1. Introduction
Running is a steady and moderately paced running motion, and it is often considered to be a popular recreational activity and common component of many fitness routines [
1]. It requires the precise coordination and synchronization of various muscles throughout the body [
2]. The complexity and high redundancy of the human musculoskeletal system execute a wide variety of daily tasks [
3]. Understanding the mechanics of motor control during movement can enhance our ability to optimize performance, prevent injuries, and develop effective rehabilitation strategies.
Muscle synergies are characterized by coordinated recruitment of specific muscle groups with precise timing and amplitude synchronization to achieve purposeful movements [
4]. This conceptual framework provides a method to deal with the complexity of motor control and has demonstrated practical applications in various fields [
5,
6,
7]. Although muscle synergies have shown great potential in various fields, some long-standing issues in the methodology of synergy analyses should be considered. No standardized criteriaregarding the selection and number of muscles have been included in studies [
8], and the processing of EMG signals [
9] makes a direct comparison and interpretation of the literature so far difficult.
Previous studies have typically focused on measuring EMGs within a limited range of eight to nineteen muscles in the arm [
10,
11], or eight to sixteen muscles during walking [
12,
13]. However, relying on a subset of muscles for a muscle synergy analysis may not fully capture the recruitment and activation patterns of all the relevant muscles [
8,
14]. Notably, the number and selection of these muscles, especially deep muscles, are essential for the results of a muscle synergy analysis [
15]. Deep muscles play acrucial role in running by providing stability, control, and efficient force transmission throughout the running motion. Deep muscles are particularly important for accurate muscle synergy analyses, and understanding the importance of deep muscles in running has practical implications for training and injury prevention.
In summary, the purpose of this study was to determine the influence of these deep muscles on a muscle synergy analysis during running. This investigation allowed us to gain insights into the influence of muscle selection on the analysis of muscle synergies and provided a basis for a more comprehensive understanding the complexity of motor control. This analysis would shed light on muscle synergy and its contribution to running, providing valuable insights into the underlying mechanisms, optimizing training strategies, and running performance.
2. Materials and Methods
We utilized publicly available datasets [
16] to analyze the running activity of one subject. The dataset consisted of ten channels of sEMG signals and kinematic and kinetic data while running at a speed of 2 m/s.
Table 1 presents the measurement of the sEMG signals and the simulated activations of twenty-three muscles, including the deep muscles. To obtain simulated muscle activations, an open-source platform OpenSim [
17] simulation was applied to calculate the activation patterns of the various muscles involved in lower limb movements, specifically the hip, knee, and ankle. In addition to the 10 sEMG measurements, we incorporated thirteen deep muscles into the synergy analysis. By integrating surface EMG measurements and simulated deep muscle activations, we calculated and examined the synergy among these 23 muscles. This approach enabled us to capture the complete information on the muscle activations during running. While data from one subject were used in this study, they provide valuable information regarding muscle synergies during running at a speed of 2 m/s.
To obtain the sEMG envelope signals, which can reflect the level of lower extremity muscle contraction,, the sEMG signals of a single-gait cycle were digitally filtered at 50 Hz with a zero-phase 4th-order Butterworth filter, full-wave rectified, and low-pass filtered at 10 Hz with a zero-phase 4th-order Butterworth filter. The envelopes were normalized to the maximum value of each muscle.
Muscle synergies were applied via a non-negative matrix factorization (NMF) algorithm to extract from the sEMGs and simulated muscle-activated signals [
6,
10]. NMF enabled the decomposition of the sEMG signals into muscle synergy vectors (
W) and temporal activity patterns (
C) through a linear combination, as illustrated by the following equation:
Here, E is an m×n representing the pre-processed EMG matrix recorded from m muscles. W is an m×s synergy matrix with reduced dimensions. The variable s represents the number of synergies to be identified from the data. C is an m×n a matrix with s activation coefficients, representing the temporal activity patterns corresponding to each synergy. Finally, e denotes the residual matrix, which accounts for any remaining noise.
To reconstruct the sEMG signals and determine the minimum number of required muscle synergies, the variability accounted for (VAF) criterion was employed [
14]. A fixed threshold of VAF > 92% was used as the basis on the VAF curve to determine the number of muscle synergies that needed to be extracted.
