Abstract
Background: Achondroplasia is a genetic condition that results in several orofacial and physical manifestations that predispose patients to dental, breathing, and sleep issues. Case Description: This report details the case of a 2-year-old girl with achondroplasia, malocclusion, speech and breathing dysfunction, and obstructive sleep apnea (OSA). Treatment involved using a myofunctional device that required chewing twice per day, two simple tongue exercises, speech-language therapy, chiropractic therapy, and continuous positive airway pressure (CPAP) for 24 months. Throughout the treatment period, the patient demonstrated significant improvements in her sleep, breathing, dental occlusion, speech, and overall confidence. Conclusion: This case report highlights how pediatric patients with achondroplasia can improve their OSA symptoms and health with conservative myofunctional therapy interventions.
INTRODUCTION
Achondroplasia is a genetic condition associated with a mutation in the fibroblast growth factor receptor 3 (FGFR3) gene. This results in impaired chondrocyte proliferation and maturation leading to decreased endochondral ossification and reduced bone growth (Rohilla et al., 2012). Achondroplasia results in several physical manifestations including stenosis of the foramen magnum, macrocephaly, mid-face hypoplasia, middle ear deformation, frontal bossing, reduced mandibular growth, and shortened limbs (Chhabra et al., 2016).
From a dental and airway perspective, the complications that patients with achondroplasia develop include increased risk of malocclusion, acute and chronic otitis media, and increased risk of obstructive sleep apnea (OSA) (Savarirayan et al., 2021). If these symptoms go unaddressed, they can lead to further complications such as cardiovascular disease, metabolic syndrome, and neurocognitive impairment (Cao et al., 2017). It is therefore essential to address the cause of these symptoms by providing long-term solutions and improving the patient’s health.
The gold standard intervention that is commonly used for sleep disordered breathing and OSA in adults is continuous positive airway pressure (CPAP) (Gay et al., 2006). Despite its wide acceptance, patients commonly complain of dryness and embarrassment when using the device, but also its tendency to increase central apneas for adult patients (Hopper & Cramer, 2023). In addition, children suffer from a significant reduction in the magnitude and direction of skeletal facial growth from prolonged CPAP use, which is exacerbated in patients with achondroplasia who already have skeletal deficiencies (Cao et al., 2017; Villa et al., 2002; Li et al., 2000). For these reasons, it is becoming more common for practitioners to address the cause of OSA symptoms and use conservative therapies such as orofacial myofunctional therapy (OMT) to improve patient outcomes (Chwieśko-Minarowska et al., 2013; De Dios & Brass, 2012; Guimarães et al., 2009; Valbuza et al., 2010; Villa et al., 2014).
OMT is demonstrated to increase oral and pharyngeal muscle tone, which improves airway patency during sleep and reduces OSA (Koka, 2021). In addition, OMT is also an important therapy to treat craniofacial and dental occlusion deficiencies, which are highly prevalent in patients with achondroplasia (Chuang et al., 2019; Benkert, 1997). It is widely adopted in OMT practices for practitioners to teach patients about correct head posture, resting tongue position on the palate, appropriate swallowing patterns, and bilateral chewing (Camacho et al., 2015; Guimarães et al., 2009). The importance of correct swallowing and chewing is also essential for managing food textures and improving nutrition, as evidenced in studies of patients with cerebral palsy (Arslan et al., 2017).
Patients with OSA and achondroplasia commonly present with forward head posture and thoracolumbar kyphosis which may negatively affect airway patency (Hopper & Cramer, 2023; Kopits, 1988). These clinical symptoms have shown to be improved with chiropractic care through spinal manipulative therapy (SMT) (Hopper & Cramer, 2023). Speech-language therapy is another complimentary therapy to improve hearing and speech impairments that result from craniofacial deficiencies and chronic otitis media (Savarirayan et al., 2021; Rosenfeld et al., 2016).
There is currently no known existing literature on the effect of chewable oral devices as an early intervention myofunctional tool for patients with achondroplasia and OSA. This case report is unique in how it addresses the underlying causes of the patient’s symptoms, and avoids invasive surgical and orthodontic treatments, which are commonly recommended (Pineau et al., 2018). This case therefore highlights a potential pathway for conservative and simple interventions for patients with achondroplasia.
