 13, 1161759. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Pavlick, A.C. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: A clinical case series. J. Immunother. Cancer 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, O.; Thakar, S.D.; Kandl, T.J.; Ford, J.; Sniegowski, M.C.; Hwu, W.J.; Esmaeli, B. Immunotherapy with Programmed Cell Death 1 Inhibitors for 5 Patients With Conjunctival Melanoma. JAMA Ophthalmol. 2018, 136, 1236–1241. [Google Scholar] [CrossRef]
- Yamada, K.; Takeuchi, M.; Fukumoto, T.; Suzuki, M.; Kato, A.; Mizuki, Y.; Yamada, N.; Kaneko, T.; Mizuki, N.; Horita, N. Immune checkpoint inhibitors for metastatic uveal melanoma: A meta-analysis. Sci. Rep. 2024, 14, 7887. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Lutzky, J.; Feun, L.; Harbour, W. 430 A phase II study of nivolumab + BMS-986016 (relatlimab) in patients with metastatic uveal melanoma(UM) (CA224–094). J. ImmunoTherapy Cancer 2020, 8, A261–A262. [Google Scholar] [CrossRef]
- Cohen, V.M.L.; O’Day, R.F. Management Issues in Conjunctival Tumours: Conjunctival Melanoma and Primary Acquired Melanosis. Ophthalmol. Ther. 2019, 8, 501–510. [Google Scholar] [CrossRef]
- Baginska, J.; Nau, A.; Gomez Diaz, I.; Giobbie-Hurder, A.; Weirather, J.; Vergara, J.; Abrecht, C.; Hallisey, M.; Dennis, J.; Severgnini, M.; et al. Ziv-aflibercept plus pembrolizumab in patients with advanced melanoma resistant to anti-PD-1 treatment. Cancer Immunol. Immunother. 2024, 73, 17. [Google Scholar] [CrossRef]
- Dummer, R.; Corrie, P.; Gutzmer, R.; Meniawy, T.M.; Del Vecchio, M.; Lebbé, C.; Guida, M.; Dutriaux, C.; Dreno, B.; Meyer, N.; et al. First-Line, Fixed-Duration Nivolumab Plus Ipilimumab Followed by Nivolumab in Clinically Diverse Patient Populations With Unresectable Stage III or IV Melanoma: CheckMate 401. J. Clin. Oncol. 2023, 41, 3917–3929. [Google Scholar] [CrossRef]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kämpgen, E.; et al. Phase II DeCOG-Study of Ipilimumab in Pretreated and Treatment-Naïve Patients with Metastatic Uveal Melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.; Ascierto, P.A.; Haanen, J.; Espinosa, E.; Demidov, L.; Garbe, C.; Guida, M.; Lorigan, P.; Chiarion-Sileni, V.; Gogas, H.; et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: A single-arm, open-label, phase II study (CheckMate 172). Eur. J. Cancer 2019, 119, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Antoun, J.; Titah, C.; Cochereau, I. Ocular and orbital side-effects of checkpoint inhibitors: A review article. Curr. Opin. Oncol. 2016, 28, 288. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wei, X. Ocular Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors in Lung Cancer. Front. Immunol. 2021, 12, 701951. [Google Scholar] [CrossRef]
- Le, I.; Dhandayuthapani, S.; Chacon, J.; Eiring, A.M.; Gadad, S.S. Harnessing the Immune System with Cancer Vaccines: From Prevention to Therapeutics. Vaccines 2022, 10, 816. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, D.; Li, Y.; Yang, L. Development of therapeutic vaccines for the treatment of diseases. Mol. Biomed. 2022, 3, 40. [Google Scholar] [CrossRef]
- Schank, T.E.; Hassel, J.C. Immunotherapies for the Treatment of Uveal Melanoma—History and Future. Cancers 2019, 11, 1048. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Bartlett, D.L.; Liu, Z.; Sathaiah, M.; Ravindranathan, R.; Guo, Z.; He, Y.; Guo, Z.S. Oncolytic viruses as therapeutic cancer vaccines. Mol. Cancer 2013, 12, 103. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene Laherparepvec (T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Lutzky, J.; Sullivan, R.J.; Cohen, J.V.; Ren, Y.; Li, A.; Haq, R. Phase 1b study of intravenous coxsackievirus A21 (V937) and ipilimumab for patients with metastatic uveal melanoma. J. Cancer Res. Clin. Oncol. 2023, 149, 6059–6066. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Mensink, H.W.; Aarntzen, E.H.J.G.; Schreibelt, G.; Keunen, J.E.E.; Coulie, P.G.; de Klein, A.; Punt, C.J.; Paridaens, D.; Figdor, C.G.; et al. Long Overall Survival After Dendritic Cell Vaccination in Metastatic Uveal Melanoma Patients. Am. J. Ophthalmol. 2014, 158, 939–947.e5. [Google Scholar] [CrossRef] [PubMed]
