Optimization of Copper-Ammonia-Sulfate Electrolyte for Maximizing Cu(I):Cu(II) Ratio Using pH and Copper Solubility
Abstract
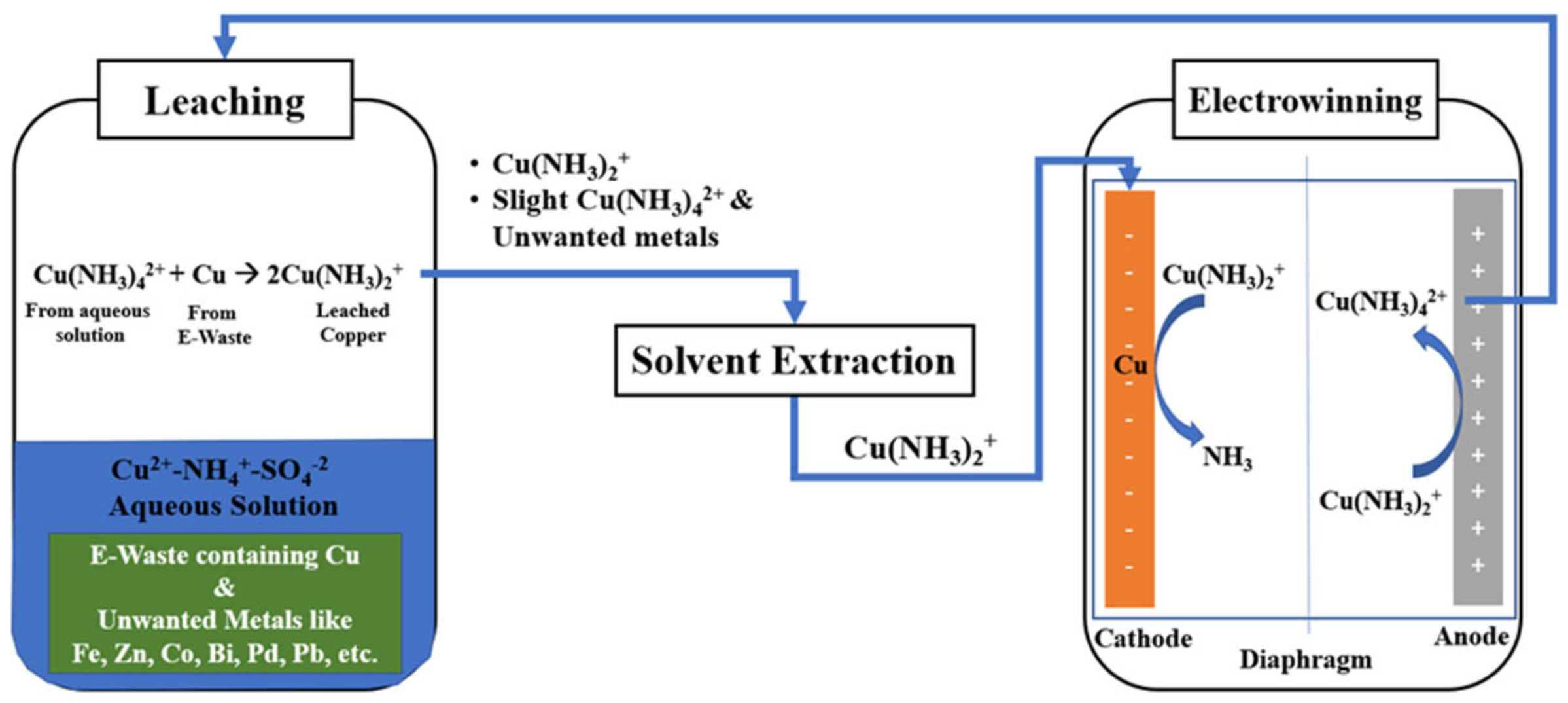
1. Introduction
- i.
- Determining the saturation level of CuSO4·5H2O.
- ii.
- Determining maximum Cu(I):Cu(II) ratio for low and high pH solutions.
- iii.
- Development of statistical models to predict i and ii.
- iv.
- Calculating K value by experimentation and using MINTEQ.
2. Materials and Methods
2.1. Scoping Experiments
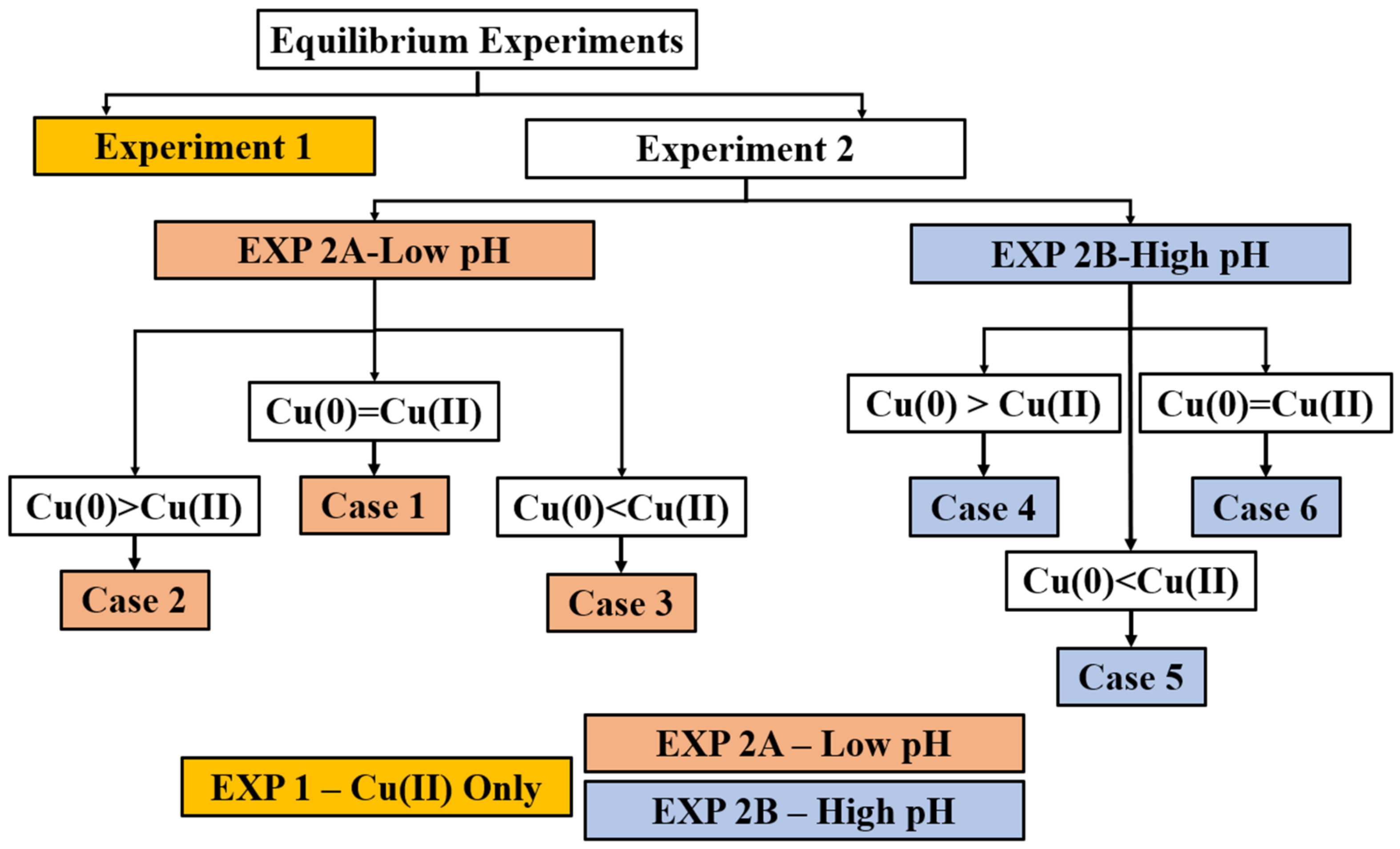
2.2. Experimental Classification
- Low pH (5.04–7.91)—2A
- ⚬
- Case 1: Cu(II) at Saturation = Cu(0)—Saturation balance between Cu(I) and Cu(II).