Patient Information
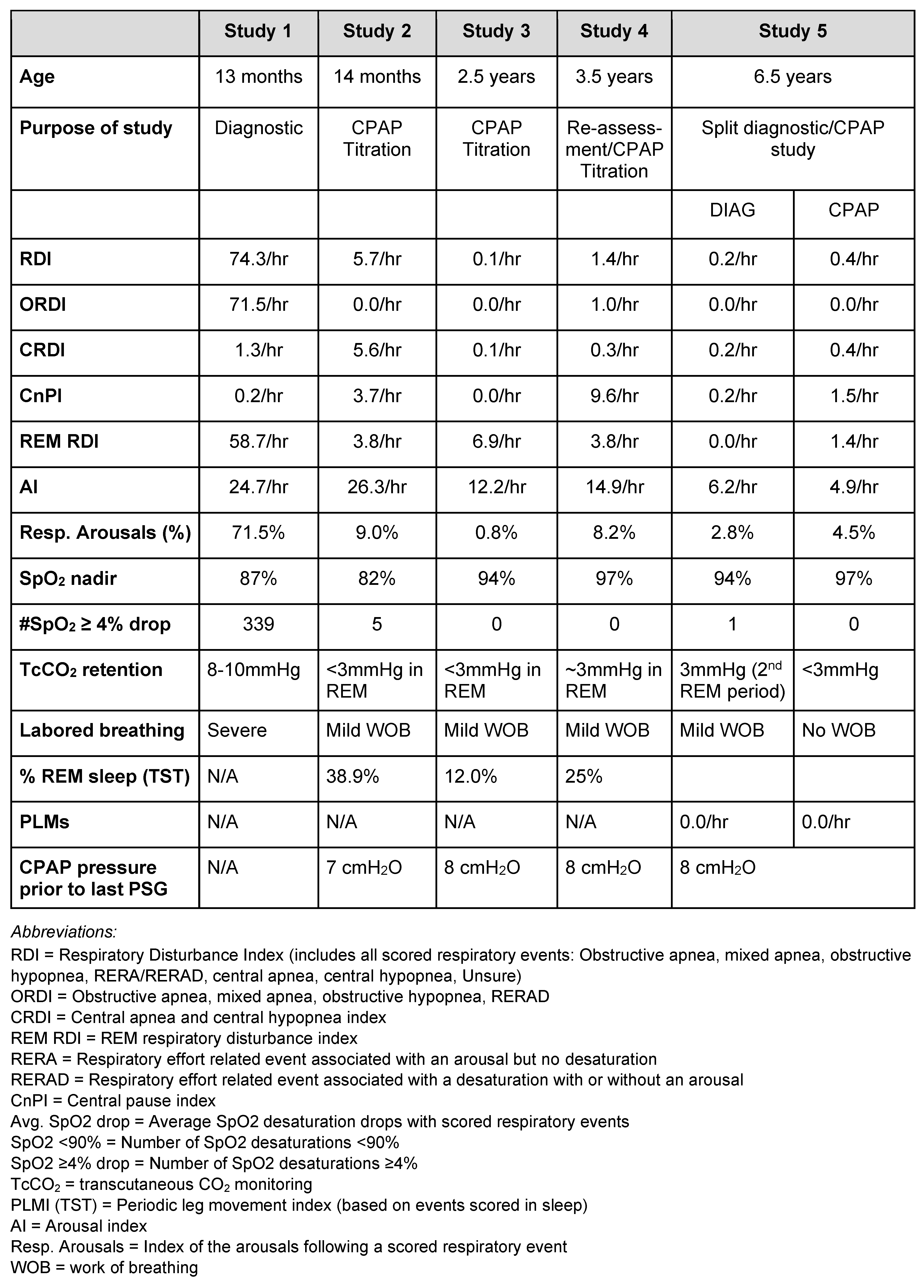
The patient is a 2.5-year-old female of Anglo-Saxon ancestry who was born in Australia. She is the youngest of three siblings and was diagnosed with achondroplasia 3 days after birth. There was no familial history of the condition. The patient was diagnosed at 13 months with OSA after a sleep study showed a respiratory disturbance index (RDI) (74.3/hr) and obstructive RDI (ORDI) (71.5/hr) (Table 1). The sleep specialists overseeing the patient’s care recommended CPAP, which demonstrated efficacy (Table 1). The specialist also recommended a tonsillectomy and tympanostomy, as routinely indicated for patients with achondroplasia (Hunter et al., 1998).

Table 1.
Comparison of sleep study results before and after myofunctional therapy and CPAP treatment.
Despite their child receiving effective CPAP therapy, the parents still reported frequent mouth breathing during sleep, night sweats, snoring, and neck hyperextension. Daytime symptoms included an inability to swallow solid food, significant speech delay, and complaints of ear discomfort. The patient’s parents were seeking broader clinical opinions and sought an initial consultation with a myofunctional dentist to address the patient’s symptoms and diagnosis.
