Artificial Intelligence in Glaucoma: A New Landscape of Diagnosis and Management
Abstract
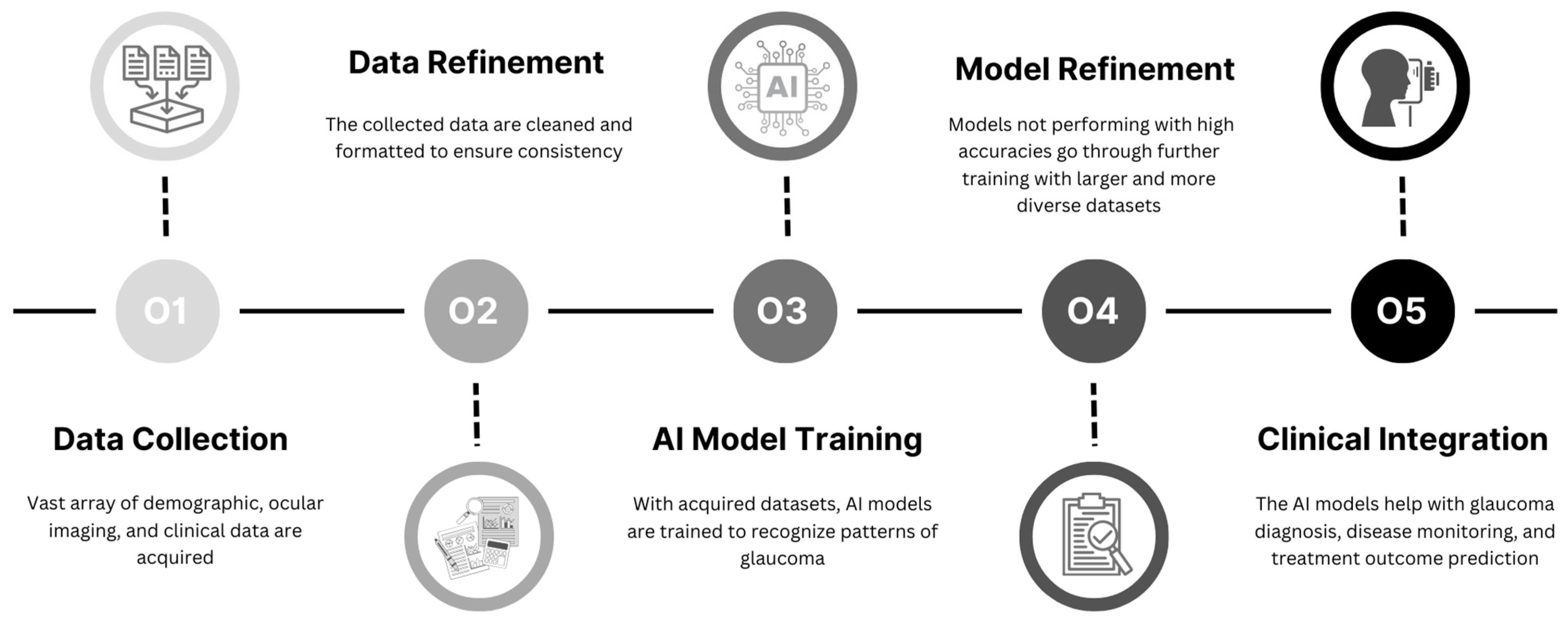
1. Introduction
1.1. Current Challenges in Glaucoma Management
1.2. Role of AI in Glaucoma
1.3. Objectives
2. AI in Glaucoma Detection and Diagnosis
2.1. AI in Functional Imaging
2.2. AI in Structural Imaging
2.3. Integrating Multiple Modalities Using AI for Detection and Diagnosis
2.4. Telemedicine and Remote Monitoring
3. AI in Monitoring Glaucoma Progression and Prediction
3.1. AI in Functional Imaging for Progression Monitoring and Prediction
3.2. AI in Structural Imaging for Progression Monitoring and Prediction
3.3. Integrating Multiple Modalities Using AI into Progression Monitoring and Prediction
4. AI in Glaucoma Treatment
4.1. AI in Surgical Interventions
4.2. Treatment Response Prediction
5. Ethical, Legal, and Social Implications
5.1. Ethical Considerations
5.2. Legal Implications
5.3. Social Implications
6. Limitations of AI in Glaucoma
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Nadler, Z.; Wollstein, G.; Ishikawa, H.; Schuman, J.S. Clinical Application of Ocular Imaging. Optom. Vis. Sci. 2012, 89, E543–E553. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Talwar, N.; LaVerne, A.M.; Nan, B.; Lichter, P.R. Trends in Use of Ancillary Glaucoma Tests for Patients with Open-Angle Glaucoma from 2001 to 2009. Ophthalmology 2012, 119, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.; Alencar, L.M. The role of standard automated perimetry and newer functional methods for glaucoma diagnosis and follow-up. Indian J. Ophthalmol. 2011, 59, S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, B.; Heijl, A. A Visual Field Index for Calculation of Glaucoma Rate of Progression. Arch. Ophthalmol. 2008, 145, 343–353. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Qu, G.; Song, D.; Yuan, Y.; Xu, Y.; Gao, K.; Luo, G.; Xiao, Z.; Lam, D.S.C.; et al. Automatic differentiation of Glaucoma visual field from non-glaucoma visual filed using deep convolutional neural network. BMC Med. Imaging 2018, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.F.; Quigley, H.A. Adherence and Persistence with Glaucoma Therapy. Surv. Ophthalmol. 2008, 53 (Suppl. S1), S57–S68. [Google Scholar] [CrossRef]
- Nordstrom, B.L.; Friedman, D.S.; Mozaffari, E.; Quigley, H.A.; Walker, A.M. Persistence and Adherence With Topical Glaucoma Therapy. Arch. Ophthalmol. 2005, 140, 598–606. [Google Scholar] [CrossRef]
- Friedman, D.S.; Nordstrom, B.; Mozaffari, E.; Quigley, H.A. Variations in Treatment among Adult-Onset Open-Angle Glaucoma Patients. Ophthalmology 2005, 112, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- De Fauw, J.; Ledsam, J.R.; Romera-Paredes, B.; Nikolov, S.; Tomasev, N.; Blackwell, S.; Askham, H.; Glorot, X.; O’donoghue, B.; Visentin, D.; et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat. Med. 2018, 24, 1342–1350. [Google Scholar] [CrossRef]