- ⚬
- Case 2: Cu(II) at Saturation < Cu(0)—Oxidizer limitation.
- ⚬
- Case 3: Cu(II) at Saturation > Cu(0)—Might show saturation balance or ran out of Cu(0)
- High pH (8.02–10.41)—2B
- ⚬
- Case 4: Cu(II) Insufficient < Cu(0) Surplus—It shows thermodynamic equilibrium between Cu(II) and Cu(I).
- ⚬
- Case 5: Cu(II) > Cu(0) Insufficient—Due to stoichiometry the Cu(0) is the limiting reagent.
- ⚬
- Case 6: Cu(II) Insufficient = Cu(0)—Thermodynamic limits.
2.3. Materials
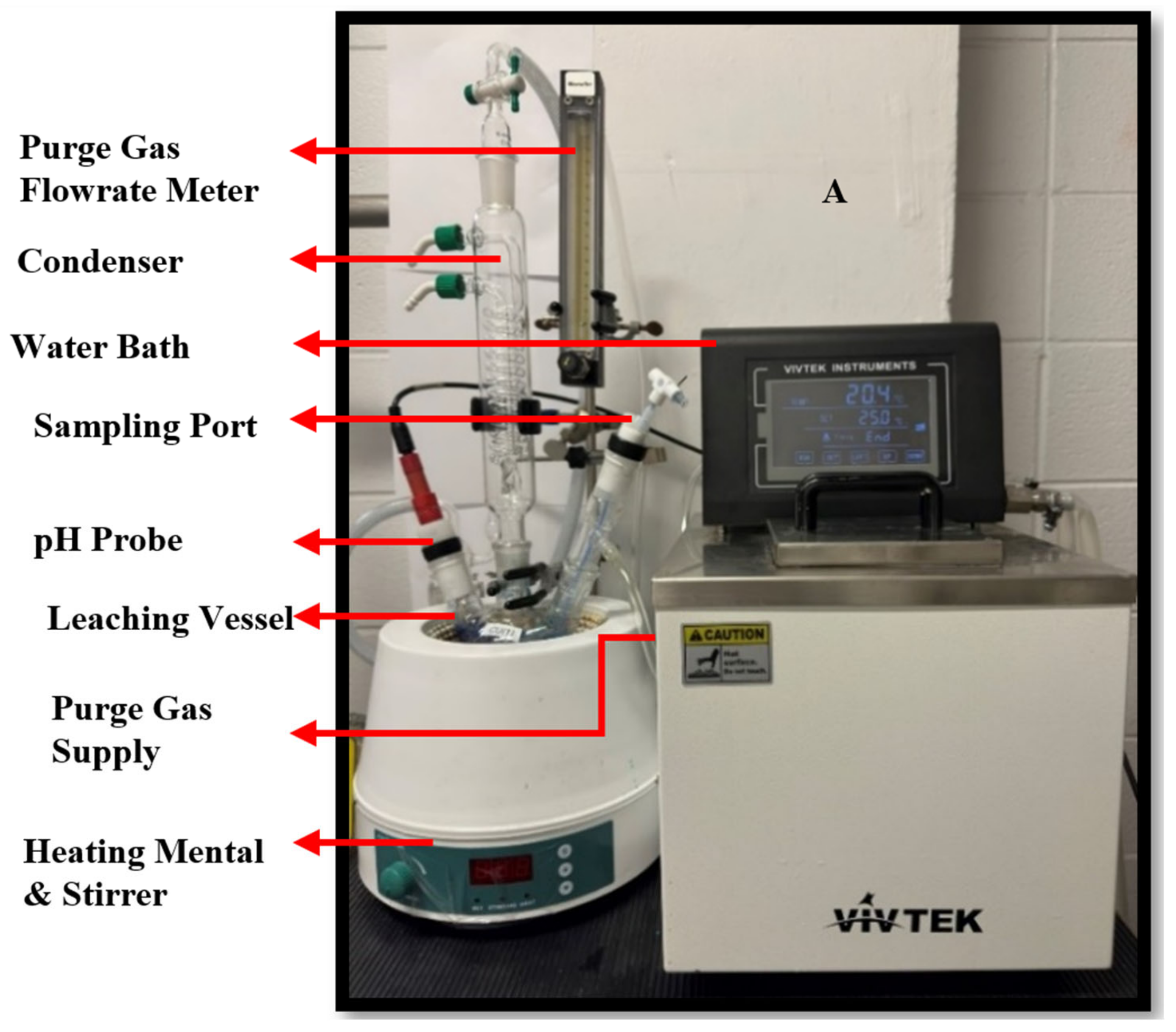
2.4. Experimental Setup and Procedure
2.5. Theoretical and Experimental Equilibrium Constant “K”
2.6. UV-Vis Spectrometry and ICP-OES Analysis
3. Results and Discussion
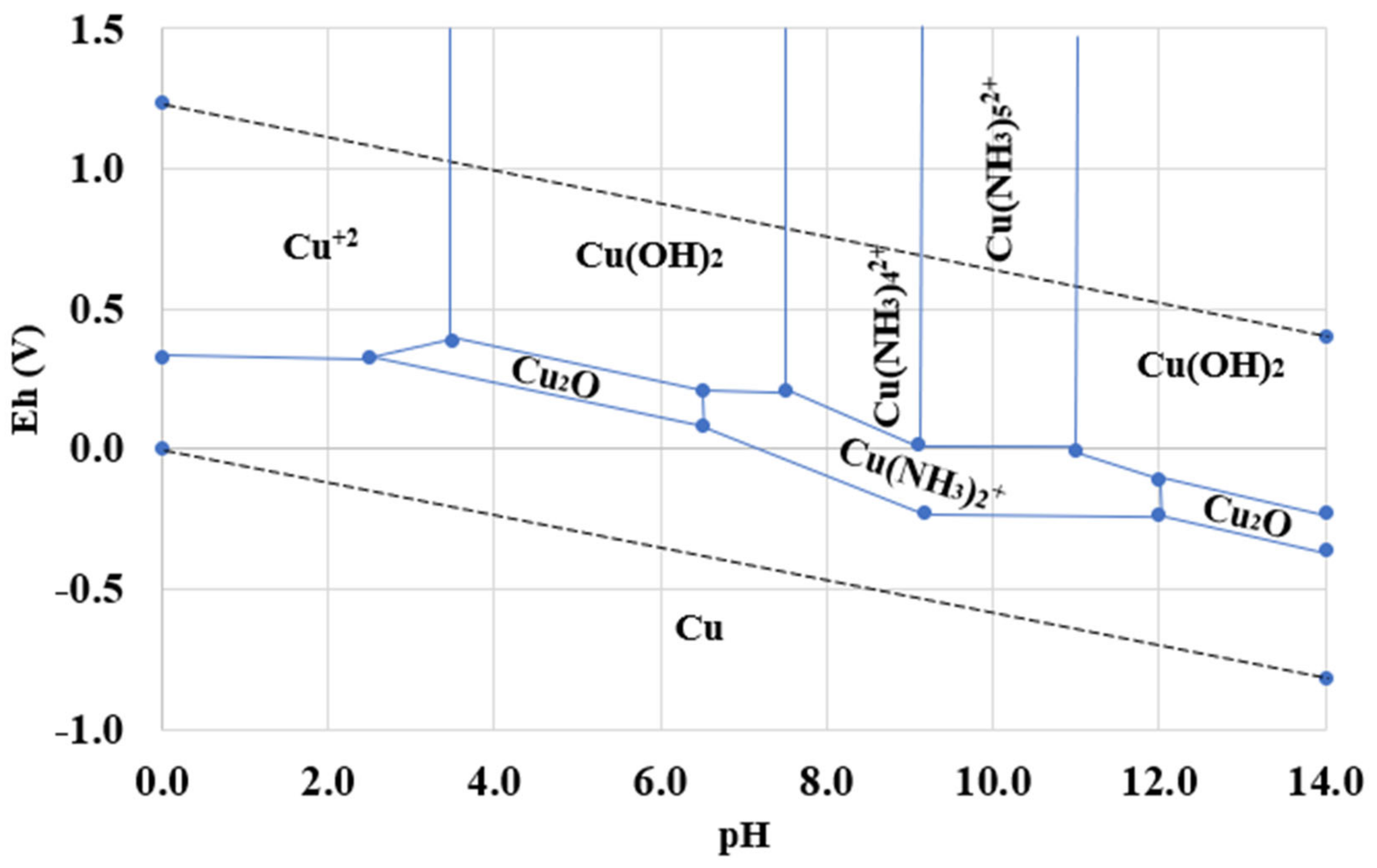
3.1. Pourbaix Diagram
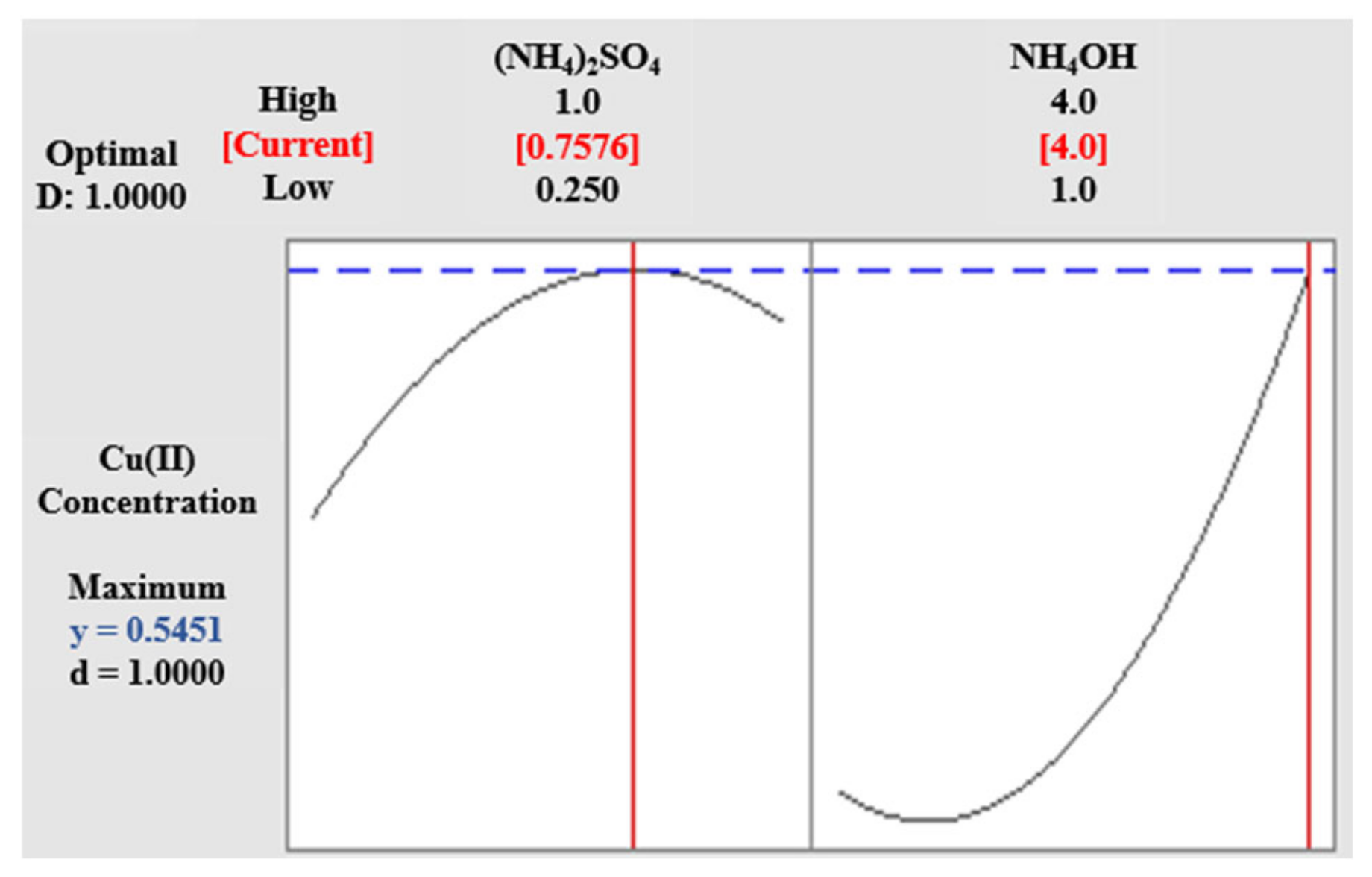
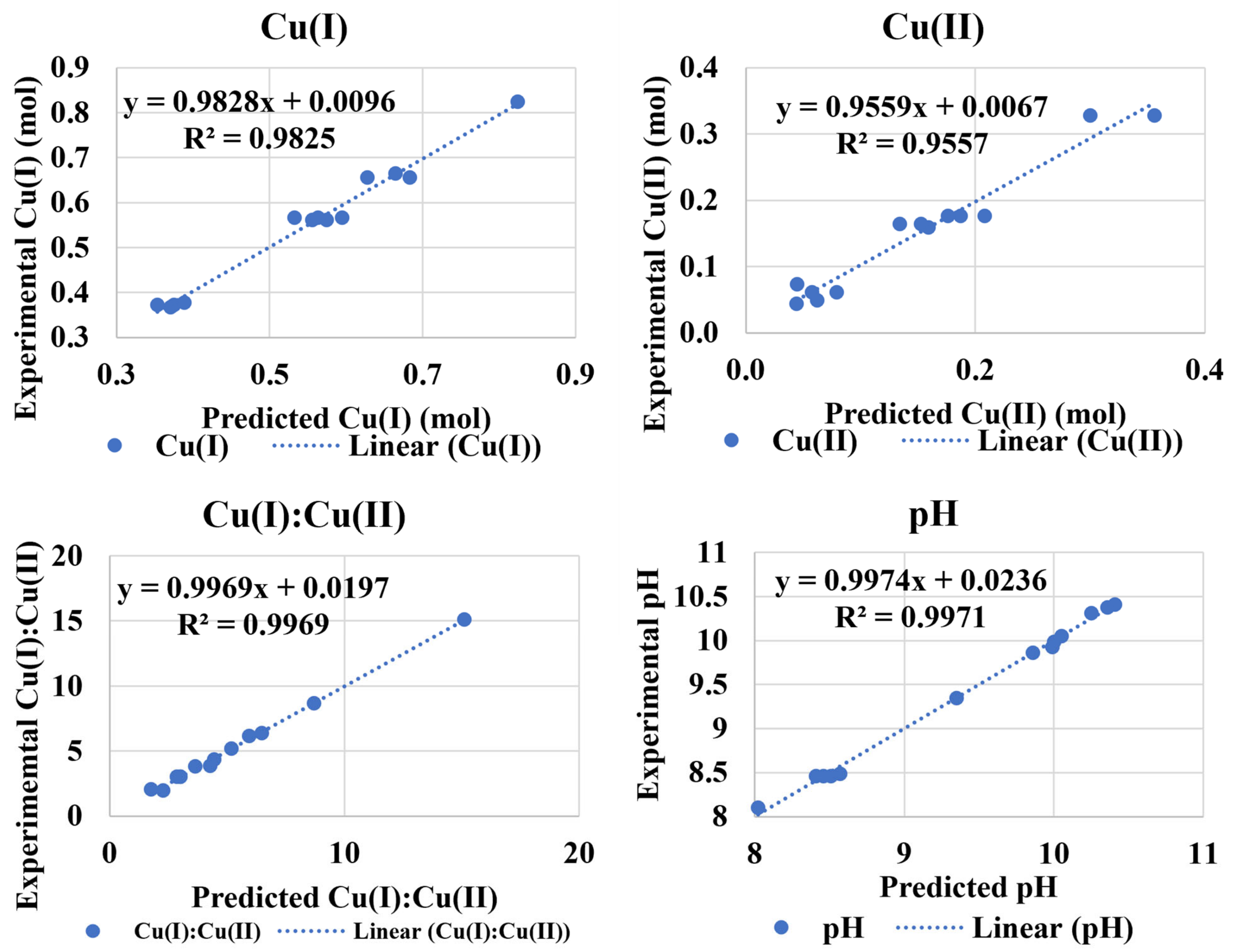
3.2. Cu(II) Solubility (Experiment 1)
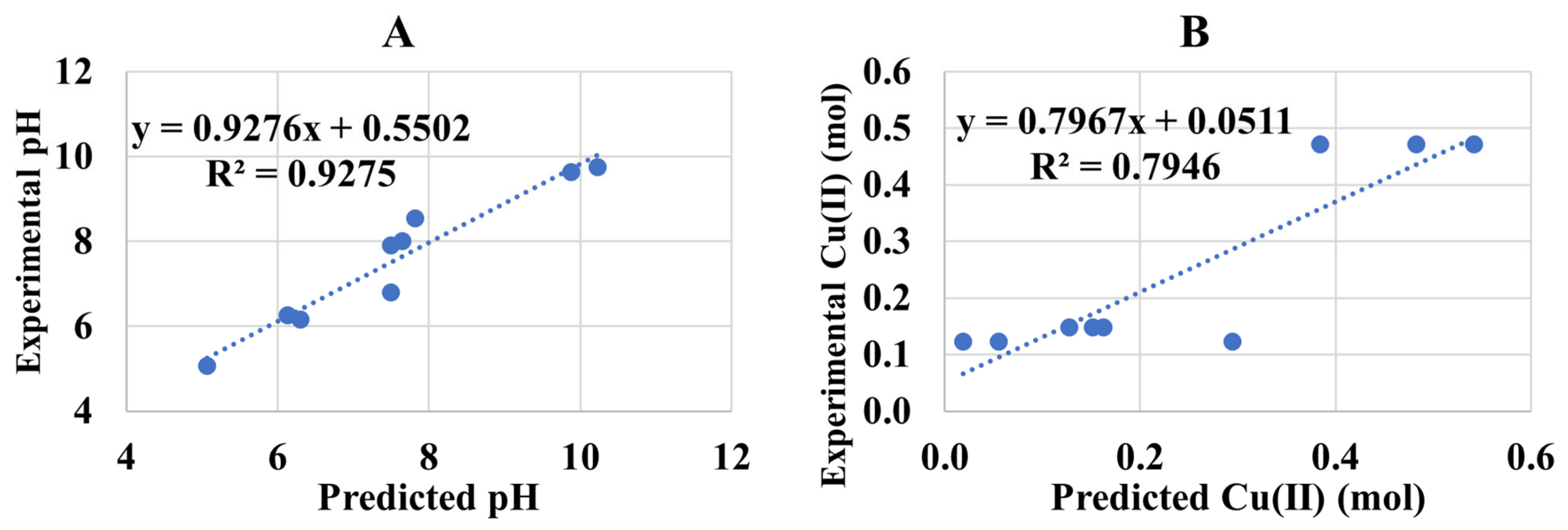
3.3. Cu(I):Cu(II) at Low pH (Experiment 2A)
Adj. R2 = 97.14%
Adj. R2 = 84.60%
Adj. R2 = 94.14%
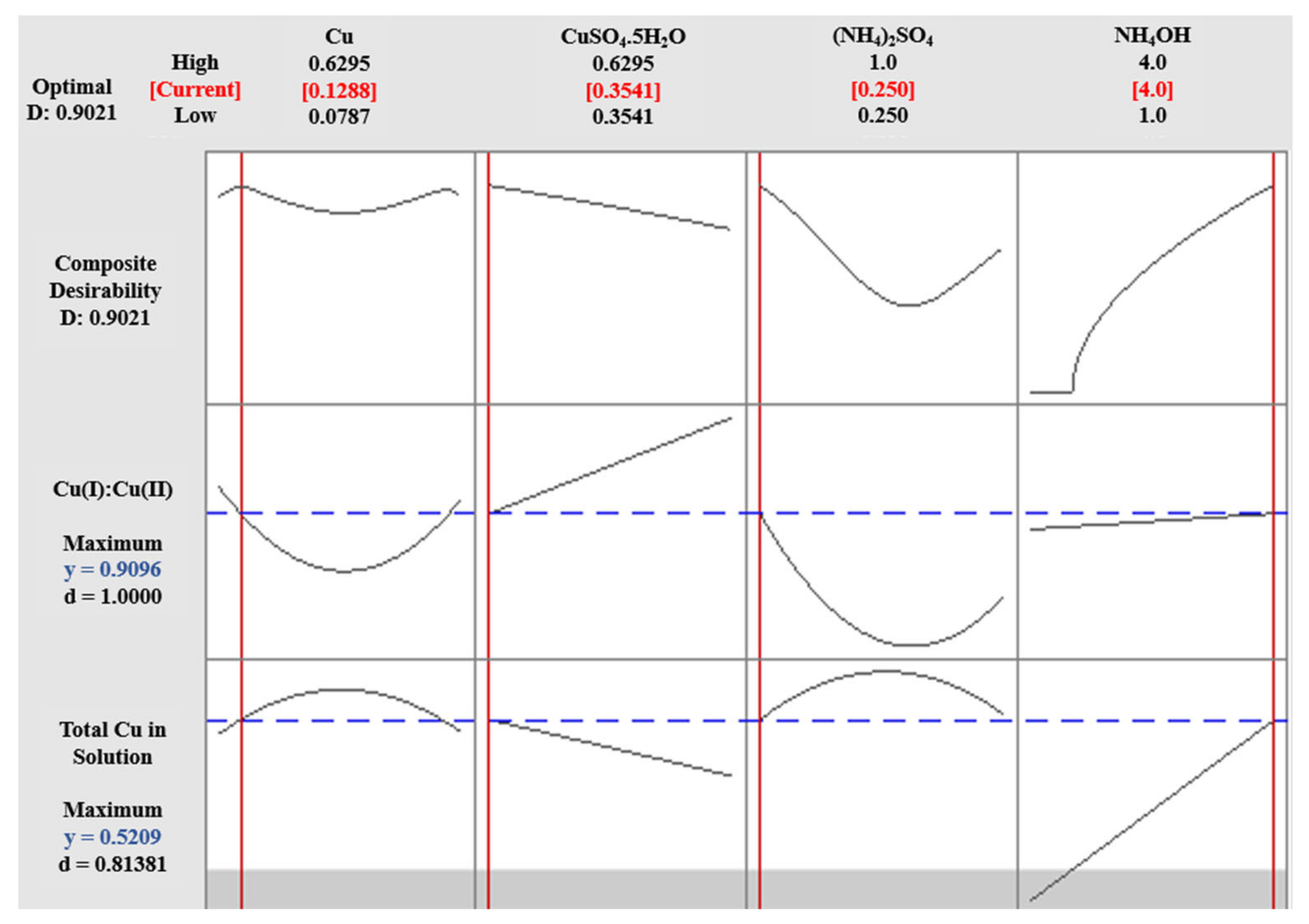
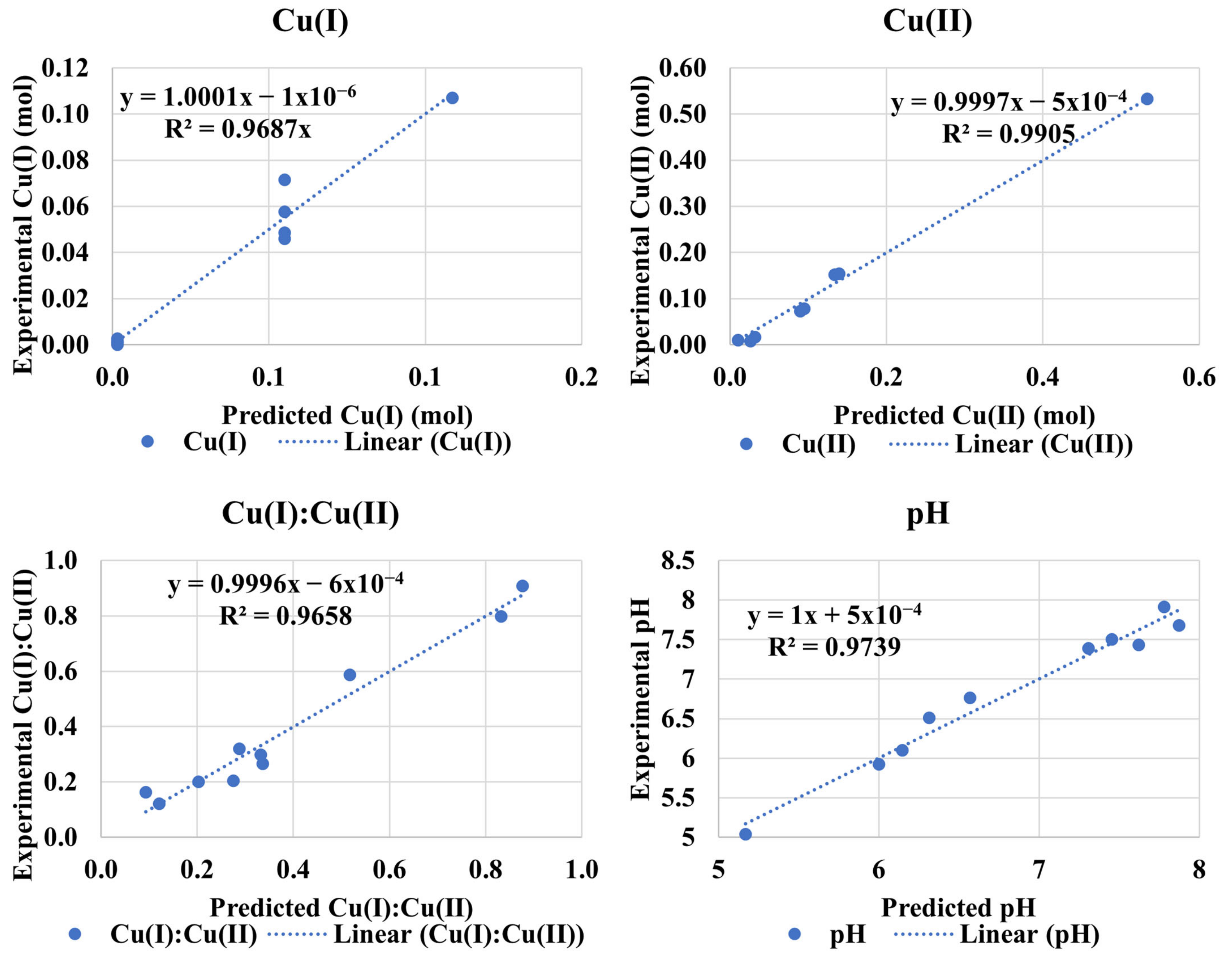
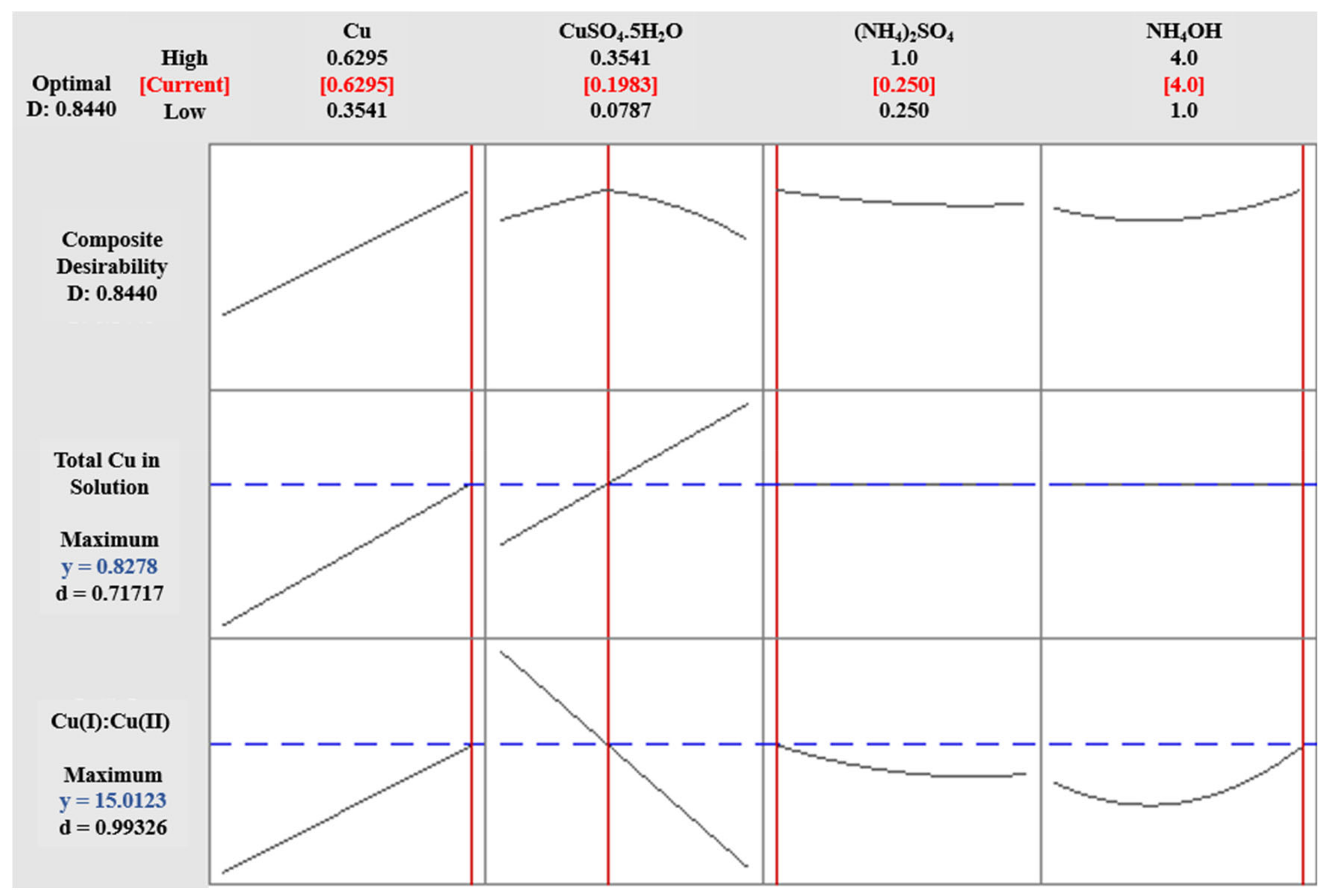
3.4. Cu(I):Cu(II) at High pH (Experiment 2B)
Adj. R2 = 96.99%
Adj. R2 = 92.41%
Adj. R2 = 99.07%
Adj. R2 = 99.13%
3.5. Equilibrium Constant (K)
Adj. R2 = 93.04%
Adj. R2 = 94.87%
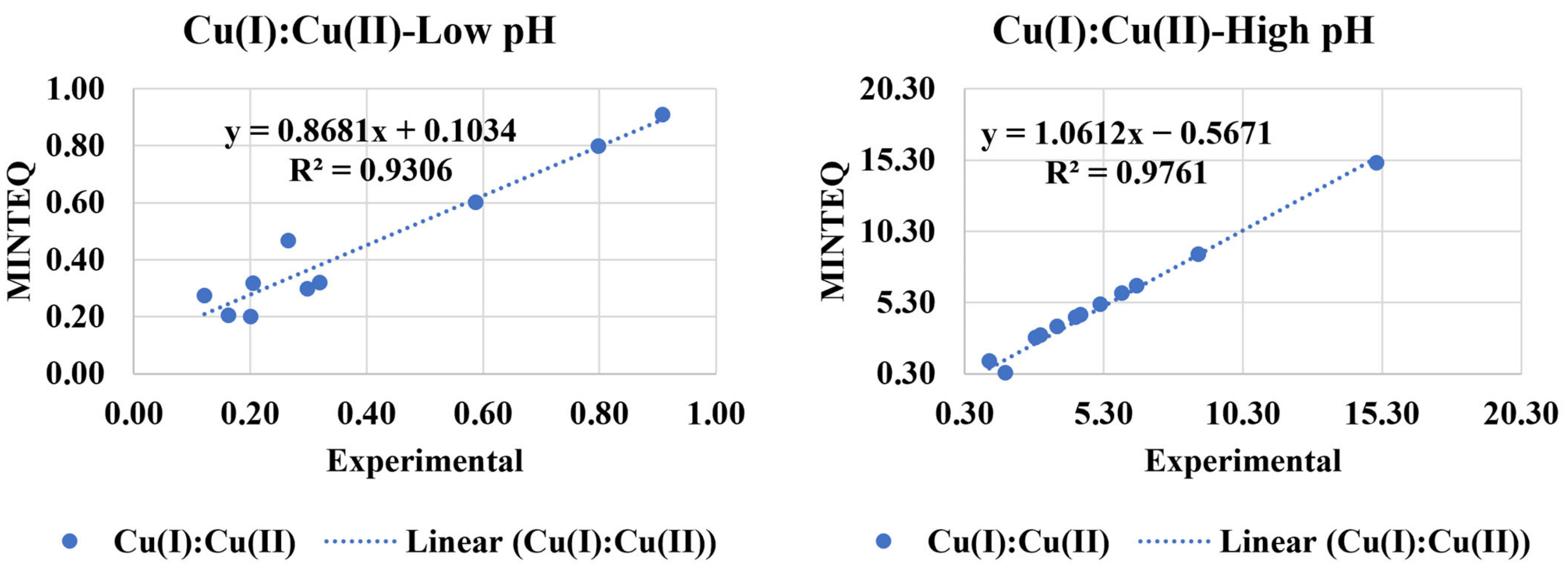