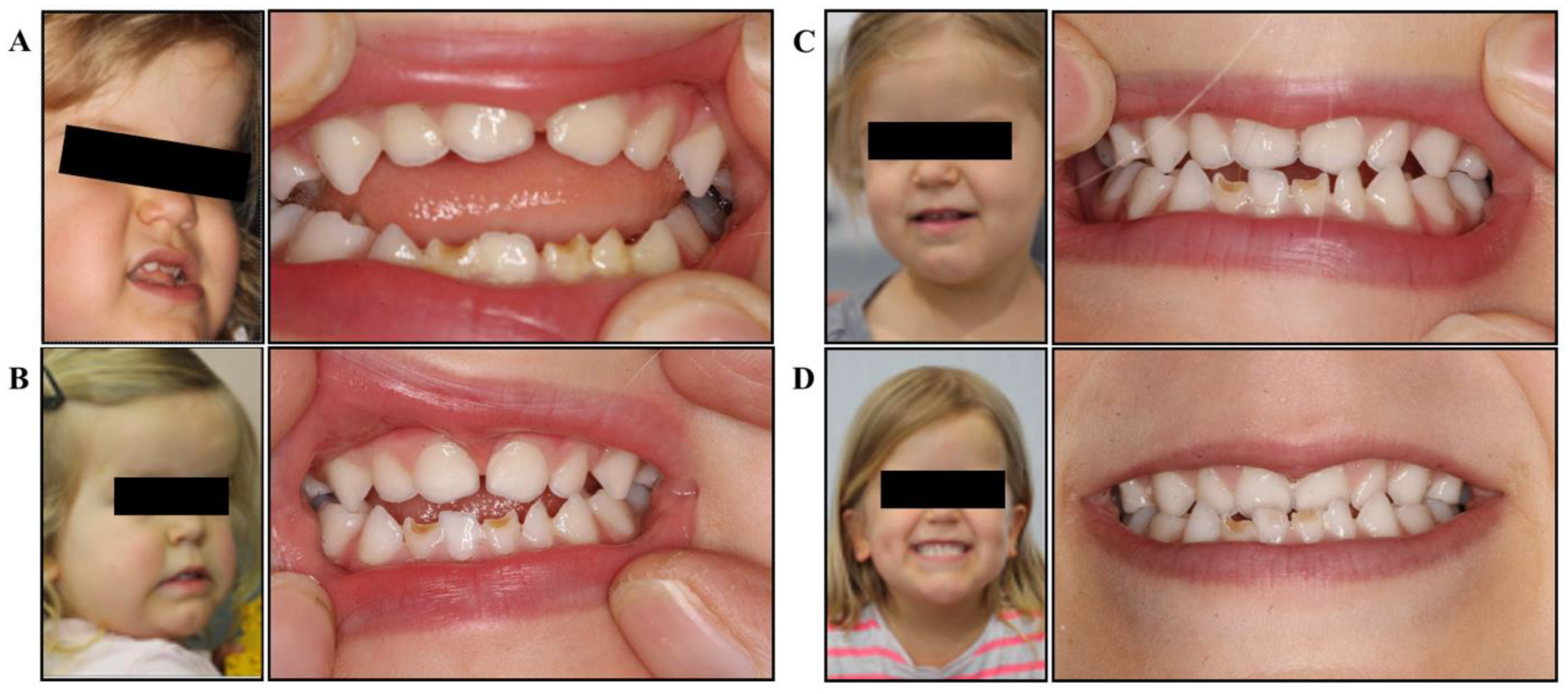
She was referred to a myofunctional dental clinic via the child’s chiropractor at 2.5 years of age. Upon presentation to the myofunctional dentist, the patient presented with typical signs and symptoms of achondroplasia (Figure 1a).
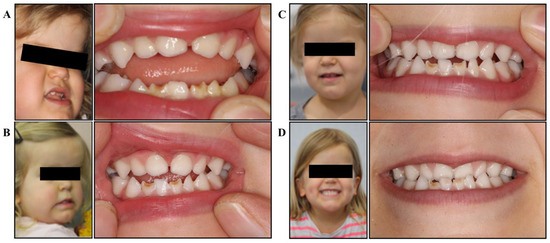
Figure 1.
Facial development and dental occlusion. (A) Initial presentation: deficient mid-face, dental crowding and protruding mandible, significant open bite, tongue thrust and low resting tongue posture; (B) after 6 months of treatment: slight improvements in mid-face development, improved orofacial tone, open bite closing, improved posterior teeth contact, improved tongue position; (C) after 12 months of treatment: significant mid-face development, improved facial symmetry, closed bite (into underbite), improved lip tone and control; (D) after 24 months of treatment: facial development becoming even more symmetrical, improved tongue control, edge-to-edge bite, maxillary development improving, upper arch growth.