- Tian, Y.; Zang, M.; Sharma, A.; Gu, S.Z.; Leshno, A.; Thakoor, K.A. Glaucoma Progression Detection and Humphrey Visual Field Prediction Using Discriminative and Generative Vision Transformers. In Ophthalmic Medical Image Analysis; Antony, B., Chen, H., Fang, H., Fu, H., Lee, C.S., Zheng, Y., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 62–71. [Google Scholar]
- Xiong, J.; Li, F.; Song, D.; Tang, G.; He, J.; Gao, K.; Zhang, H.; Cheng, W.; Song, Y.; Lin, F.; et al. Multimodal Machine Learning Using Visual Fields and Peripapillary Circular OCT Scans in Detection of Glaucomatous Optic Neuropathy. Ophthalmology 2021, 129, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Banitt, M.R.; Ventura, L.M.; Feuer, W.J.; Savatovsky, E.; Luna, G.; Shif, O.; Bosse, B.; Porciatti, V. Progressive Loss of Retinal Ganglion Cell Function Precedes Structural Loss by Several Years in Glaucoma Suspects. Investig. Opthalmol. Vis. Sci. 2013, 54, 2346–2352. [Google Scholar] [CrossRef]
- Gajendran, M.K.; Rohowetz, L.J.; Koulen, P.; Mehdizadeh, A. Novel Machine-Learning Based Framework Using Electroretinography Data for the Detection of Early-Stage Glaucoma. Front. Neurosci. 2022, 16, 869137. [Google Scholar] [CrossRef]
- Miguel-Jiménez, J.M.; Blanco, R.; De-Santiago, L.; Fernández, A.; Rodríguez-Ascariz, J.M.; Barea, R.; Martín-Sánchez, J.L.; Amo, C.; Sánchez-Morla, E.; Boquete, L. Continuous-wavelet-transform analysis of the multifocal ERG waveform in glaucoma diagnosis. Med. Biol. Eng. Comput. 2015, 53, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Cheng, Y.; Hsu, W.; Lee, M.L. Integrated Optic Disc and Cup Segmentation with Deep Learning. In Proceedings of the 2015 IEEE 27th International Conference on Tools with Artificial Intelligence (ICTAI), Vietri sul Mare, Italy, 9–11 November 2015; pp. 162–169. [Google Scholar] [CrossRef]
- Zilly, J.; Buhmann, J.M.; Mahapatra, D. Glaucoma detection using entropy sampling and ensemble learning for automatic optic cup and disc segmentation. Comput. Med. Imaging Graph. 2017, 55, 28–41. [Google Scholar] [CrossRef]
- Miri, M.S.; Abràmoff, M.D.; Kwon, Y.H.; Sonka, M.; Garvin, M.K. A machine-learning graph-based approach for 3D segmentation of Bruch’s membrane opening from glaucomatous SD-OCT volumes. Med. Image Anal. 2017, 39, 206–217. [Google Scholar] [CrossRef]
- Antony, B.J.; Miri, M.S.; Abràmoff, M.D.; Kwon, Y.H.; Garvin, M.K. Automated 3D segmentation of multiple surfaces with a shared hole: Segmentation of the neural canal opening in SD-OCT volumes. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2014: 17th International Conference, Boston, MA, USA, 14–18 September 2014; Proceedings, Part I 17; Lecture Notes in Computer Science. Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 739–746. [Google Scholar] [CrossRef]
- Koozekanani, D.; Boyer, K.; Roberts, C. Retinal thickness measurements from optical coherence tomography using a Markov boundary model. IEEE Trans. Med. Imaging 2001, 20, 900–916. [Google Scholar] [CrossRef]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014, 98 (Suppl. S2), ii15–ii19. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Jammal, A.A.; Medeiros, F.A. A Deep Learning Algorithm to Quantify Neuroretinal Rim Loss from Optic Disc Photographs. Arch. Ophthalmol. 2019, 201, 9–18. [Google Scholar] [CrossRef]
