Tongue Control for Speech and Swallowing in Healthy Younger and Older Subjects
Abstract
INTRODUCTION
Changes in tongue tissue due to aging
Changes in tongue function due to aging
CURRENT INVESTIGATION
Methods
Participants
Procedures
Instrumentation
Data collection procedures
Data processing
Dependent variables
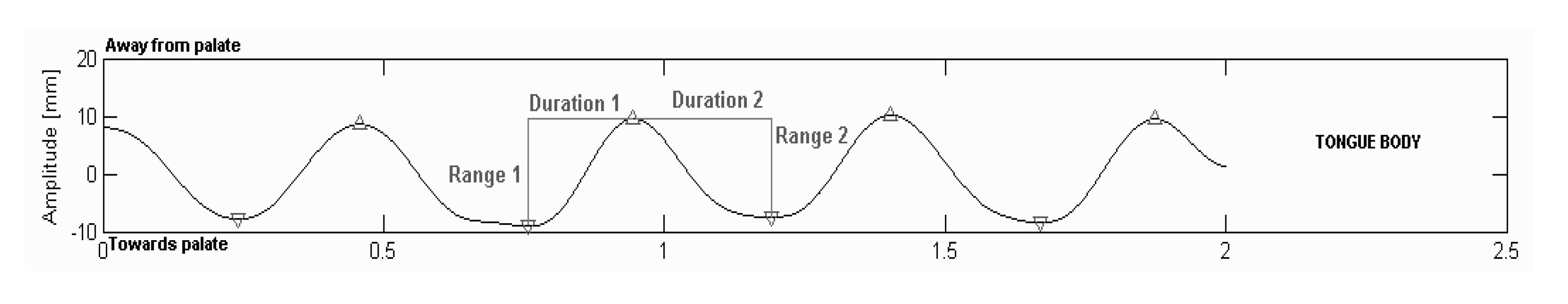
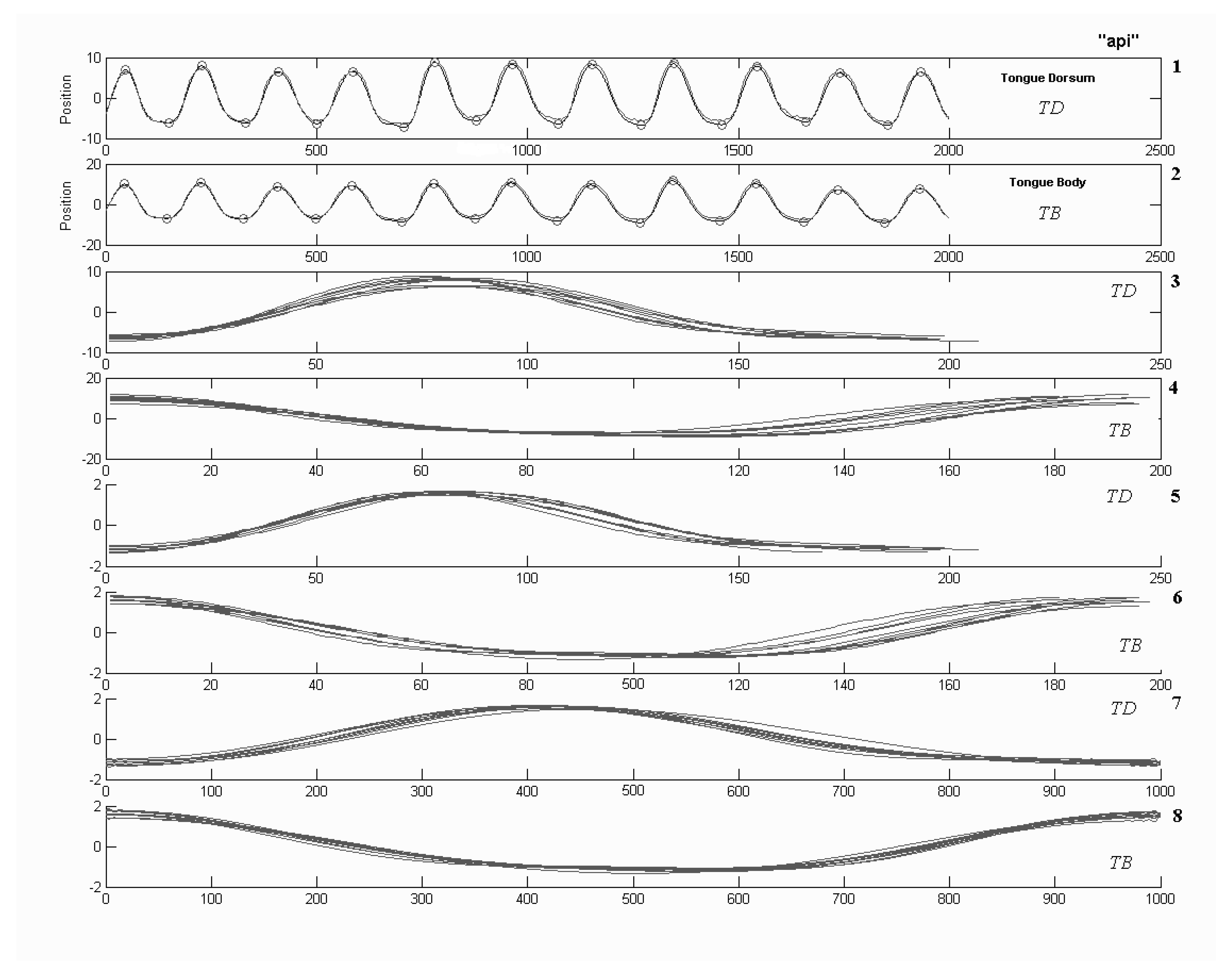
Analyses
RESULTS
| OF | OM | YF | YM TD | OF | OM | YF | YM TB | ||
|---|---|---|---|---|---|---|---|---|---|
| API | 12.97 | 14.01 | 12.50 | 14.07 | 13.46 | 14.14 | 13.39 | 19.23 | RANGE (in mm) |
| 4.78 | 4.18 | 2.86 | 4.85 | 4.25 | 2.88 | 4.03 | 7.28 | ||
| IPA | 13.67 | 15.73 | 10.09 | 12.53 | 13.12 | 15.16 | 10.59 | 17.63 | |
| 3.44 | 6.09 | 3.85 | 4.09 | 3.19 | 5.00 | 4.52 | 7.46 | ||
| PTK | 8.87 | 11.68 | 9.51 | 8.65 | 8.04 | 11.78 | 7.35 | 8.74 | |
| 3.26 | 3.52 | 3.73 | 2.54 | 2.98 | 2.53 | 2.54 | 2.97 | ||
| SWAL | 8.83 | 10.66 | 7.30 | 8.56 | 9.47 | 9.52 | 5.44 | 8.43 | |
| 4.73 | 5.61 | 3.91 | 2.89 | 5.91 | 5.73 | 2.80 | 3.94 | ||
| API | 289.11 | 261.16 | 256.03 | 310.50 | 288.46 | 260.82 | 256.19 | 327.64 | DURATION (in msec) |
| 62.54 | 37.21 | 22.24 | 130.95 | 62.17 | 37.20 | 22.05 | 180.14 | ||