3.6. Cu(I):Cu(II) Using Visual MINTEQ
4. Conclusions
5. Future Work
- A more detailed study focusing on the effects of NH4+ and SO4−2 on the solubility of copper in the copper-ammonia-sulfate system is still needed. This area presents promising research opportunities for future research work.
- Most of the available speciation software databases lack different Cu+1 and Cu+2 complexes with ammonia. There is a need for detailed fundamental studies to determine the thermodynamic properties of respective different Cu+1 and Cu+2 complexes with ammonia in order to update the databases.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, Y.; Kwon, O.H.; Lee, J.; Yoo, K. The ammonia leaching of alloy produced from waste printed circuit boards smelting process. Geosyst. Eng. 2013, 16, 216–224. [Google Scholar] [CrossRef]
- Oishi, T.; Koyama, K.; Alam, S.; Tanaka, M.; Lee, J.C. Recovery of high purity copper cathode from printed circuit boards using ammoniacal sulfate or chloride solutions. Hydrometallurgy 2007, 89, 82–88. [Google Scholar] [CrossRef]
- Ramos, A.; Miranda-Hernández, M.; González, I. Influence of Chloride and Nitrate Anions on Copper Electrodeposition in Ammonia Media. J. Electrochem. Soc. 2001, 148, C315. [Google Scholar] [CrossRef]