- Braeu, F.A.; Chuangsuwanich, T.; Tun, T.A.; Perera, S.A.; Husain, R.; Kadziauskienė, A.; Schmetterer, L.; Thiéry, A.H.; Barbastathis, G.; Aung, T.; et al. Three-Dimensional Structural Phenotype of the Optic Nerve Head as a Function of Glaucoma Severity. JAMA Ophthalmol. 2023, 141, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Tanito, M.; Mitsuhashi, K.; Fujino, Y.; Matsuura, M.; Murata, H.; Asaoka, R. Development of a deep residual learning algorithm to screen for glaucoma from fundus photography. Sci. Rep. 2018, 8, 14665. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.U.; Ballios, B.G.; Christakis, P.G.; Kaplan, A.J.; Mathew, D.J.; Tone, S.O.; Wan, M.J.; A Micieli, J.; Wong, J.C.Y. Ensemble of deep convolutional neural networks is more accurate and reliable than board-certified ophthalmologists at detecting multiple diseases in retinal fundus photographs. Br. J. Ophthalmol. 2023, 108, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Belghith, A.; Bowd, C.; Medeiros, F.A.; Weinreb, R.N.; Zangwill, L.M. Automated segmentation of anterior lamina cribrosa surface: How the lamina cribrosa responds to intraocular pressure change in glaucoma eyes? In Proceedings of the 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), New York, NY, USA, 16–19 April 2015; pp. 222–225. [Google Scholar] [CrossRef]
- Cunefare, D.; Huckenpahler, A.L.; Patterson, E.J.; Dubra, A.; Carroll, J.; Farsiu, S. RAC-CNN: Multimodal deep learning based automatic detection and classification of rod and cone photoreceptors in adaptive optics scanning light ophthalmoscope images. Biomed. Opt. Express 2019, 10, 3815–3832. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, X.; Hoshi, S.; Zhang, Y. High-speed adaptive optics ophthalmoscopy for investigation of retinal hemodynamics in the living human eye. In Optics in Health Care and Biomedical Optics XII; SPIE: Cergy-Pontoise, France, 2022; Volume 12320, p. 1232019. [Google Scholar] [CrossRef]
- oltanian-Zadeh, S.; Kurokawa, K.; Liu, Z.; Zhang, F.; Saeedi, O.; Hammer, D.X.; Miller, D.T.; Farsiu, S. Weakly supervised individual ganglion cell segmentation from adaptive optics OCT images for glaucomatous damage assessment. Optica 2021, 8, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Soltanian-Zadeh, S.; Liu, Z.; Villanueva, R.; Saeedi, O.; Kurokawa, K.; Jung, H.W.; Miller, D.T.; Hammer, D.; Farsiu, S. Automatic cellular level differentiation of glaucomatous and healthy eyes via deep learning-based adaptive optics OCT analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 877. [Google Scholar]
- Wang, Y.; Zhen, L.; Tan, T.-E.; Fu, H.; Feng, Y.; Wang, Z.; Xu, X.; Goh, R.S.M.; Ng, Y.; Calhoun, C.; et al. Geometric Correspondence-Based Multimodal Learning for Ophthalmic Image Analysis. IEEE Trans. Med. Imaging 2024, 43, 1945–1957. [Google Scholar] [CrossRef]