- Bol, K.F.; Bosch, T.v.d.; Schreibelt, G.; Mensink, H.W.; Keunen, J.E.E.; Kiliç, E.; Japing, W.J.; Geul, K.W.; Westdorp, H.; Boudewijns, S.; et al. Adjuvant Dendritic Cell Vaccination in High-Risk Uveal Melanoma. Ophthalmology 2016, 123, 2265–2267. [Google Scholar] [CrossRef] [PubMed]
- Barbari, C.; Fontaine, T.; Parajuli, P.; Lamichhane, N.; Jakubski, S.; Lamichhane, P.; Deshmukh, R.R. Immunotherapies and Combination Strategies for Immuno-Oncology. Int. J. Mol. Sci. 2020, 21, 5009. [Google Scholar] [CrossRef]
- Rohaan, M.W.; Berg, J.H.v.d.; Kvistborg, P.; Haanen, J.B.A.G. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: A viable treatment option. J. Immunother. Cancer 2018, 6, 102. [Google Scholar] [CrossRef]
- Forsberg, E.M.V.; Lindberg, M.F.; Jespersen, H.; Alsén, S.; Bagge, R.O.; Donia, M.; Svane, I.M.; Nilsson, O.; Ny, L.; Nilsson, L.M.; et al. HER2 CAR-T Cells Eradicate Uveal Melanoma and T-cell Therapy–Resistant Human Melanoma in IL2 Transgenic NOD/SCID IL2 Receptor Knockout Mice. Cancer Res. 2019, 79, 899–904. [Google Scholar] [CrossRef]
- Chandran, S.S.; Somerville, R.P.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; PharmD, D.Z.; Paria, B.C.; et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: A single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Ryan, J.S.; John, M.K.; Anthony, M.J.; Joseph, J.S.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef]
- Middleton, M.R.; McAlpine, C.; Woodcock, V.K.; Corrie, P.; Infante, J.R.; Steven, N.M.; Thomas, R.; Alan, A.; Alexander, N.S.; Omid, H.; et al. Tebentafusp, A TCR/Anti-CD3 Bispecific Fusion Protein Targeting gp100, Potently Activated Antitumor Immune Responses in Patients with Metastatic Melanoma. Clin. Cancer Res. 2020, 26, 5869–5878. [Google Scholar] [CrossRef]
- Cruz-Gálvez, C.C.; Ordaz-Favila, J.C.; Villar-Calvo, V.M.; Cancino-Marentes, M.E.; Bosch-Canto, V. Retinoblastoma: Review and new insights. Front. Oncol 2022, 12, 963780. [Google Scholar] [CrossRef] [PubMed]
- Byroju, V.V.; Nadukkandy, A.S.; Cordani, M.; Kumar, L.D. Retinoblastoma: Present scenario and future challenges. Cell Commun. Signal. 2023, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Mei, J.; Ye, L. Immunotherapies of retinoblastoma: Effective methods for preserving vision in the future. Front. Oncol. 2022, 12, 949193. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Kandalam, M.; Harilal, A.; Verma, R.S.; Krishnan, U.M.; Swaminathan, S.; Krishnakumar, S. EpCAM is a putative stem marker in retinoblastoma and an effective target for T-cell-mediated immunotherapy. Mol. Vis. 2012, 18, 290–308. [Google Scholar]
- Ghassemi, S.; Martinez-Becerra, F.J.; Master, A.M.; Richman, S.A.; Heo, D.; Leferovich, J.; Tu, Y.; García-Cañaveras, J.C.; Ayari, A.; Lu, Y.; et al. Enhancing Chimeric Antigen Receptor T Cell Anti-tumor Function through Advanced Media Design. Mol. Ther. Methods Clin. Dev. 2020, 18, 595–606. [Google Scholar] [CrossRef]
- Wilson, M.W.; Goldsmith, Z.; Coppess, W.; Gao, B.; McEwen, M.; Irvine, A.; Brennan, R.C.; Morales, V.M. PDGF-PDGFR Signaling Sustain Angiogenesis in an Autocrine and Paracrine Fashion in Retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2016, 57. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2560037 (accessed on 18 June 2024).