- Rudnik, E.; Pierzynka, M.; Handzlik, P. Ammoniacal leaching and recovery of copper from alloyed low-grade e-waste. J. Mater. Cycles Waste Manag. 2016, 18, 318–328. [Google Scholar] [CrossRef]
- Koyama, K.; Tanaka, M.; Miyasaka, Y.; Lee, J.C. Electrolytic copper deposition from ammoniacal alkaline solution containing Cu(I). Mater. Trans. 2006, 47, 2076–2080. [Google Scholar] [CrossRef]
- Oishi, T.; Koyama, K.; Konishi, H.; Tanaka, M.; Lee, J.C. Influence of ammonium salt on electrowinning of copper from ammoniacal alkaline solutions. Electrochim. Acta 2007, 53, 127–132. [Google Scholar] [CrossRef]
- Werner, J.; Bertucci, L.; Hubert, K.; Ali, Z. Extraction of Copper from a Feed Material for the Production of Metallic Copper; World Intellectual Property Organization: Alexandria, VA, USA, 2024. [Google Scholar]
- Sun, Z.H.I.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Visser, G.; Yang, Y. Selective copper recovery from complex mixtures of end-of-life electronic products with ammonia-based solution. Hydrometallurgy 2015, 152, 91–99. [Google Scholar] [CrossRef]
- Radmehr, V.; Koleini, S.M.J.; Khalesi, M.R.; Tavakoli Mohammadi, M.R. Ammonia Leaching: A New Approach of Copper Industry in Hydrometallurgical Processes. J. Inst. Eng. (India) Ser. D 2013, 94, 95–104. [Google Scholar] [CrossRef]