- Mariottoni, E.B.; Datta, S.; Dov, D.; Jammal, A.A.; Berchuck, S.; Tavares, I.M.; Carin, L.; Medeiros, F.A. Artificial Intelligence Mapping of Structure to Function in Glaucoma. Transl. Vis. Sci. Technol. 2020, 9, 19. [Google Scholar] [CrossRef]
- Wang, R.; Bradley, C.; Herbert, P.; Hou, K.; Ramulu, P.; Breininger, K.; Unberath, M.; Yohannan, J. Deep learning-based identification of eyes at risk for glaucoma surgery. Sci. Rep. 2024, 14, 599. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Ho, H.-C.; Chen, Y.-W.; Lee, C.-K.; Chen, P.-J.; Lai, F.; Jang, J.-S.R.; Ko, M.-L. Use of multimodal dataset in AI for detecting glaucoma based on fundus photographs assessed with OCT: Focus group study on high prevalence of myopia. BMC Med. Imaging 2022, 22, 206. [Google Scholar] [CrossRef]
- Benzebouchi, N.E.; Azizi, N.; Ashour, A.S.; Dey, N.; Sherratt, R.S. Multi-modal classifier fusion with feature cooperation for glaucoma diagnosis. J. Exp. Theor. Artif. Intell. 2019, 31, 841–874. [Google Scholar] [CrossRef]
- Bhuiyan, A.; Govindaiah, A.; Smith, R.T. An Artificial-Intelligence- and Telemedicine-Based Screening Tool to Identify Glaucoma Suspects from Color Fundus Imaging. J. Ophthalmol. 2021, 2021, 6694784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Tailor, P.; Verma, R.; Zhang, I.; Schott, B.; Ye, C.; Szirth, B.; Habiel, M.; Khouri, A.S. Implementation of deep learning artificial intelligence in vision-threatening disease screenings for an underserved community during COVID-19. J. Telemed. Telecare 2023, 1357633X231158832. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, M.; Gonzalez-Hernandez, D.; Betancor-Caro, N.; Guedes-Guedes, I.; Guldager, M.K.; de la Rosa, M.G. Glaucoma Incidence and Progression in Diabetics: The Canary Islands Study Using the Laguna ONhE Application. J. Clin. Med. 2022, 11, 7294. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Luo, J.; Cui, T.; Liu, H.; Tang, H.; Zeng, Y.; Liu, C.; Li, Y.; Jian, J.; Wu, J.; et al. Soft Electronics for Health Monitoring Assisted by Machine Learning. Nano-Micro Lett. 2023, 15, 66. [Google Scholar] [CrossRef]
- Jones, P.R.; Campbell, P.; Callaghan, T.; Jones, L.; Asfaw, D.S.; Edgar, D.F.; Crabb, D.P. Glaucoma Home Monitoring Using a Tablet-Based Visual Field Test (Eyecatcher): An Assessment of Accuracy and Adherence Over 6 Months. Arch. Ophthalmol. 2020, 223, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bekollari, M.; Dettoraki, M.; Stavrou, V.; Glotsos, D.; Liaparinos, P. Computer-Aided Discrimination of Glaucoma Patients from Healthy Subjects Using the RETeval Portable Device. Diagnostics 2024, 14, 349. [Google Scholar] [CrossRef]
- Payne, N.; Gangwani, R.; Barton, K.; Sample, A.P.; Cain, S.M.; Burke, D.T.; Newman-Casey, P.A.; Shorter, K.A. Medication Adherence and Liquid Level Tracking System for Healthcare Provider Feedback. Sensors 2020, 20, 2435. [Google Scholar] [CrossRef]