3. Results
Our study identified four synergies that accounted for 92% of the total variance in the muscle activity when ten muscles were analyzed. These muscle synergies, represented by synergy vectors W1–W4 (
Figure 1), are essential for the coordination of muscle actions during running. The activation of the Gasmed1 and Tibant muscles primarily characterizes synergy vector 1. These muscles contribute to hip abduction and dorsiflexion. The synergy activation of these muscles is essential to stabilizing the pelvis and hip joints during running, providing significant leg propulsion, and ensuring balance and postural stability throughout running. Synergy Vector 2 is mainly characterized by the synchronized activation of the Gasmax1, Gasmed1, Soleus, Vaslat, and Vasmed muscles, contributing to hip abduction, hip extension, and knee extension actions. This synergy helps to maintain stability, generate power, and prevent excessive joint movement or rotation during running. Synergy vector 3 is characterized by the activation of the hamstrings and soleus muscles. The coordinated activation of these muscles contributes to specific aspects of running mechanics. Synergy vector 4 primarily consists of the activity of Bflth and Glamax, which contribute to knee flexion and hip extension.
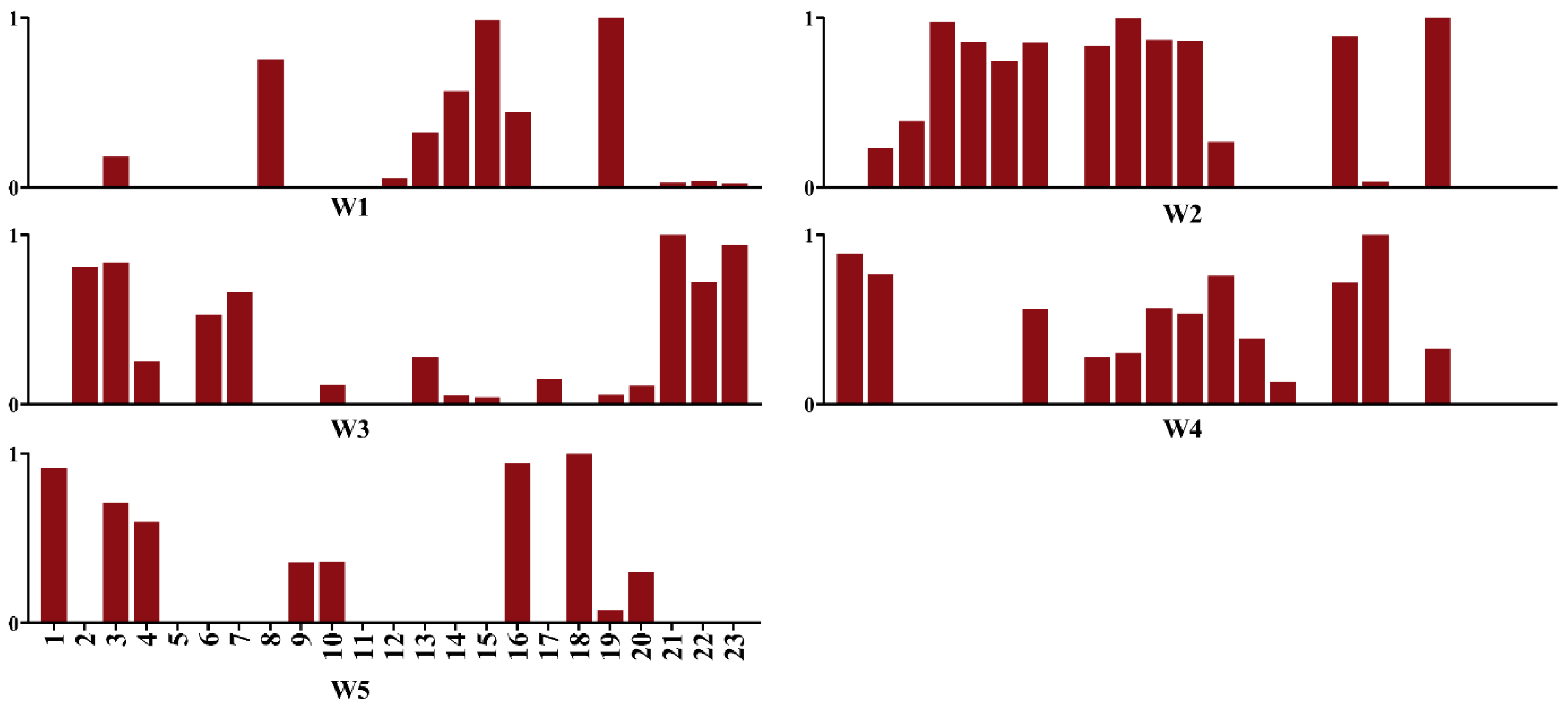
Then, we calculated from all 23 muscles, including 13 deep muscles, to identify the muscle synergies. As shown in
Figure 2, the results showed that these five synergies accounted for 92% of the variability in muscle activation during running. Importantly, when comparing the results obtained with the 23 muscles to those obtained with 10 muscles, we observed differences in the grouping and relative weights of the muscles. The simulation study further demonstrated the contribution of the deep muscles to muscle synergy analysis, leading to the identification of an additional synergy vector. Notably, deep dominant muscles in the synergy vectors highlighted the inclusion of muscles not considered in the experimental analysis, such as the Semimem and Semiten muscles. These findings underscore the importance of considering a comprehensive set of muscles, including deep muscles, to capture the full complexity of muscle synergies in the context of the activity being studied.