- Konishi, H.; Bitoh, T.; Ono, H.; Oishi, T.; Koyama, K.; Tanaka, M. Behavior of Copper Dissolution in an Ammonia Solution Containing Ammonium Chloride or Sulfate. J. Jpn. Soc. Exp. Mech. 2014, 14, s205–s209. [Google Scholar]
- Koyama, K.; Tanaka, M.; Lee, J.C. Copper leaching behavior from waste printed circuit board in ammoniacal alkaline solution. Mater. Trans. 2006, 47, 1788–1792. [Google Scholar] [CrossRef]
- Sun, Z.H.I.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Yang, Y. Complex electronic waste treatment—An effective process to selectively recover copper with solutions containing different ammonium salts. Waste Manag. 2016, 57, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Werner, J.; Ali, Z.A.; Bertucci, L.; Groppo, J. Kinetics and Modeling of Counter-Current Leaching of Waste Random-Access Memory Chips in a Cu-NH3-SO4 System Utilizing Cu(II) as an Oxidizer. Materials 2023, 16, 6274. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Yévenes, L.; Ram, R. The aqueous chemistry of the copper-ammonia system and its implications for the sustainable recovery of copper. Clean. Eng. Technol. 2022, 9, 100515. [Google Scholar] [CrossRef]
- Meng, X.; Han, K.N. Principles and applications of ammonia leaching of metals—A review. Miner. Process. Extr. Metall. Rev. 1996, 16, 23–61. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Venkatesan, P.; Jin, W.; Xiao, Y.; Sietsma, J.; Yang, Y. Electrochemistry during efficient copper recovery from complex electronic waste using ammonia based solutions. Front. Chem. Sci. Eng. 2017, 11, 308–316. [Google Scholar] [CrossRef]