- Yousefi, S.; Kiwaki, T.; Zheng, Y.; Sugiura, H.; Asaoka, R.; Murata, H.; Lemij, H.; Yamanishi, K. Detection of Longitudinal Visual Field Progression in Glaucoma Using Machine Learning. Arch. Ophthalmol. 2018, 193, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Elze, T.; Pasquale, L.R.; Shen, L.Q.; Chen, T.C.; Wiggs, J.L.; Bex, P.J. Patterns of functional vision loss in glaucoma determined with archetypal analysis. J. R. Soc. Interface 2015, 12, 20141118. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713, discussion 829–830. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Smith, S.; Fingert, J.; Gordon, M.; Kass, M.; Scheetz, T.; Segrè, A.V.; Wiggs, J.; Elze, T.; Zebardast, N. Machine Learning–Derived Baseline Visual Field Patterns Predict Future Glaucoma Onset in the Ocular Hypertension Treatment Study. Investig. Opthalmol. Vis. Sci. 2024, 65, 35. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.T.M.; Han, J.C.; Park, D.Y.; Shin, J. Multimodal Deep Learning Model of Predicting Future Visual Field for Glaucoma Patients. IEEE Access 2023, 11, 19049–19058. [Google Scholar] [CrossRef]
- Mariottoni, E.B.; Datta, S.; Shigueoka, L.S.; Jammal, A.A.; Tavares, I.M.; Henao, R.; Carin, L.; Medeiros, F.A. Deep Learning–Assisted Detection of Glaucoma Progression in Spectral-Domain OCT. Ophthalmol. Glaucoma 2023, 6, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Tandon, A.K.; Sun, G.; Dinkin, M.J.; Oliveira, C. Early Detection of Optic Nerve Changes on Optical Coherence Tomography Using Deep Learning for Risk-Stratification of Papilledema and Glaucoma. J. Neuro-Ophthalmol. 2023, 44, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Normando, E.M.; Yap, T.E.; Maddison, J.; Miodragovic, S.; Bonetti, P.; Almonte, M.; Mohammad, N.G.; Ameen, S.; Crawley, L.; Ahmed, F.; et al. A CNN-aided method to predict glaucoma progression using DARC (Detection of Apoptosing Retinal Cells). Expert Rev. Mol. Diagn. 2020, 20, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Su, Y.; Lin, F.; Li, Z.; Song, Y.; Nie, S.; Xu, J.; Chen, L.; Chen, S.; Li, H.; et al. A deep-learning system predicts glaucoma incidence and progression using retinal photographs. J. Clin. Investig. 2022, 132, e157968. [Google Scholar] [CrossRef]
- Lin, T.P.; Hui, H.Y.; Ling, A.; Chan, P.P.; Shen, R.; Wong, M.O.; Chan, N.C.; Leung, D.Y.; Xu, D.; Lee, M.L.; et al. Risk of Normal Tension Glaucoma Progression from Automated Baseline Retinal-Vessel Caliber Analysis: A Prospective Cohort Study. Arch. Ophthalmol. 2022, 247, 111–120. [Google Scholar] [CrossRef]
- Hussain, S.; Chua, J.; Wong, D.; Lo, J.; Kadziauskiene, A.; Asoklis, R.; Barbastathis, G.; Schmetterer, L.; Yong, L. Predicting glaucoma progression using deep learning framework guided by generative algorithm. Sci. Rep. 2023, 13, 19960. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.; Hou, K.; Bradley, C.; Hager, G.; Boland, M.V.; Ramulu, P.; Unberath, M.; Yohannan, J. Forecasting Risk of Future Rapid Glaucoma Worsening Using Early Visual Field, OCT, and Clinical Data. Ophthalmol. Glaucoma 2023, 6, 466–473. [Google Scholar] [CrossRef]