| IPA | 307.92 | 258.87 | 264.19 | 272.96 | 308.39 | 258.85 | 258.48 | 273.42 | |
| 66.33 | 65.85 | 33.66 | 85.95 | 65.77 | 65.85 | 36.51 | 86.35 | ||
| PTK | 325.72 | 289.51 | 269.58 | 283.20 | 349.09 | 289.00 | 269.14 | 276.98 | |
| 50.12 | 36.60 | 29.71 | 111.48 | 91.52 | 36.27 | 29.83 | 90.67 | ||
| SWAL | 1100.76 | 1055.78 | 1046.16 | 591.47 | 1134.87 | 1088.23 | 1078.34 | 634.69 | |
| 359.32 | 369.97 | 429.76 | 169.08 | 361.21 | 332.23 | 635.35 | 189.07 | ||
| API | 6.37 | 5.23 | 6.10 | 7.31 | 4.35 | 4.37 | 5.40 | 6.56 | cSTI (a.u.) |
| 3.35 | 2.33 | 2.36 | 4.56 | 2.13 | 1.55 | 1.63 | 4.67 | ||
| IPA | 6.93 | 6.98 | 9.84 | 7.64 | 6.94 | 6.57 | 7.55 | 6.08 | |
| 3.76 | 4.00 | 5.71 | 3.20 | 3.75 | 4.06 | 3.30 | 2.90 | ||
| PTK | 9.55 | 6.82 | 10.23 | 12.29 | 11.56 | 5.97 | 11.14 | 11.50 | |
| 6.26 | 2.85 | 4.09 | 7.31 | 8.08 | 3.14 | 4.53 | 5.85 | ||
| SWAL | 21.09 | 17.49 | 18.45 | 15.43 | 19.81 | 20.69 | 18.00 | 17.38 | |
| 9.49 | 5.27 | 6.62 | 6.56 | 7.95 | 10.93 | 6.99 | 10.32 |
| RANGE | DURATION | cSTI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TD | df1,df2 | F | p-value | df1,df2 | F | p-value | df1,df2 | F | p-value | |