- Clarkson, A.H.; Paine, S.W.; Kendall, N.R. Evaluation of the solubility of a range of copper sources and the effects of iron & sulphur on copper solubility under rumen simulated conditions. J. Trace Elem. Med. Biol. 2021, 68, 126815. [Google Scholar] [CrossRef]
- Oishi, T.; Koyama, K.; Tanaka, M.; Lee, J.C. Influence of electrolyte on an energy-saving copper recycling process using ammoniacal alkaline solutions. Mater. Trans. 2006, 47, 2871–2876. [Google Scholar] [CrossRef][Green Version]
- Guspita, D.; Ulianas, A. Optimization of complex NH3 with Cu2+ ions to determine levels of ammonia by UV-Vis spectrophotometer. J. Phys. Conf. Ser. 2020, 1481, 012040. [Google Scholar] [CrossRef]
- Nila, C.; Gonzfilez, I. The role of pH and Cu(II) concentration in the electrodeposition of Cu(II) in NH4Cl solutions. J. Electroanal. Chem. 1996, 401, 171–182. [Google Scholar] [CrossRef]
- Lin, P.; Ali, Z.A.; Werner, J. Investigation of the Bimodal Leaching Response of RAM Chip Gold Fingers in Ammonia Thiosulfate Solution. Materials 2023, 16, 4940. [Google Scholar] [CrossRef]