- Qidwai, U.; Qidwai, U.; Sivapalan, T.; Ratnarajan, G. iMIGS: An innovative AI based prediction system for selecting the best patient-specific glaucoma treatment. MethodsX 2023, 10, 102209. [Google Scholar] [CrossRef]
- Conlon, R.; Saheb, H.; Ahmed, I.I.K. Glaucoma treatment trends: A review. Can. J. Ophthalmol. 2016, 52, 114–124. [Google Scholar] [CrossRef]
- Ciociola, E.C.; Fernandez, E.; Kaufmann, M.; Klifto, M.R. Future directions of glaucoma treatment: Emerging gene, neuroprotection, nanomedicine, stem cell, and vascular therapies. Curr. Opin. Ophthalmol. 2023, 35, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Chen, A.; Song, X.; Weiskopf, N.G.; Chiang, M.F.; Hribar, M.R. Prediction of multiclass surgical outcomes in glaucoma using multimodal deep learning based on free-text operative notes and structured EHR data. J. Am. Med. Inform. Assoc. 2023, 31, 456–464. [Google Scholar] [CrossRef]
- Wang, S.Y.; Tseng, B.; Hernandez-Boussard, T. Deep Learning Approaches for Predicting Glaucoma Progression Using Electronic Health Records and Natural Language Processing. Ophthalmol. Sci. 2022, 2, 100127. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.L.; Marks, C.; Kuo, T.-T.; Ohno-Machado, L.; Weinreb, R.N. Machine Learning-Based Predictive Modeling of Surgical Intervention in Glaucoma Using Systemic Data From Electronic Health Records. Arch. Ophthalmol. 2019, 208, 30–40. [Google Scholar] [CrossRef]
- Lin, K.Y.; Urban, G.; Yang, M.C.; Lee, L.-C.; Lu, D.-W.; Alward, W.L.M.; Baldi, P. Accurate Identification of the Trabecular Meshwork under Gonioscopic View in Real Time Using Deep Learning. Ophthalmology 2022, 129, 402–412. [Google Scholar] [CrossRef]
- Nespolo, R.G.; Yi, D.; Cole, E.; Valikodath, N.; Luciano, C.; Leiderman, Y.I. Evaluation of Artificial Intelligence–Based Intraoperative Guidance Tools for Phacoemulsification Cataract Surgery. JAMA Ophthalmol. 2022, 140, 170–177. [Google Scholar] [CrossRef]
- Banna, H.U.; Zanabli, A.; McMillan, B.; Lehmann, M.; Gupta, S.; Gerbo, M.; Palko, J. Evaluation of machine learning algorithms for trabeculectomy outcome prediction in patients with glaucoma. Sci. Rep. 2022, 12, 2473. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Xiao, Y.; Hou, B.; Wanyan, T.; Sharma, M.M.; Wang, Z.; Wang, F.; Van Tassel, S.; Peng, Y. Evaluate underdiagnosis and overdiagnosis bias of deep learning model on primary open-angle glaucoma diagnosis in under-served populations. AMIA Summits Transl. Sci. Proc. 2023, 2023, 370–377. [Google Scholar] [PubMed]
- Prabhakar, B.; Singh, R.K.; Yadav, K.S. Artificial intelligence (AI) impacting diagnosis of glaucoma and understanding the regulatory aspects of AI-based software as medical device. Comput. Med. Imaging Graph. 2020, 87, 101818. [Google Scholar] [CrossRef] [PubMed]