- Cacciavillano, W.; Sampor, C.; Venier, C.; Gabri, M.R.; de Dávila, M.T.G.; Galluzzo, M.L.; Guthmann, M.D.; Fainboim, L.; Alonso, D.F.; Chantada, G.L.; et al. A Phase I Study of the Anti-Idiotype Vaccine Racotumomab in Neuroblastoma and Other Pediatric Refractory Malignancies. Pediatr. Blood Cancer 2015, 62, 2120–2124. [Google Scholar] [CrossRef]
- Eckardt, A.M.; Lemound, J.; Rana, M.; Gellrich, N.C. Orbital lymphoma: Diagnostic approach and treatment outcome. World J. Surg. Oncol. 2013, 11, 73. [Google Scholar] [CrossRef]
- Lee Boniao, E.; Allen, R.C.; Sundar, G. Targeted therapy and immunotherapy for orbital and periorbital tumors: A major review. Orbit 2023, 43, 656–673. [Google Scholar] [CrossRef]
- Fernández, C.A.; Henry, R.K.; Shields, C.L.; Bilyk, J.R.; Lally, S.E.; Eagle, R.C.; Milman, T. Ocular adnexal lymphoma: A single-institution retrospective study. Saudi J. Ophthalmol. 2022, 35, 230–238. [Google Scholar] [CrossRef]
- Savino, G.; Battendieri, R.; Balia, L.; Colucci, D.; Larocca, L.M.; Laurenti, L.; De Padua, L.; Blasi, M.A.; Balestrazzi, E. Evaluation of intraorbital injection of rituximab for treatment of primary ocular adnexal lymphoma: A pilot study. Cancer Sci. 2011, 102, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Geng, H.; Fraser, E.J.; Formaker, P.; Chen, L.; Sharma, J.; Killea, P.; Choi, K.; Ventura, J.; Kurhanewicz, J.; et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018, 2, 1595–1607. [Google Scholar] [CrossRef]
- Ghesquieres, H.; Chevrier, M.; Laadhari, M.; Chinot, O.; Choquet, S.; Moluçon-Chabrot, C.; Beauchesne, P.; Gressin, R.; Morschhauser, F.; Schmitt, A.; et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: A multicenter prospective ‘proof of concept’ phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA). Ann. Oncol. 2019, 30, 621–628. [Google Scholar]
- Craig, A.; Güney, E.; Pekmezci, M.; Bloomer, M.; Laszik, Z.; Ohgami, R.S.; Toland, A.; Vogel, H.; Forns, T.; Wang, E.; et al. Utility of PD-1, PD-L1, and IDO-1 Stains in Ocular Extranodal Marginal Zone Lymphoma (MZL) and Diffuse Large B-cell Lymphoma (DLBCL). Appl. Immunohistochem. Mol. Morphol. 2024, 32, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Abadir, E.; McCluskey, P.; Hamad, N.; Lo, T.H.; Heydon, P. Presumptive recurrence of intraocular lymphoma despite chimeric antigen receptor T-cell therapy. Retin. Cases Brief Rep. 2023, 17, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Masalkhi, M.; Wahhoud, N.; Elhassadi, E. Next-gen oncology: The role of CAR-T cells against ocular lymphoma and myeloma. Eye 2024, 38, 3416–3417. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.; Shao, K.; Feng, P.W.; Falcone, M.; Feng, H. Periocular and ocular surface nonmelanoma skin cancer. Clin. Dermatol. 2024, 42, 71–77. [Google Scholar] [CrossRef]