Clinical Presentation
The myofunctional dentist assessed the patient at 2.5 years of age and documented the following clinical presentation (Figure 1a):
- Anterior open bite
- Hypoplasic lower anterior teeth
- Forward tongue rest position and tongue thrust on swallow
- Mouth breathing
- Poor lip and oral tone
- Open bite
- Borderline non-verbal with extremely minimal speech. Parents needed to interpret the sounds the patient made and communicate this to the practitioner
- Typical features of patient with achondroplasia including macrocephaly, mid-face hypoplasia, anterior cross bite, frontal bossing, and protrusive mandible
- Extremely introverted disposition
- Patient chewed pacifier
Assessments and Therapeutic Intervention
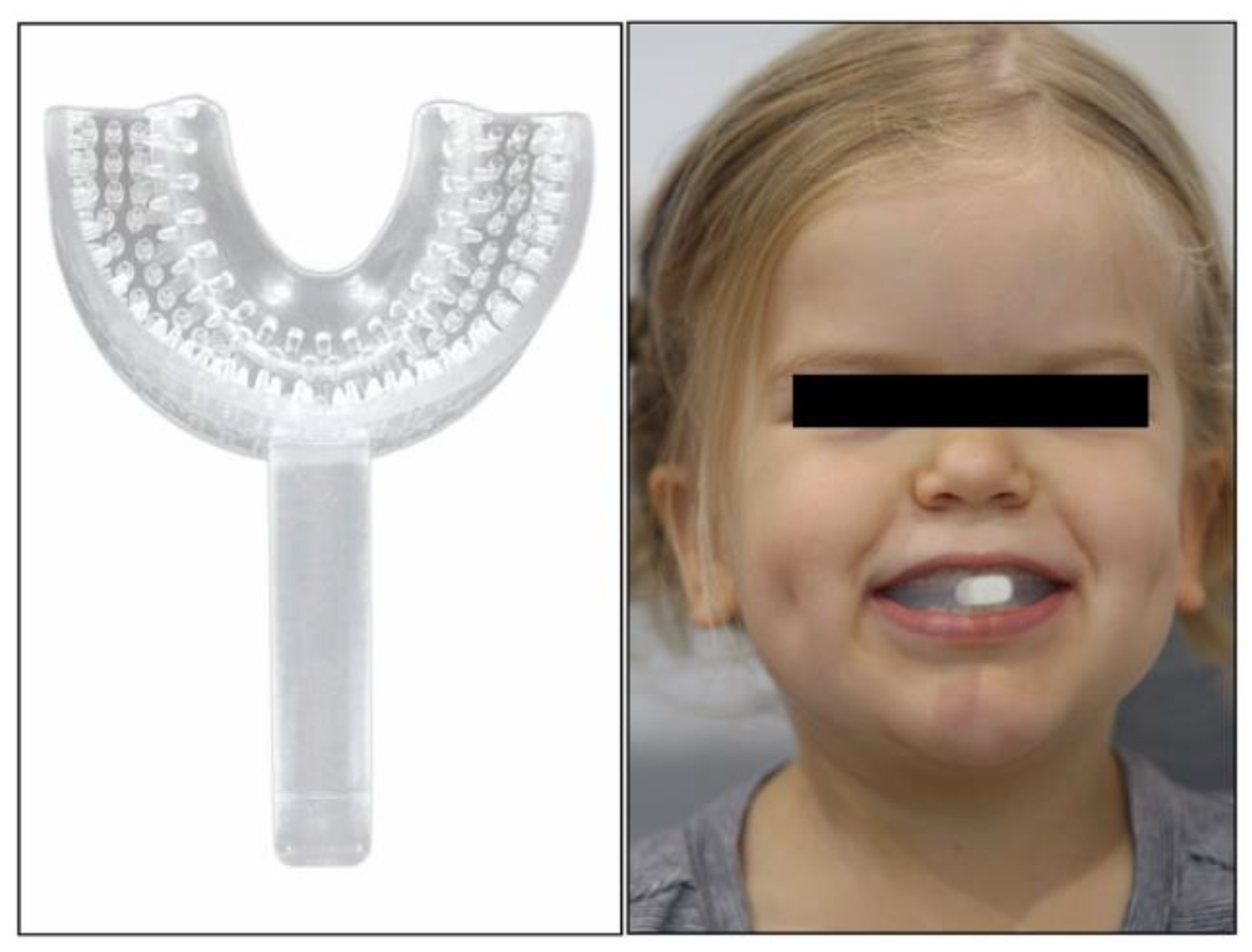
During the initial examination, the myofunctional dentist devised a protocol to improve the patient’s symptoms. The protocol, listed here, included the prescription of a “Mini” and “Small” myofunctional chewing device (Figure 2, Myo Munchee, Carrington, Australia) and tongue exercises:
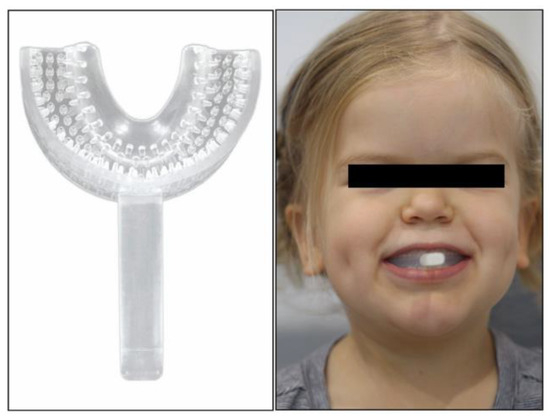
Figure 2.
