Preliminary Visual Outcomes of a Novel Non-Diffractive Extended Monofocal Intraocular Lens (Evolux) 3 Months After Cataract Surgery
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alio, J.L.; Plaza-Puche, A.B.; Férnandez-Buenaga, R.; Pikkel, J.; Maldonado, M. Multifocal intraocular lenses: An overview. Surv. Ophthalmol. 2017, 62, 611–634. [Google Scholar] [CrossRef] [PubMed]
- MacRae, S.; Holladay, J.T.; Glasser, A.; Calogero, D.; Hilmantel, G.; Masket, S.; Stark, W.; Tarver, M.E.; Nguyen, T.; Eydelman, M. Special Report: American Academy of Ophthalmology Task Force Consensus Statement for Extended Depth of Focus Intraocular Lenses. Ophthalmology 2017, 124, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Blancafort Alias, S.; Del Campo Carrasco, Z.; Salvador-Miras, I.; Luna Mariné, S.; Gómez Prieto, M.J.; Liñán Martín, F.; Salvà Casanovas, A. Exploring Vision-Related Quality of Life: A Qualitative Study Comparing Patients’ Experience of Cataract Surgery with a Standard Monofocal IOL and an Enhanced Monofocal IOL. Clin. Ophthalmol. 2022, 16, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Toto, F.; Grzybowski, A.; Alio, J.L. Extended Depth-of-Field Intraocular Lenses: An Update. Asia-Pac. J. Ophthalmol. 2020, 9, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Breyer, D.R.; Kaymak, H.; Ax, T.; Kretz, F.T.; Auffarth, G.U.; Hagen, P.R. Multifocal Intraocular Lenses and Extended Depth of Focus Intraocular Lenses. Asia-Pac. J. Ophthalmol. 2017, 6, 339–349. [Google Scholar] [CrossRef]
- Sifi Internal Data. Available online: https://www.sifigroup.com/s/news/test-news-MCDWV3QW4Z3NGTTHPLVHGULGS26U?language=ro (accessed on 20 July 2024).
- Wang, L.; Yeu, E.; Koch, D.D. Wavefront-Modified IOLs. In Cataract and Refractive Surgery; Springer: Berlin/Heidelberg, Germany, 2008; pp. 81–93. [Google Scholar] [CrossRef]
- Beiko, G.H.H. Personalized correction of spherical aberration in cataract surgery. J. Cataract Refract. Surg. 2007, 33, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Santaella, R.M.; Booth, M.; Koch, D.D. Higher-order aberrations from the internal optics of the eye. J. Cataract Refract. Surg. 2005, 31, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, T.; Petermann, K.; Böhm, M.; Hemkeppler, E.; Ahmad, W.; Hinzelmann, L.; Pawlowicz, K.; Jandewerth, T.; Lwowski, C. Nondiffractive wavefront-shaping extended depth-of-focus intraocular lens: Visual performance and patient-reported outcomes. J. Cataract Refract. Surg. 2022, 48, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.M.; Schickhardt, S.K.; Wang, Q.; Friedmann, E.; Khoramnia, R.; Auffarth, G.U. Quantitative evaluation of microvacuole formation in five intraocular lens models made of different hydrophobic materials. PLoS ONE 2021, 16, e0250860. [Google Scholar] [CrossRef] [PubMed]
- Werner, L.; Thatthamla, I.; Ong, M.; Schatz, H.; Garcia-Gonzalez, M.; Gros-Otero, J.; Cañones-Zafra, R.; Teus, M.A. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J. Cataract Refract. Surg. 2019, 45, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Ale, J.B. Intraocular lens tilt and decentration: A concern for contemporary IOL designs. Nepal. J. Ophthalmol. 1970, 3, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J.; Aramberri, J.; Haigis, W.; Olsen, T.; Savini, G.; Shammas, H.J.; Bentow, S. Protocols for Studies of Intraocular Lens Formula Accuracy. Am. J. Ophthalmol. 2015, 160, 403–405.e1. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, C.; Pesudovs, K.; Moore, J.E. The Development of an Instrument to Measure Quality of Vision: The Quality of Vision (QoV) Questionnaire. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5537. [Google Scholar] [CrossRef] [PubMed]
- Nanavaty, M.A. Evolving generation of new Extended Depth of Focus intraocular lenses. Eye 2024, 38, 1–3. [Google Scholar] [CrossRef]
- Mencucci, R.; Cennamo, M.; Venturi, D.; Vignapiano, R.; Favuzza, E. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: Preliminary results. J. Cataract Refract. Surg. 2020, 46, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Nicula, C.A.; Pop, R.N.; Nicula, P. Evaluation of a Novel Enhanced Monofocal Extended Depth of Focus IOL with a Non-Diffractive Surface Design—First Results. 2023. Available online: https://escrs.conference2web.com/#!resources/evaluation-of-a-novel-enhanced-monofocal-extended-depth-of-focus-iol-with-a-non-diffractive-surface-design-first-results (accessed on 20 July 2024).