- Basti, S.; Macsai, M.S. Ocular Surface Squamous Neoplasia: A Review. Cornea 2003, 22, 687. [Google Scholar] [CrossRef]
- Vann, R.R.; Karp, C.L. Perilesional and topical interferon alfa-2b for conjunctival and corneal neoplasia. Ophthalmology 1999, 106, 91–97. [Google Scholar] [CrossRef]
- Schechter, B.A.; Schrier, A.; Nagler, R.S.; Smith, E.F.; Velasquez, G.E. Regression of presumed primary conjunctival and corneal intraepithelial neoplasia with topical interferon alpha-2b. Cornea 2002, 21, 6–11. [Google Scholar] [CrossRef]
- Nägeli, M.; Mangana, J.; Chaloupka, K.; Dummer, R. Cutaneous SCC with orbital invasion: Case series. J. Eur. Acad. Dermatol. Venereol. 2022, 36 (Suppl. S1), 59–62. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, A.; Feizi, S. Subconjunctival bevacizumab injection for ocular surface squamous neoplasia. Cornea 2013, 32, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.A.; Çiloğlu, E.; Esen, E.; Şimdivar, G.H. Use of topical bevacizumab for conjunctival intraepithelial neoplasia. Cornea 2014, 33, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- van Poppelen, N.M.; van Ipenburg, J.A.; van den Bosch, Q.; Vaarwater, J.; Brands, T.; Eussen, B.; Magielsen, F.; Dubbink, H.J.; Paridaens, D.; Brosens, E.; et al. Molecular Genetics of Conjunctival Melanoma and Prognostic Value of TERT Promoter Mutation Analysis. Int. J. Mol. Sci. 2021, 22, 5784. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular immune privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef]
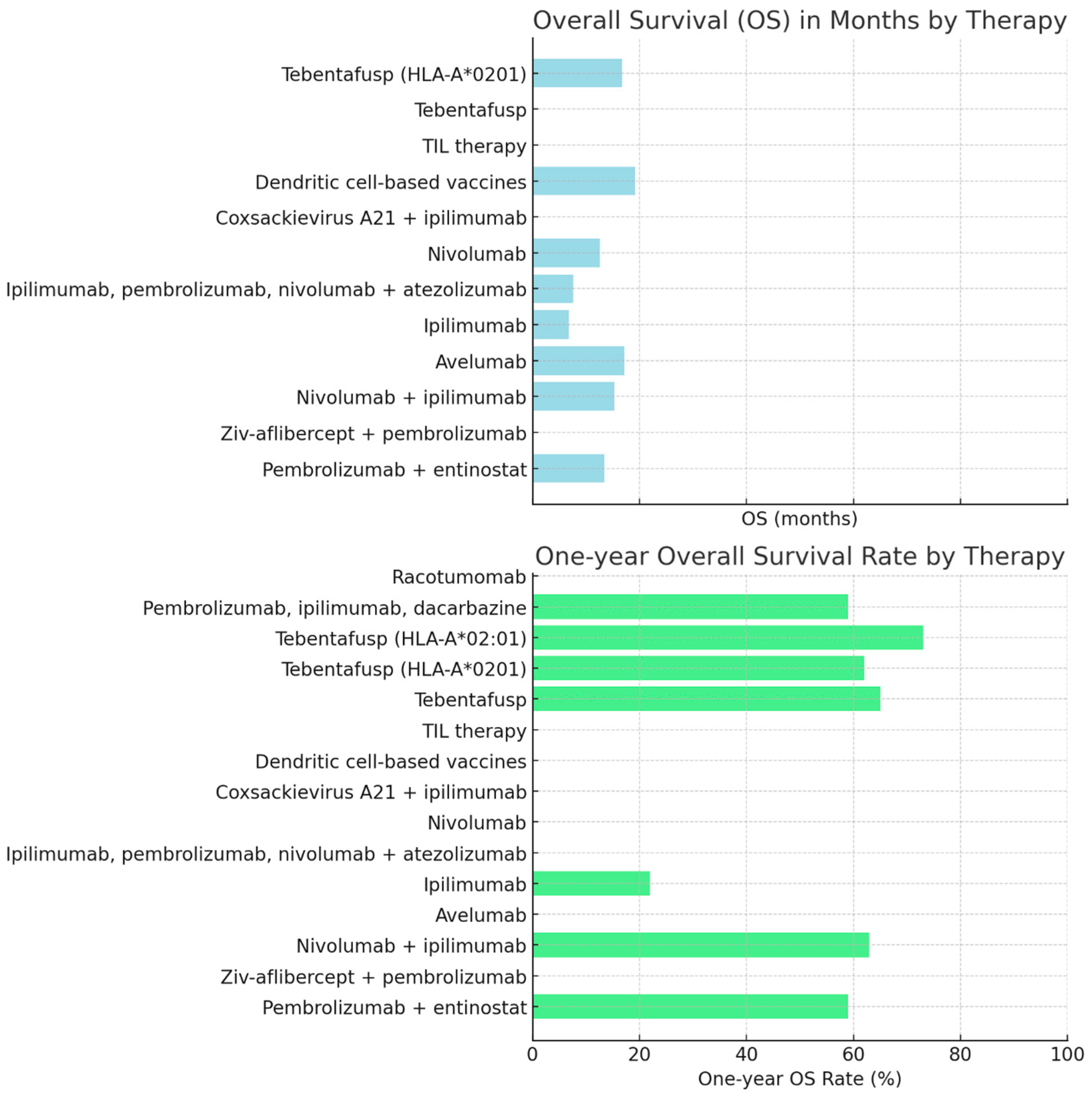
| Type of Immunotherapy | Cancer Type | Overall Survival (OS) | One-Year OS Rate | Two-Year OS Rate | PFS | Stable Disease Rate | Other Response Rates | References |
|---|---|---|---|---|---|---|---|---|
| Pembrolizumab + entinostat | Metastatic uveal melanoma | 13.4 months | 59% | N/A * | N/A | N/A | N/A | [1] |
| Ziv-aflibercept + pembrolizumab | Ocular melanoma | N/A | N/A | N/A | N/A | Stable disease in both patients | N/A | [2] |
| Nivolumab + ipilimumab, followed by nivolumab | Ocular/uveal melanoma | 15.3 months | 63% | 36% | N/A | N/A | N/A | [3] |
| Avelumab | Ocular melanoma | 17.2 months | N/A | N/A | N/A | N/A | N/A | [4] |
| Ipilimumab | Metastatic uveal melanoma | 6.8 months | 22% | 7% | 2.8 months | N/A | N/A | [5] |
| Ipilimumab, pembrolizumab, nivolumab + atezolizumab | Metastatic uveal melanoma | 7.6 months | N/A | N/A | 2.6 months | 14.3% | N/A | [6] |
| Nivolumab | Ocular melanoma | 12.6 months | N/A | N/A | N/A | N/A | 18-month OS rate = 34.8% | [7] |
| Coxsackievirus A21 (V937) vaccine + ipilimumab | Metastatic uveal melanoma | N/A | N/A | N/A | N/A | No meaningful clinical benefit | N/A | [8] |