Myo Munchee, myofunctional chewing device, being chewed by the patient.
- Replace pacifier with Myo Munchee chewing.
- Myo Munchee chewing: Block practice of chewing twice per day, building to 10 minutes of active use each session.
- Tongue exercises: repeat each exercise 10 times 2x per day.
- Make /n/ sound by placing tongue tip at incisive papilla.
- Perform tongue clicks with tongue tip at the incisive papilla.
- The Myo Munchee size was changed at 18 months from a “Mini” size to a “Small” size. There was no change to the rest of this protocol.
- Progress was evaluated every 6 months during follow-up appointments, and treatment was modified as necessary.
- Adherence was assessed when the patient visited the clinic. Patient was highly adherent as witnessed by parents and dentist. She did not require any extrinsic motivators to complete treatments.
The myofunctional dental treatment was in combination with chiropractic and speech-language pathology treatment, as described below:
- Chiropractic
- Assessments: pelvic and spinal assessments
- Treatment: low force technique and ArthostimTM instrument adjustment of spine and pelvis, and extremity mobilization.
- Timeline: initiated within the first month of life
- Frequency of appointments: every 3-4 weeks.
- Speech-language pathology
- Assessments:
- Preschool Language Scales Fifth Edition (PLS-5) (Zimmerman et al., 2002)
- The Articulation Survey (Atkin & Fisher, 1996)
- Treatment: rounding of lips for /ʃ/ (“sh”), adding word-final consonants, improve contact of lips to ensure bilabial production, encourage speaking and engagement with others
- Timeline: received care over 24-month period
- Frequency of appointments: every 2-4 weeks
Results and Outcomes
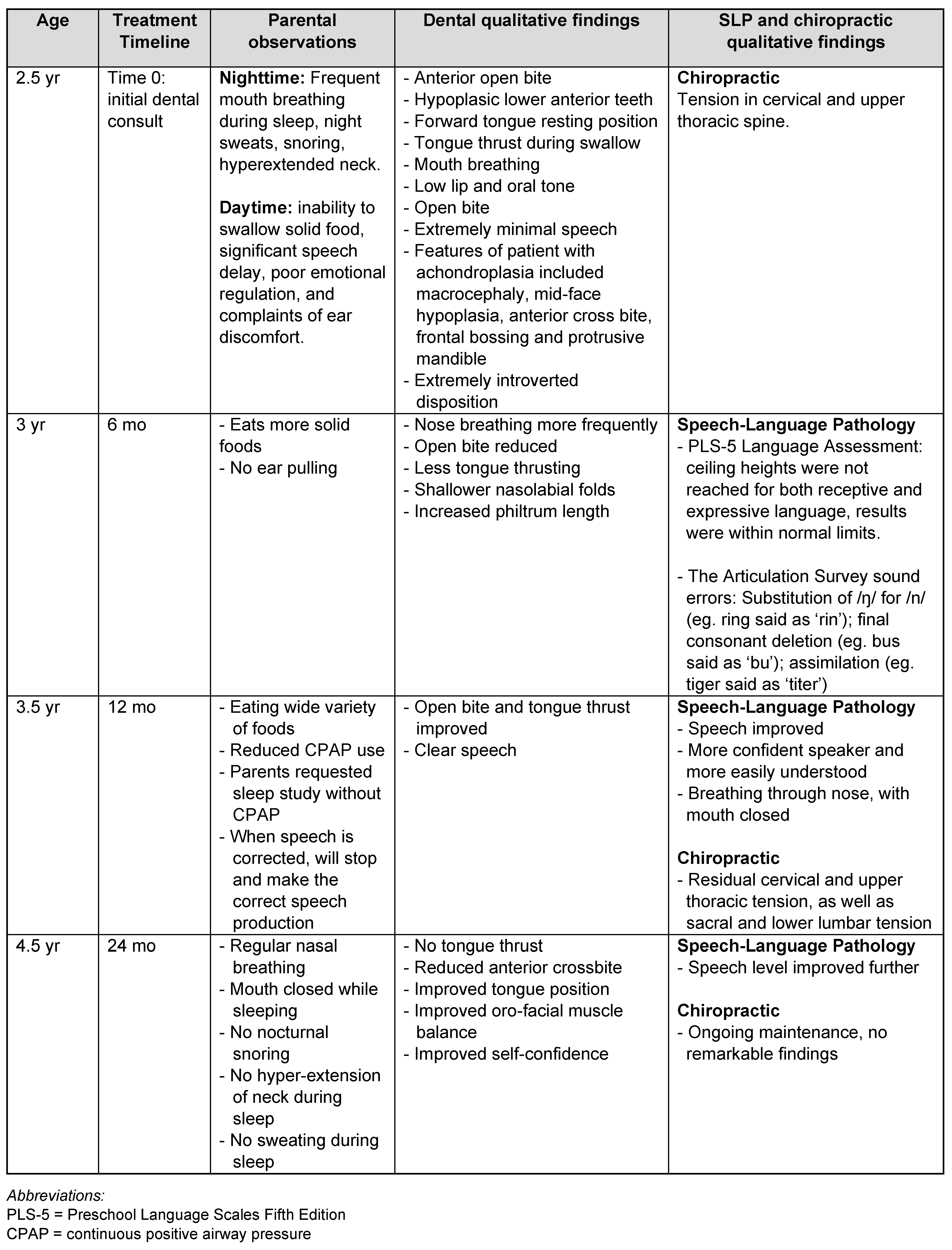
Over the course of the treatment, all practitioners observed an improvement in the patient’s symptoms from her initial presentation (Table 2 and Figure 1). In particular, the patient’s occlusion, tongue thrust, and resting mouth posture all improved over the 24- month period. Furthermore, the speech-language pathologist noted a significant improvement in the patient’s language and articulation, as well as her confidence in communicating (Table 2).