| AGE COHORT [A] | 1,17 | 1.77 0.201 1,17 | 2.46 0.135 1,17 | 0.48 | 0.499 | |||||
| GENDER [B] | 1,17 | 1.5 0.237 1,17 | 2 0.175 1,17 | 0.9 | 0.356 | |||||
| TASK [C] | 3,51 | 15.12 0.000*** 3,51 | 143.77 0.000*** 3,51 | 65.81 | 0.000*** | |||||
| A X B | 1,17 | 0.11 0.741 1,17 | 0.34 0.570 1,17 | 0.31 | 0.586 | |||||
| A X C | 3,51 | 1.03 0.389 3,51 | 4.99 0.004** 3,51 | 3.06 | 0.036* | |||||
| B X C | 3,51 | 0.17 0.917 3,51 | 4.85 0.005** 3,51 | 1.31 | 0.280 | |||||
| A X B X C | 3,51 | 0.54 0.658 3,51 | 4.54 0.007** 3,51 | 0.88 | 0.460 | |||||
| TB | ||||||||||
| AGE COHORT [A] | 1,17 | 0.17 0.682 1,17 | 1.93 0.183 1,17 | 0.13 | 0.724 | |||||
| GENDER [B] | 1,17 | 6.57 0.020* 1,17 | 1.51 0.236 1,17 | 0.37 | 0.552 | |||||
| TASK [C] | 3,51 | 26.07 0.000*** 3,51 | 141.22 0.000*** 3,51 | 45.88 | 0.000*** | |||||
| A X B | 1,17 | 1.34 0.262 1,17 | 0.15 0.708 1,17 | 0.23 | 0.636 | |||||
| A X C | 3,51 | 2.69 0.056 3,51 | 4.28 0.009** 3,51 | 1.45 | 0.240 | |||||
| B X C | 3,51 | 0.91 0.441 3,51 | 4.17 0.01** 3,51 | 0.45 | 0.716 | |||||
| A X B X C | 3,51 | 1.35 0.269 3,51 | 4.07 0.011* 3,51 | 0.69 | 0.564 | |||||
DISCUSSION
CONCLUSIONS
References
- Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiology & Behaviour 92: 129–135.