4. Conclusions
In this study, we examined the impact of the selection of muscles on a muscle synergy analysis. Specifically, we compared the results obtained from 23 simulated muscles with the traditional approach of using recorded sEMG signals. Additional synergies were observed when more deep muscles were involved in the analysis. This suggests that deeper muscle activation patterns and coordination play a significant role in the overall muscle synergy dynamics during a task. These results highlight the importance of considering the contribution of deep muscles when studying muscle synergies and provide further insights into the complexity of motor control during the examined activity. Neglecting specific muscles may lead to an underestimation of the complexity of the muscle coordination, which could have implications for both laboratory research and clinical applications. It is important to note that these preliminary findings warrant further investigation, using high-density experimental data for validation.
Author Contributions
Y.C.: conceptualization, methodology, data curation, writing—original draft preparation. W.C.: writing—review and editing. Y.W.: validation; H.L.: conception and design of the word. X.W.: editing. R.L.: supervision, writing, editing, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Science and Technology Research Project of Education Department of Liaoning Province under Grant jyt-dldxfw202005, in part by the Dalian Mentoring Program Project in the field of life and health under Grant 2022ZXYG28, and in part by the China Postdoctoral Science Foundation Funded Project under Grant 2020M670714.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Lai, A.; Lichtwark, G.A.; Schache, A.G.; Lin, Y.-C.; Brown, N.A.; Pandy, M.G. In vivo behavior of the human soleus muscle with increasing walking and running speeds. J. Appl. Physiol. 2015, 118, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Hamill, J.; Busa, M.A.; Emmerik, R. Changes in coordination and variability during running as a function of head stability demands. Hum. Mov. Sci. 2020, 73, 102673. [Google Scholar] [CrossRef] [PubMed]
- Stanev, D.; Moustakas, K. Modeling musculoskeletal kinematic and dynamic redundancy using null space projection. PLoS ONE 2019, 14, e0209171. [Google Scholar] [CrossRef] [PubMed]
- Shuman, B.R.; Goudriaan, M.; Desloovere, K.; Schwartz, M.H.; Steele, K.M. Muscle Synergy Constraints Do Not Improve Estimates of Muscle Activity from Static Optimization During Gait for Unimpaired Children or Children with Cerebral Palsy. Front. Neurorobotics 2019, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- d’Avella, A.; Fernandez, L.; Portone, A.; Lacquaniti, F. Modulation of phasic and tonic muscle synergies with reaching direction and speed. J. Neurophysiol. 2008, 100, 1433–1454. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Zoppirolli, C.; Bortolan, L.; Schena, F.; Pellegrini, B.; Rainoldi, A. Muscle synergies and activation in Nordic walking compared with conventional walking. Gait Posture 2017, 57, 26–27. [Google Scholar] [CrossRef]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The number and choice of muscles impact the results of muscle synergy analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [PubMed]
- Shuman, B.R.; Schwartz, M.H.; Steele, K.M. Electromyography Data Processing Impacts Muscle Synergies during Gait for Unimpaired Children and Children with Cerebral Palsy. Front. Comput. Neurosci. 2017, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- d’Avella, A.; Portone, A.; Fernandez, L.; Lacquaniti, F. Control of fast-reaching movements by muscle synergy combinations. J. Neurosci. 2006, 26, 7791–7810. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rymer, W.Z.; Beer, R.F. Robustness of muscle synergies underlying three-dimensional force generation at the hand in healthy humans. J. Neurophysiol. 2012, 107, 2123–2142. [Google Scholar] [CrossRef] [PubMed]
- Torres-Oviedo, G.; Macpherson, J.M.; Ting, L.H. Muscle synergy organization is robust across a variety of postural perturbations. J. Neurophysiol. 2006, 96, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Safavynia, S.A.; Torres-Oviedo, G.; Ting, L.H. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top. Spinal Cord Inj. Rehabil. 2011, 17, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Turpin, N.A.; Uriac, S.; Dalleau, G. How to improve the muscle synergy analysis methodology? Eur. J. Appl. Physiol. 2021, 121, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Tomita, A.; Ando, R.; Watanabe, K.; Akima, H. Similarity of muscle synergies extracted from the lower limb including the deep muscles between level and uphill treadmill walking. Gait Posture 2018, 59, 134–139. [Google Scholar] [PubMed]
- Hamner, S.R.; Delp, S.L. Muscle contributions to fore-aft and vertical body mass center accelerations over a range of running speeds. J. Biomech. 2013, 46, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Thelen, D.G. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).