- Black, D.A.; Bala, C.; Alarcon, A.; Vilupuru, S. Tolerance to refractive error with a new extended depth of focus intraocular lens. Eye 2024, 38, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, V.; Mazzini, C.; Franchini, A. Clinical Results of a New Advanced Monofocal IOL. 2023. Available online: https://escrs.conference2web.com/#!resources/clinical-results-of-a-new-advanced-monofocal-iol (accessed on 20 July 2024).
- Pagnacco, C.; Bonacci, E.; Kilian, R.; Polinelli, E.; Marchini, G.; Pedrotti, E. Visual Performance, Subjective Satisfaction and Quality of Life Effect of a New Extended Monofocal Intraocular Lens (IOL). 2024. Available online: https://escrs.conference2web.com/#!resources/visual-performance-subjective-satisfaction-and-quality-of-life-effect-of-a-new-extended-monofocal-intraocular-lens-iol-6de64f2e-50b8-4e95-a5b7-6f16e577baaf (accessed on 20 July 2024).
- Escandón-García, S.; Ribeiro, F.J.; McAlinden, C.; Queirós, A.; González-Méijome, J.M. Through-Focus Vision Performance and Light Disturbances of 3 New Intraocular Lenses for Presbyopia Correction. J. Ophthalmol. 2018, 2018, 6165493. [Google Scholar] [CrossRef]
- Gundersen, K.; Potvin, R. Trifocal intraocular lenses: A comparison of the visual performance and quality of vision provided by two different lens designs. Clin. Ophthalmol. 2017, 11, 1081–1087. [Google Scholar] [CrossRef]