- Alfonso, P. J., and P. van Lieshout. 1997. Edited by W. Hulstijn, H. F. Peters and P. H. H. M. van Lieshout. Spatial and temporal variability in obstruent gestural specification by stutterers and controls: Comparisons across sessions. In Speech production: Motor control, brain research and fluency disorders. Elsevier Publishers: Amsterdam: pp. 151–160. [Google Scholar]
- Baker, K. K., L. O. Ramig, S. Sapir, E. S. Luschei, and M. E. Smith. 2001. Control of vocal loudness in young and old adults. Journal of Speech Language and Hearing Research 44: 297–305. [Google Scholar]
- Bassler, R. 1987. Histopathology of different types of atrophy of the human tongue. Pathology, Research and Practice 182: 87–97. [Google Scholar] [CrossRef] [PubMed]
- Baum, B. J., A. J. Caruso, J. A. Ship, and A. Wolff. 1991. Edited by A.S. Papas, L. C. Niessen and H. H. Chauncey. Oral physiology. In Geriatric dentistry: Aging and oral health. pp. 71–82. [Google Scholar]
- Caruso, A. J., P. B. Mueller, and B. B. Shadden. 1995. Effects of aging on speech and voice. Physical and Occupational Therapy in Geriatrics 13: 63–79. [Google Scholar]
- Crow, H. C., and J. A. Ship. 1996. Tongue strength and endurance in different aged individuals. Journal of Gerontology: Medical Sciences 51: M247–M250. [Google Scholar]
- Edstrom, E., M. Altun, E. Bergman, H. Johnson, S. Kullberg, and V. Ramirez-Leon.
- Flanagan, K. P., and J. S. Dembowski. 2002. Kinematics of normal lingual diadokokinesis. The Journal of the Acoustical Society of America 111: 2476. [Google Scholar]
- Goozee, J. V., D. K. Stephenson, B. E. Murdoch, R. E. Darnell, and L. L. Lapointe. 2005. Lingual kinematic strategies used to increase speech rate: Comparison between younger and older adults. Clinical Linguistics and Phonetics 19: 319–334. [Google Scholar] [PubMed]
- Hasegawa-Johnson, M. 1998. Electromagnetic exposure safety of the Carstens articulograph AG100. Journal of the.Acoustical Society of America 104: 2529–2532. [Google Scholar] [CrossRef]
- Hirai, T., O. Tanaka, H. Koshino, and T. Yajima. 1991. Ultrasound Observations of Tongue Motor Behavior. Journal of Prosthetic Dentistry 65: 840–844. [Google Scholar]
- Linville, S. E., and J. Rens. 2001. Vocal tract resonance analysis of aging voice using long-term average spectra. Journal of Voice 15: 323–330. [Google Scholar]
- Logemann, J. A., B. R. Pauloski, A. W. Rademaker, and P. J. Kahrilas. 2002. Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis. Journal of Speech, Language, and Hearing Research 45: 434–445. [Google Scholar] [CrossRef]
- Logemann, J. A., B. R. Pauloski, A. W. Rademaker, L. A. Colangelo, P. J. Kahrilas, and C. H. Smith. 2000. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech, Language, and Hearing Research 43: 1264–1274. [Google Scholar] [CrossRef]
- McClean, M. D., and S. M. Tasko. 2003. Association of orofacial muscle activity and movement during changes in speech rate and intensity. Journal of Speech, Language, and Hearing Research 46: 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, M. A., J. A. Hind, E. B. Roecker, M. Carnes, J. Doyle, G. A. Dengel, and et al. 2000. Age effects on the temporal evolution of isometric and swallowing pressure. Journal of Gerontology: Medical Sciences 55: M634–M640. [Google Scholar] [CrossRef] [PubMed]
- Perkell, J. S., M. Zandipour, M. L. Matthies, and H. Lane. 2002. Economy of effort in different speaking conditions. I. A preliminary study of intersubject differences and modeling issues. Journal of the Acoustical Society of America 112: 1627–1641. [Google Scholar] [CrossRef]