| Dendritic-cell-based vaccines/gp100 + tyrosinase | Metastatic uveal melanoma | 19.2 months | N/A | N/A | N/A | N/A | N/A | [9] |
| TIL therapy | Metastatic uveal melanoma | N/A | N/A | N/A | N/A | N/A | Partial response = 30%, objective tumor regression = 35%, complete response = 5% | [10] |
| Tebentafusp | Uveal melanoma | N/A | 65% | N/A | N/A | N/A | N/A | [11] |
| Tebentafusp | HLA-A*0201 uveal melanoma | 16.8 months | 62% | N/A | 2.8 months | 45% | N/A | [12] |
| Tebentafusp | HLA-A*02:01-metastatic uveal melanoma | N/A | 73% | N/A | N/A | N/A | 6-month PFS = 31% | [13] |
| Pembrolizumab, ipilimumab, or dacarbazine | HLA-A*02:01-metastatic uveal melanoma | N/A | 59% | N/A | N/A | N/A | 6-month PFS = 19% | [13] |
| Racotumomab | N-glycolyl GM3 neuroectodermal malignancies | N/A | N/A | N/A | N/A | N/A | Immune response with favorable toxicity profile | [14] |
| Drug Class | Clinical Use | Adverse Effects |
|---|---|---|
| Immune checkpoint inhibitors | Metastatic melanoma, non-small-cell lung cancer, renal cell carcinoma [15] | Dry eye disease, uveitis [16], retinopathy [17], ophthalmoplegia [13] |
| Vaccine therapies | Refractory melanoma [18], prostate cancer [18] | Injection site pain, headache, influenza-like illness [19] |
| Adoptive cell transfer therapies | Metastatic melanoma, cervical SCC, cholangiocarcinoma [20], Epstein–Barr virus (EBV)-induced post-transplant lymphoproliferative disease [21] | Cytokine release syndrome and prolonged B-cell depletion [22], capillary leak syndrome [20] |
| Bispecific T-cell engagers | Uveal melanoma [11] | Rash, pruritis, pyrexia, periorbital edema, fatigue, nausea [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masalkhi, M.; Wahoud, N.; Moran, B.; Elhassadi, E. Immunotherapy in Ophthalmic Oncology: Current Trends and Future Directions. J. Clin. Transl. Ophthalmol. 2025, 3, 1. https://doi.org/10.3390/jcto3010001
Masalkhi M, Wahoud N, Moran B, Elhassadi E. Immunotherapy in Ophthalmic Oncology: Current Trends and Future Directions. Journal of Clinical & Translational Ophthalmology. 2025; 3(1):1. https://doi.org/10.3390/jcto3010001
Chicago/Turabian StyleMasalkhi, Mouayad, Noura Wahoud, Bridget Moran, and Ezzat Elhassadi. 2025. "Immunotherapy in Ophthalmic Oncology: Current Trends and Future Directions" Journal of Clinical & Translational Ophthalmology 3, no. 1: 1. https://doi.org/10.3390/jcto3010001
APA StyleMasalkhi, M., Wahoud, N., Moran, B., & Elhassadi, E. (2025). Immunotherapy in Ophthalmic Oncology: Current Trends and Future Directions. Journal of Clinical & Translational Ophthalmology, 3(1), 1. https://doi.org/10.3390/jcto3010001