- Makhotkina, N.Y.; Nijkamp, M.D.; Berendschot, T.T.; van den Borne, B.; Aelen-van Kruchten, M.; van Vught, L.; Beenakker, J.W.; Krijgh, E.; Aslam, T.; Pesudovs, K.; et al. Measuring quality of vision including negative dysphotopsia. Acta Ophthalmol. 2024, 102, e510–e519. [Google Scholar] [CrossRef]

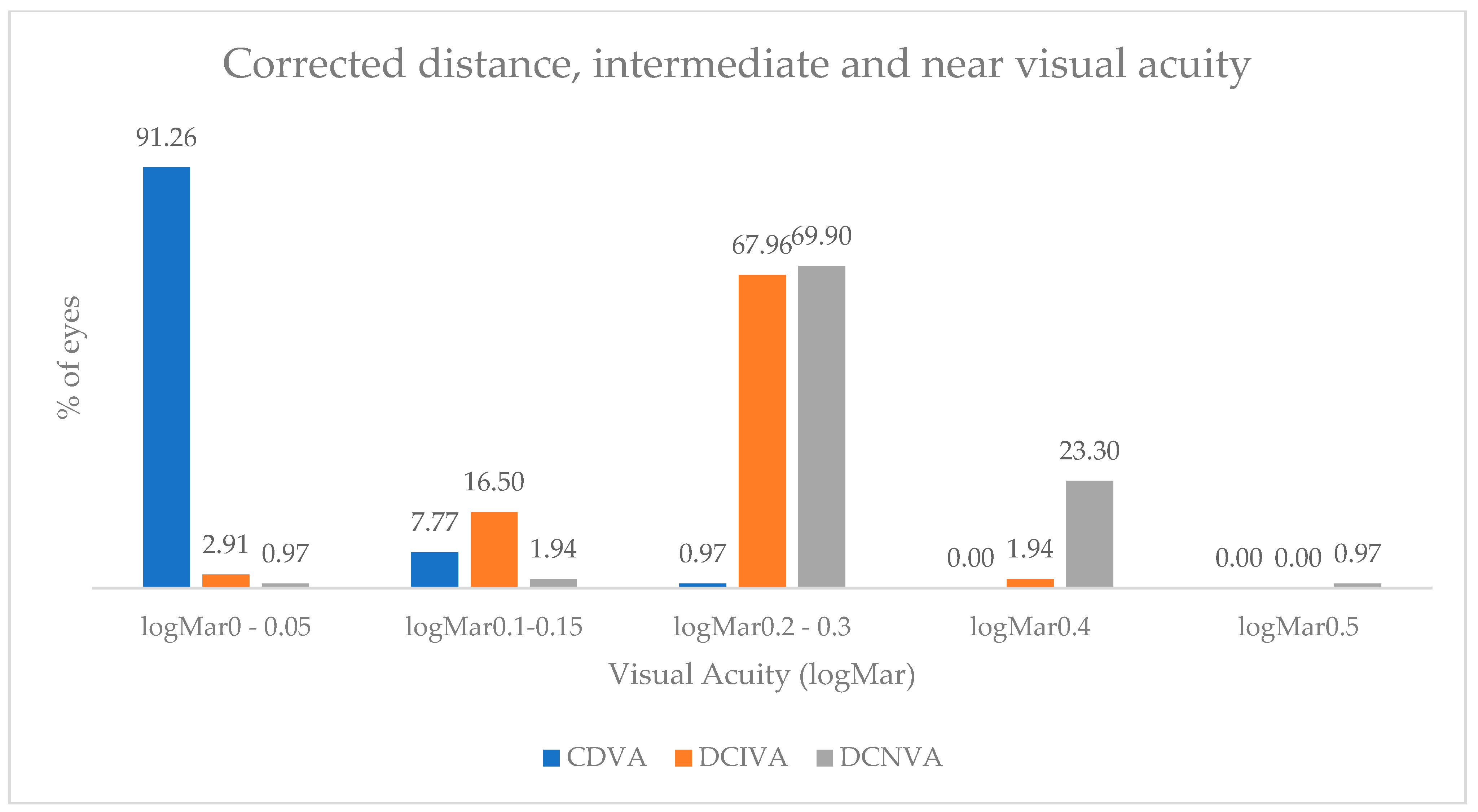

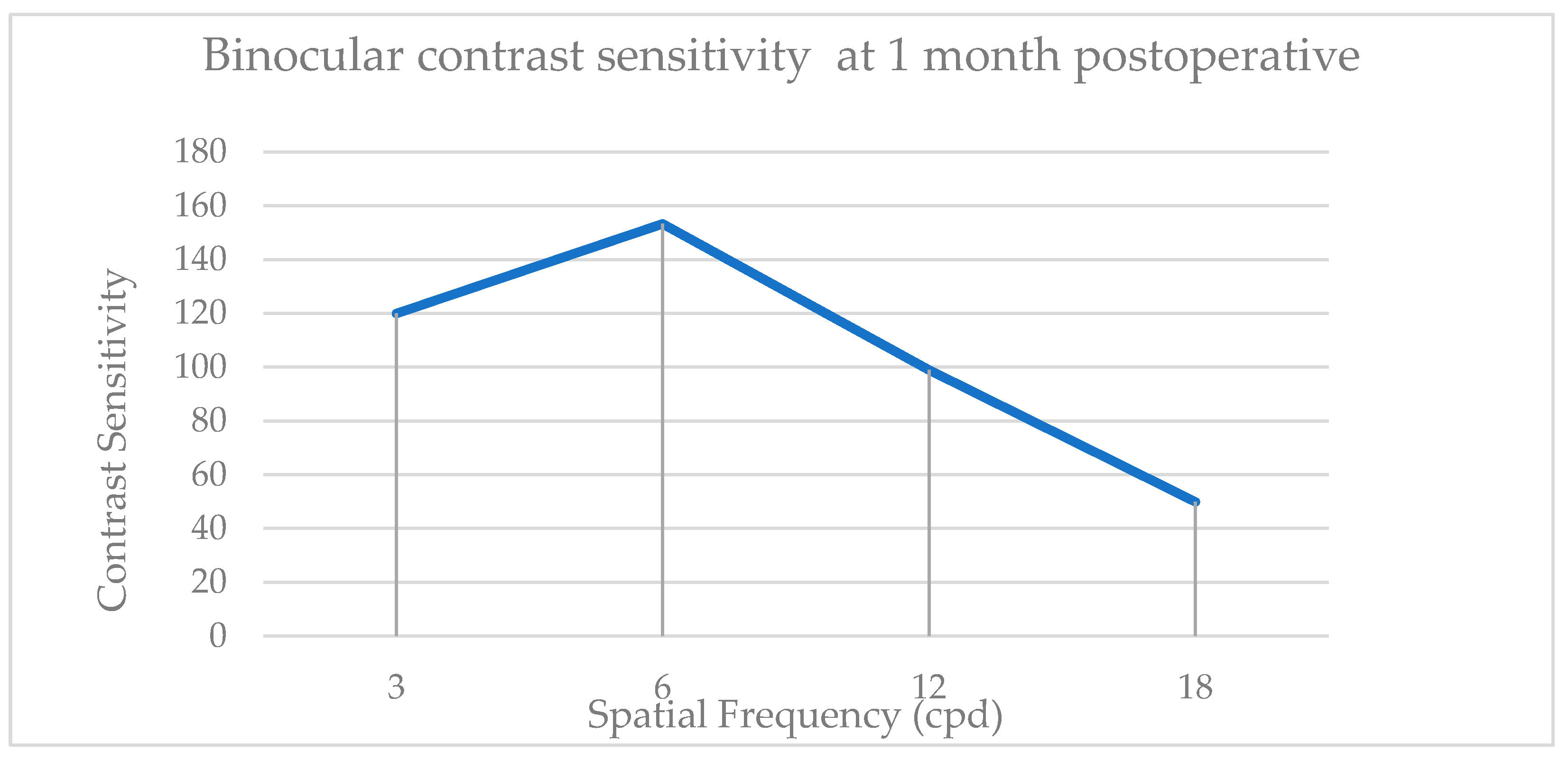
| Biometry Data | Mean | SD | 95% CI |
|---|---|---|---|
| K1 (D) | 43.03 | ±1.47 | 42.76–43.32 |
| K2 (D) | 43.57 | ±1.48 | 43.28–43.85 |
| Axial length (mm) | 23.42 | ±0.79 | 23.27–23.57 |
| ACD (mm) | 3.20 | ±0.45 | 3.11–3.29 |
| LT (mm) | 4.38 | ±0.54 | 4.27–4.48 |
| WTW (mm) | 11.71 | ±0.42 | 11.63–11.80 |
| CCT (um) | 543.15 | ±30.4 | 537.25–549.05 |
| IOL power (D) | 21 | ±1.73 | 20.67–21.33 |
| Mean VA (logMAR) | SD | |
|---|---|---|
| UDVA | 0.08 | ±0.08 |
| CDVA | 0.02 | ±0.04 |
| UIVA | 0.2 | ±0.09 |
| DCIVA | 0.2 | ±0.07 |
| UNVA | 0.3 | ±0.09 |
| DCNVA | 0.3 | ±0.07 |
| No. of Eyes | ||||||
|---|---|---|---|---|---|---|
| UDVA | CDVA | UIVA | DCIVA | UNVA | DCNVA | |
| logMAR 0 | 33 | 72 | 4 | 3 | 1 | 1 |
| logMAR 0.05 | 24 | 22 | 0 | 0 | 0 | 0 |
| logMAR 0.1 | 9 | 0 | 5 | 8 | 1 | 1 |
| logMAR 0.15 | 26 | 8 | 10 | 9 | 1 | 1 |
| logMAR 0.2 | 7 | 0 | 43 | 48 | 16 | 14 |
| logMAR 0.3 | 2 | 1 | 23 | 22 | 43 | 58 |
| logMAR 0.4 | 1 | 0 | 7 | 2 | 34 | 24 |
| logMAR 0.5 | 1 | 0 | 1 | 0 | 4 | 1 |