- Maliha, G.; Gerke, S.; Cohen, I.G.; Parikh, R.B. Artificial Intelligence and Liability in Medicine: Balancing Safety and Innovation. Milbank Q. 2021, 99, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Bin, K.J.; Melo, A.A.R.; da Rocha, J.G.M.F.; de Almeida, R.P.; Junior, V.C.; Maia, F.L.; de Faria, E.; Pereira, A.J.; Battistella, L.R.; Ono, S.K. The Impact of Artificial Intelligence on Waiting Time for Medical Care in an Urgent Care Service for COVID-19: Single-Center Prospective Study. JMIR Form. Res. 2022, 6, e29012. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, D.; Li, W.; Dong, B.; Wang, H.; Yuan, J.; Li, B.; Shi, L.; Lin, X.; Zhao, L.; et al. Artificial intelligence-assisted reduction in patients’ waiting time for outpatient process: A retrospective cohort study. BMC Health Serv. Res. 2021, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.; Rohren, E. Social Asymmetry, Artificial Intelligence and the Medical Imaging Landscape. Semin. Nucl. Med. 2022, 52, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.M. The Problem of Overfitting. J. Chem. Inf. Comput. Sci. 2003, 44, 1–12. [Google Scholar] [CrossRef]
- Long, E.; Wan, P.; Zhuo, Y. Predicting the Real-World Future of Glaucoma Patients? Cautions Are Required for Machine Learning. Transl. Vis. Sci. Technol. 2017, 6, 3. [Google Scholar] [CrossRef]
- Christopher, M.; Nakahara, K.; Bowd, C.; Proudfoot, J.A.; Belghith, A.; Goldbaum, M.H.; Rezapour, J.; Weinreb, R.N.; Fazio, M.A.; Girkin, C.A.; et al. Effects of Study Population, Labeling and Training on Glaucoma Detection Using Deep Learning Algorithms. Transl. Vis. Sci. Technol. 2020, 9, 27. [Google Scholar] [CrossRef]
- Zhu, Y.; Salowe, R.; Chow, C.; Li, S.; Bastani, O.; O’brien, J.M. Advancing Glaucoma Care: Integrating Artificial Intelligence in Diagnosis, Management, and Progression Detection. Bioengineering 2024, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.M.; Prybutok, V. Balancing Privacy and Progress: A Review of Privacy Challenges, Systemic Oversight, and Patient Perceptions in AI-Driven Healthcare. Appl. Sci. 2024, 14, 675. [Google Scholar] [CrossRef]
- Prakash, S.; Balaji, J.N.; Joshi, A.; Surapaneni, K.M. Ethical Conundrums in the Application of Artificial Intelligence (AI) in Healthcare—A Scoping Review of Reviews. J. Pers. Med. 2022, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Ruamviboonsuk, P.; Ruamviboonsuk, V.; Tiwari, R. Recent evidence of economic evaluation of artificial intelligence in ophthalmology. Curr. Opin. Ophthalmol. 2023, 34, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mursch-Edlmayr, A.S.; Ng, W.S.; Diniz-Filho, A.; Sousa, D.C.; Arnold, L.; Schlenker, M.B.; Duenas-Angeles, K.; Keane, P.A.; Crowston, J.G.; Jayaram, H. Artificial Intelligence Algorithms to Diagnose Glaucoma and Detect Glaucoma Progression: Translation to Clinical Practice. Transl. Vis. Sci. Technol. 2020, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Wang, M.; Frueh, L.; Rosner, B.; Wiggs, J.L.; Elze, T.; Pasquale, L.R. Cohort Study of Race/Ethnicity and Incident Primary Open-Angle Glaucoma Characterized by Autonomously Determined Visual Field Loss Patterns. Transl. Vis. Sci. Technol. 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Luo, Y.; Tian, Y.; Shen, L.; Elze, T.; Zebardast, N.; Eslami, M.; Kazeminasab, S.; Boland, M.V.; Friedman, D.S.; et al. Equitable Artificial Intelligence for Glaucoma Screening with Fair Identity Normalization. medRxiv 2023. [Google Scholar] [CrossRef]