Table 2.
Timeline of parental and practitioner observations and measurements of patient’s outcomes.
The sleep-study results (Table 1) revealed substantial sleep improvements from the myofunctional therapy. After only 12 months of treatment, obstructive sleep events decreased significantly from sleep study 1 (ORDI 71.5/hr) to sleep study 4 (ORDI 1.0/hr). Furthermore, the arousal index (14.9/hr) and oximetry (nadir 97%) improved. After 48 months of treatment, the diagnostic part of study 5 showed that there was no significant obstruction (ORDI 0.0/hr), no elevation of arousal index, normal oximetry (nadir 94%), and normal transcutaneous CO2 (TcCO2) baseline with a 3mmHg rise in the 2nd REM period. There were no significant central apneas or periodic limb movements. Additionally, the sleep specialist recorded nasal airflow, snoring and occasional stridor, and mild work of breathing (WOB) during sleep. During the CPAP part of Study 5, titration between 7-8 cmH2O resolved all snoring and WOB. Furthermore, the sleep specialist noted a reduction in tonsil and adenoid size, removing the indication for tympanostomy insertion. These results demonstrate the efficacy of combining CPAP and myofunctional therapy for improving the sleep of patients with achondroplasia and OSA.
Parents’ perspectives
The parents’ primary objective when seeking treatment was to reduce the amount and severity of medical intervention. Namely, they sought to avoid tonsillectomy and tympanostomy insertion, and to prevent orthodontic surgery in the future. While tonsillectomy and tympanostomy are indicated and recommended for patients with achondroplasia, the parents wanted to try conservative treatment as an alternative (Hunter et al., 1998). They also researched palatal expansion as a method for correcting sleep-breathing problems while typically being advised that nothing could be done while the child was so young.
Discovering how easy the myofunctional chewing device was to implement, the parents were motivated to try the simple and non-invasive treatment, especially as it was age appropriate and very easy for their child. For these parents, understanding functional approaches to treatment and the impact on their child’s health were significant motivators. From the positive outcomes in speech, breathing, and sleep, the parents have become advocates of the myofunctional chewing device as an inexpensive and conservative intervention for special needs children.
Discussion
This case presents a novel approach of how a myofunctional chewing device can be used to improve OSA, dental and speech deficiencies for pediatric patients with achondroplasia. This case highlights a simple, non-invasive and accessible 24- month intervention. In a relatively short period of time, the practitioners and parents saw significant improvements in the patient’s sleep, ear health, oral muscle balance, occlusion, swallowing, and speech.
With a combination of therapies (OMT, dental, chiropractic, sleep, and speech-language pathology), the patient improved her initial presenting symptoms considerably. We propose that the treatment had the following effects on the patient. Firstly, as demonstrated by previous studies that assessed the impact of OMT and chewing on oral musculature, the patient chewing the myofunctional device and performing the simple tongue exercises likely improved her tongue and lip tone and function (Koka, 2021; Chuang et al., 2019; Benkert, 1997; Camacho et al., 2015; Guimarães et al., 2009). As described in the myofunctional dentist’s qualitative findings (Table 2), the patient improved her ability to close the lips and rest the tongue at the roof of the palate to facilitate nasal breathing, improved her swallowing function by increasing muscle tone, and eliminated tongue thrusting. As evidenced in the literature, the improvements in oral muscle tone likely contributed to the positive development of her occlusion, avoiding tympanostomy, and reducing OSA severity (Chwieśko-Minarowska et al., 2013; De Dios & Brass, 2012; Guimarães et al., 2009; Valbuza et al., 2010; Villa et al., 2014). Secondly, the speech-language therapy significantly improved the patient’s ability and confidence to communicate, leading to improved engagement with people around her. Finally, the SMT may have improved the patients forward head posture and thoracolumbar kyphosis, leading to improved airway patency and reducing OSA severity (Hopper & Cramer, 2023). These conservative therapies all had a complementary and positive effect on the patient’s airway, oral musculature and communication.