- Halpern, J. Kinetics of the Dissolution of Copper in Aqueous Ammonia. J. Electrochem. Soc. 1953, 100, 421. [Google Scholar] [CrossRef]
- Free, M. Hydrometallurgy: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]

| Reaction | logK |
|---|---|
| Cu2+ + NH3 → Cu(NH3)2+ | 4.19 |
| Cu2+ + 2NH3 → Cu(NH3)22+ | 7.74 |
| Cu2+ + 3NH3 → Cu(NH3)32+ | 10.69 |
| Cu2+ + 4NH3 → Cu(NH3)42+ | 12.88 |
| Cu2+ + 5NH3 → Cu(NH3)52+ | 12.88 |
| Cu+ + NH3 → Cu(NH3)+ | 5.78 |
| Cu+ + 2NH3 → Cu(NH3)2+ | 10.56 |
| Species | (J/mol) | (J/mol) | S0 (J/mol·K) |
|---|---|---|---|
| Cu(NH3)+ | −10,469 | 149.5 | |
| Cu(NH3)2+ | −65,285 | 242.5 | |
| Cu(NH3)2+ | 15,578 | −38,945 | 12.1 |
| Cu(NH3)22+ | −30,486 | −142,378 | 111.4 |
| Cu(NH3)32+ | −73,199 | −245,812 | 199.7 |
| Cu(NH3)42+ | −111,390 | −348,827 | 273.9 |
| Component | Units | Lower Limit | Center Point | Upper Limit |
|---|---|---|---|---|
| (NH4)2SO4 | (mol) | 0.25 | 0.625 | 1.00 |
| NH4OH | (mol) | 1.00 | 2.5 | 4.00 |
| Constant | ||||
| CuSO4.5H2O | (mol) | 0.6295 | ||
| Temperature | (°C) | 25.0 ± 1.0 | ||
| Component | Units | Lower Limit | Center Point | Upper Limit | |
|---|---|---|---|---|---|
| (NH4)2SO4 | (mol) | 0.25 | 0.625 | 1.00 | |
| NH4OH | (mol) | 1.00 | 2.5 | 4.00 | |
| Metallic Copper | (mol) | 0.0787 | 0.3541 | 0.6295 | |
| Constant | Units | Lower Limit | Upper Limit | ||
| CuSO4.5H2O | (mol) | 0.3541 | 0.6295 | ||