- Vieira, C.M.; Oliveira, M.V.D.C.; Guimarães, M.D.P.; Rocha, L.; Dias, D.R.C. Applied Explainable Artificial Intelligence (XAI) in the classification of retinal images for support in the diagnosis of Glaucoma. In Proceedings of the 29th Brazilian Symposium on Multimedia and the Web, Ribeirão Preto, Brazil, 23–27 October 2023; Association for Computing Machinery: New York, NY, USA, 2023; pp. 82–90. [Google Scholar] [CrossRef]
- Kamal, S.; Dey, N.; Chowdhury, L.; Hasan, S.I.; Santosh, K. Explainable AI for Glaucoma Prediction Analysis to Understand Risk Factors in Treatment Planning. IEEE Trans. Instrum. Meas. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Li, C. Glaucoma Detection Based on Optical Coherence Tomography Imaging. Master’s Thesis, Nanyang Technological University, Singapore, 2023. [Google Scholar] [CrossRef]
- Mehta, P.; Petersen, C.A.; Wen, J.C.; Banitt, M.R.; Chen, P.P.; Bojikian, K.D.; Egan, C.; Lee, S.-I.; Balazinska, M.; Lee, A.Y.; et al. Automated Detection of Glaucoma with Interpretable Machine Learning Using Clinical Data and Multimodal Retinal Images. Arch. Ophthalmol. 2021, 231, 154–169. [Google Scholar] [CrossRef]
- Ran, A.R.; Cheung, C.Y.; Wang, X.; Chen, H.; Luo, L.-Y.; Chan, P.P.; Wong, M.O.M.; Chang, R.T.; Mannil, S.S.; Young, A.L.; et al. Detection of glaucomatous optic neuropathy with spectral-domain optical coherence tomography: A retrospective training and validation deep-learning analysis. Lancet Digit. Health 2019, 1, e172–e182. [Google Scholar] [CrossRef] [PubMed]
- Rai, A. Explainable AI: From black box to glass box. J. Acad. Mark. Sci. 2020, 48, 137–141. [Google Scholar] [CrossRef]
- Hossain, I.; Zamzmi, G.; Mouton, P.R.; Salekin, S.; Sun, Y.; Goldgof, D. Explainable AI for Medical Data: Current Methods, Limitations, and Future Directions. ACM Comput. Surv. 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, P.X.; Ramalingam, V.; Balas, M.; Pickel, L.; Mathew, D.J. Artificial Intelligence in Glaucoma: A New Landscape of Diagnosis and Management. J. Clin. Transl. Ophthalmol. 2024, 2, 47-63. https://doi.org/10.3390/jcto2020005
Ji PX, Ramalingam V, Balas M, Pickel L, Mathew DJ. Artificial Intelligence in Glaucoma: A New Landscape of Diagnosis and Management. Journal of Clinical & Translational Ophthalmology. 2024; 2(2):47-63. https://doi.org/10.3390/jcto2020005
Chicago/Turabian StyleJi, Patrick Xiang, Vethushan Ramalingam, Michael Balas, Lauren Pickel, and David J. Mathew. 2024. "Artificial Intelligence in Glaucoma: A New Landscape of Diagnosis and Management" Journal of Clinical & Translational Ophthalmology 2, no. 2: 47-63. https://doi.org/10.3390/jcto2020005
APA StyleJi, P. X., Ramalingam, V., Balas, M., Pickel, L., & Mathew, D. J. (2024). Artificial Intelligence in Glaucoma: A New Landscape of Diagnosis and Management. Journal of Clinical & Translational Ophthalmology, 2(2), 47-63. https://doi.org/10.3390/jcto2020005