| Mean ± SD | Median | Mode | Range | |
|---|---|---|---|---|
| Frequency | 30.20 ± 16 | 32 | 37 | 0–67 |
| Severity | 17.19 ± 12.45 | 22 | 0 | 0–44 |
| Bothersome | 15.45 ± 12.94 | 14 | 0 | 0–53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stejar, L.D.; Barac, R.; Preoteasa, D. Preliminary Visual Outcomes of a Novel Non-Diffractive Extended Monofocal Intraocular Lens (Evolux) 3 Months After Cataract Surgery. J. Clin. Transl. Ophthalmol. 2024, 2, 171-180. https://doi.org/10.3390/jcto2040014
Stejar LD, Barac R, Preoteasa D. Preliminary Visual Outcomes of a Novel Non-Diffractive Extended Monofocal Intraocular Lens (Evolux) 3 Months After Cataract Surgery. Journal of Clinical & Translational Ophthalmology. 2024; 2(4):171-180. https://doi.org/10.3390/jcto2040014
Chicago/Turabian StyleStejar, Laura Denisa, Ramona Barac, and Dana Preoteasa. 2024. "Preliminary Visual Outcomes of a Novel Non-Diffractive Extended Monofocal Intraocular Lens (Evolux) 3 Months After Cataract Surgery" Journal of Clinical & Translational Ophthalmology 2, no. 4: 171-180. https://doi.org/10.3390/jcto2040014
APA StyleStejar, L. D., Barac, R., & Preoteasa, D. (2024). Preliminary Visual Outcomes of a Novel Non-Diffractive Extended Monofocal Intraocular Lens (Evolux) 3 Months After Cataract Surgery. Journal of Clinical & Translational Ophthalmology, 2(4), 171-180. https://doi.org/10.3390/jcto2040014