| Temperature | (°C) | 25.0 ± 1 | |||
| Component | Units | Lower Limit | Center Point | Upper Limit | |
|---|---|---|---|---|---|
| (NH4)2SO4 | (mol) | 0.25 | 0.625 | 1.00 | |
| NH4OH | (mol) | 1.00 | 2.5 | 4.00 | |
| Cu-Components | Units | Lower Limit | Upper Limit | ||
| CuSO4.5H2O | (mol) | 0.0787 | 0.3541 | ||
| Metallic Copper | (mol) | 0.3541 | 0.6295 | ||
| Temperature | (°C) | 25.0 ± 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, Z.A.; Werner, J.M. Optimization of Copper-Ammonia-Sulfate Electrolyte for Maximizing Cu(I):Cu(II) Ratio Using pH and Copper Solubility. Waste 2024, 2, 397-413. https://doi.org/10.3390/waste2040022
Ali ZA, Werner JM. Optimization of Copper-Ammonia-Sulfate Electrolyte for Maximizing Cu(I):Cu(II) Ratio Using pH and Copper Solubility. Waste. 2024; 2(4):397-413. https://doi.org/10.3390/waste2040022
Chicago/Turabian StyleAli, Zulqarnain Ahmad, and Joshua M. Werner. 2024. "Optimization of Copper-Ammonia-Sulfate Electrolyte for Maximizing Cu(I):Cu(II) Ratio Using pH and Copper Solubility" Waste 2, no. 4: 397-413. https://doi.org/10.3390/waste2040022
APA StyleAli, Z. A., & Werner, J. M. (2024). Optimization of Copper-Ammonia-Sulfate Electrolyte for Maximizing Cu(I):Cu(II) Ratio Using pH and Copper Solubility. Waste, 2(4), 397-413. https://doi.org/10.3390/waste2040022