Limitations and Future Studies
Limitations of this study include the involvement of only a single individual, meaning that these results may not be generalizable. Despite rigorous objective medical assessments with sleep and CPAP studies, the clinical assessment by the dentist did not involve standardized assessments, but instead relied on the dentist’s clinical observations. As this is only a brief study involving a 24-month health intervention, it is unclear what the long-term health benefits will be.
For future research, it would be wise to separate each intervention component to understand their individual contributions to the patient’s outcomes. That said, it may be difficult to determine which intervention led to which outcome. Additionally, including a larger treatment cohort would help with supporting the clinical significance and generalizability of the outcomes.
Conclusion
A patient with achondroplasia and OSA responded favorably to a conservative therapeutic approach over a 24-month period. The patient’s dental occlusion, sleep quality, speech, and other health outcomes improved significantly after implement- ing a combination of myofunctional exercises with a myofunctional chewing device, chiropractic, CPAP, and speech therapies.
Author Contributions
Conceptualization: D.M., M.B. Data collection: D.M. Data analysis and interpretation: D.M., M.B., I.B. Writing/manuscript preparation: D.M., M.B., I.B. Critical revision: D.M., M.B., I.B.
Funding
This project received no external funding.
Data Availability Statement
Data generated or analyzed for this project are included in this published article.
Acknowledgments
We appreciate Mr. Cole Clayton’s assistance with manuscript preparation.
Consent to Participate
Informed written consent was provided by the parents of the patient.
Conflicts of Interest
Dr. Donny Mandrawa lectures on behalf of Myo Munchee. Dr. Mary Bourke is the CEO of Myo Munchee. Mr. Ignatius Bourke is an employee of Myo Munchee.
References
- Atkin, M., and J. Fisher. 1996. Articulation Assessment survey—Articulation survey. Melbourne: Royal Children’s Hospital. [Google Scholar]
- Benkert, K. 1997. The effectiveness of orofacial myofunctional therapy in improving dental occlusion. International Journal of Orofacial Myology 23, 1: 35–46. [Google Scholar] [CrossRef]
- Bernkopf, E., G. Cristalli, G. C. de Vincentiis, G. Bernkopf, and V. Capriotti. 2022. Temporomandibular Joint and Otitis Media: A Narrative Review of Implications in Etiopathogenesis and Treatment. Medicina 58, 12: 1806. [Google Scholar] [CrossRef]
- Camacho, M., V. Certal, J. Abdullatif, S. Zaghi, C. M. Ruoff, R. Capasso, and C. A. Kushida. 2015. Myofunctional Therapy to Treat Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Sleep 38, 5: 669–675. [Google Scholar] [CrossRef]
- Cao, M. T., J. M. Sternbach, and C. Guilleminault. 2017. Continuous positive airway pressure therapy in obstuctive sleep apnea: benefits and alternatives. Expert Review of Respiratory Medicine 11, 4: 259–272. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N., A. Chhabra, and R. Mehta. 2016. Craniofacial manifestations and dental considerations in association with achondroplasia: Clinical insight and report of a case. SRM Journal of Research in Dental Sciences 7, 4: 264. [Google Scholar] [CrossRef]
- Chuang, L.-C., Y.-J. Hwang, Y.-C. Lian, M. Hervy-Auboiron, P. Pirelli, Y.-S. Huang, and C. Guilleminault. 2019. Changes in craniofacial and airway morphology as well as quality of life after passive myofunctional therapy in children with obstructive sleep apnea: a comparative cohort study. Sleep and Breathing 23, 4: 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Chwieśko-Minarowska, S., Ł. Minarowski, A. Kuryliszyn-Moskal, J. Chwieśko, and E. Chyczewska. 2013. Rehabilitation of patients with obstructive sleep apnea syndrome. International Journal of Rehabilitation Research. Internationale Zeitschrift Fur Rehabilitationsforschung. Revue Internationale de Recherches de Readaptation 36, 4: 291–297. [Google Scholar] [CrossRef]
- De Dios, J. A. A., and S. D. Brass. 2012. New and Unconventional Treatments for Obstructive Sleep Apnea. Neurotherapeutics 9, 4: 702–709. [Google Scholar] [CrossRef]
- Gay, P., T. Weaver, D. Loube, and C. Iber. 2006. Evaluation of Positive Airway Pressure Treatment for Sleep Related Breathing Disorders in Adults. Sleep 29, 3: 381–401. [Google Scholar] [CrossRef]
- Guimarães, K. C., L. F. Drager, P. R. Genta, B. F. Marcondes, and G. Lorenzi-Filho. 2009. Effects of Oropharyngeal Exercises on Patients with Moderate Obstructive Sleep Apnea Syndrome. American Journal of Respiratory and Critical Care Medicine 179, 10: 962–966. [Google Scholar] [CrossRef]
- Hopper, D. E., and G. Cramer. 2023. Conservative Treatment Using Chiropractic Care and Orofacial Myofunctional Therapy for Obstructive Sleep Apnea: A Case Report. Journal of Chiropractic Medicine 22, 3. [Google Scholar] [CrossRef]
- Hunter, A. G., A. Bankier, J. G. Rogers, D. Sillence, and C. I. Scott. 1998. Medical complications of achondroplasia: a multicentre patient review. Journal of Medical Genetics 35, 9: 705–712. [Google Scholar] [CrossRef]
- Koka, V., A. De Vito, G. Roisman, M. Petitjean, G. R. Filograna Pignatelli, D. Padovani, and W. Randerath. 2021. Orofacial Myofunctional Therapy in Obstructive Sleep Apnea Syndrome: A Pathophysiological Perspective. Medicina 57, 4: 323. [Google Scholar] [CrossRef] [PubMed]
- Kopits, S. E. 1988. Thoracolumbar Kyphosis and Lumbosacral Hyperlordosis in Achondroplastic Children. Springer EBooks: pp. 241–255. [Google Scholar] [CrossRef]
- Li, K. K., R. W. Riley, and C. Guilleminault. 2000. An Unreported Risk in the Use of Home Nasal Continuous Positive Airway Pressure and Home Nasal Ventilation in Children. Chest 117, 3: 916–918. [Google Scholar] [CrossRef]
- Pineau, M., E. Farrow, R. Nicot, and J. Ferri. 2018. Achondroplasia. Journal of Craniofacial Surgery 29, 8: 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, S., A. Kaushik, V. C. Vinod, R. Tanwar, and M. Kumar. 2012. Orofacial manifestations of achondroplasia. EXCLI Journal 11: 538–542. Available online: https://www.ncbi.nlm.nih.gov/pmc/ articles/PMC4897656/.
- Rosenfeld, R. M., J. J. Shin, S. R. Schwartz, R. Coggins, L. Gagnon, J. M. Hackell, D. Hoelting, L. L. Hunter, A. W. Kummer, S. C. Payne, D. S. Poe, M. Veling, P. M. Vila, S. A. Walsh, and M. D. Corrigan. 2016. Clinical Practice Guideline. Otolaryngology–Head and Neck Surgery 154, 2: 201–214. [Google Scholar] [CrossRef]
- Savarirayan, R., P. Ireland, M. Irving, D. Thompson, I. Alves, W. A. R. Baratela, J. Betts, M. B. Bober, S. Boero, J. Briddell, J. Campbell, P. M. Campeau, P. Carl-Innig, M. S. Cheung, M. Cobourne, V. Cormier-Daire, M. Deladure-Molla, M. del Pino, H. Elphick, and V. Fano. 2021. International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nature Reviews Endocrinology 18, 3: 1–17. [Google Scholar] [CrossRef]
- Serel Arslan, S., N. Demir, and A. A. Karaduman. 2016. Effect of a new treatment protocol called Functional Chewing Training on chewing function in children with cerebral palsy: a double-blind randomised controlled trial. Journal of Oral Rehabilitation 44, 1: 43–50. [Google Scholar] [CrossRef]
- Valbuza, J. S., M. M. de Oliveira, C. F. Conti, L. B. F. Prado, L. B. C. de Carvalho, and G. F. do Prado. 2010. Methods for increasing upper airway muscle tonus in treating obstructive sleep apnea: systematic review. Sleep and Breathing 14, 4: 299–305. [Google Scholar] [CrossRef]
- Villa, M. P., L. Brasili, A. Ferretti, O. Vitelli, J. Rabasco, A. R. Mazzotta, N. Pietropaoli, and S. Martella. 2014. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep and Breathing 19, 1: 281–289. [Google Scholar] [CrossRef] [PubMed]
- Villa, M. P., J. Pagani, R. Ambrosio, R. Ronchetti, and E. Bernkopf. 2002. Mid-face hypoplasia after long-term nasal ventilation. American Journal of Respiratory and Critical Care Medicine 166, 8: 1142–1143. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, I. L., V. G. Steiner, and R. E. Pond. 2002. Preschool Language Scale, Fourth Edition. PsycTESTS Dataset. [Google Scholar] [CrossRef]
© 2024 by the authors. 2024 Donny Mandrawa, Mary Bourke, Ignatius Bourke.