- Price, P. A. S., and B. W. Darvell. 1981. Force and Mobility in the Aging Human Tongue. Medical Journal of Australia 1: 75–78. [Google Scholar] [CrossRef]
- Robbins, J., R. Levine, J. Wood, E. B. Roecker, and E. Luschei. 1995. Age effects on lingual pressure generation as a risk factor for dysphagia. Journal of Gerontology: Medical Sciences 50: M257–M262. [Google Scholar] [CrossRef] [PubMed]
- Rother, P., B. Wohlgemuth, W. Wolff, and I. Rebentrost. 2002. Morphometrically observable aging changes in the human tongue. Annals of Anatomy 184: 159–164. [Google Scholar] [CrossRef]
- Schönle, P. W., K. Grabe, P. Wenig, J. Hohne, J. Schrader, and B. Conrad. 1987. Electromagnetic Articulography-Use of Alternating Magnetic-Fields for Tracking Movements of Multiple Points Inside and Outside the Vocal-Tract. Brain and Language 31: 26–35. [Google Scholar] [CrossRef]
- Smith, A., and L. Goffman. 1998. Stability and patterning of speech movement sequences in children and adults. Journal of Speech, Language, and Hearing Research 41: 18–30. [Google Scholar] [CrossRef]
- Smith, A., L. Goffman, H. N. Zelaznik, G. S. Ying, and C. McGillem. 1995. Spatiotemporal stability and patterning of speech movement sequences. Experimental Brain Research 104: 493–501. [Google Scholar]
- Smith, C. H., J. A. Logemann, W. R. Burghardt, S. G. Zecker, and A. W. Rademaker. 2006. Oral and oropharyngeal perceptions of fluid viscosity across the age span. Dysphagia 21: 209–217. [Google Scholar]
- Sonies, B., and A. J. Caruso. 1990. Edited by E. Cherow. The aging process and its potential impact on measures of oral sensorimotor function. In Proceedings of the research symposium on communication sciences and disorders and aging. ASHA Reports #19. American Speech-Language-Hearing Association: Rockville, MD. [Google Scholar]
- Sonies, B. C., B. J. Baum, and T. H. Shawker. 1984. Tongue motion in elderly adults: Initial in situ observations. Journal of Gerontology 39: 279–283. [Google Scholar] [PubMed]
- Sonies, B. C., J. A. Ship, and B. J. Baum. 1989. Relationship between saliva production and oropharyngeal swallow in healthy, different-aged adults. In Dysphagia. Mosby Year Book Inc: St.Louis: vol. 4, pp. 85–89. [Google Scholar]
- Steele, C. M., and P. H. van Lieshout. 2004a. Use of electromagnetic midsagittal articulography in the study of swallowing. Journal of Speech Language And Hearing Research 47: 342–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steele, C. M., and P. H. H. M. van Lieshout. 2004b. Influence of bolus consistency on lingual behaviors in sequential swallowing. Dysphagia 19: 192–206. [Google Scholar]
- Tasko, S. M., R. D. Kent, and J. R. Westbury. 2002. Variability in tongue movement kinematics during normal liquid swallowing. Dysphagia 17: 126–138. [Google Scholar] [PubMed]
- Tracy, J. F., J. A. Logemann, P. J. Kahrilas, P. Jacob, M. Kobara, and C. Krugler. 1989. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia 4: 90–94. [Google Scholar]
- Van Lieshout, P., and W. Moussa. 2000. The assessment of speech motor behaviors using electromagnetic articulography. The Phonetician 81: 9–22. [Google Scholar]
- Van Lieshout, P. H. H. M., A. Bose, P. A. Square, and C. M. Steele. 2007. Speech motor control in fluent and dysfluent speech production of an individual with apraxia of speech and Broca’s aphasia. Clinical Linguistics & Phonetics 21: 159–188. [Google Scholar]
- Van Lieshout, P. H. H. M., C. A. W. Rutjens, and P. H. M. Spauwen. 2002. The dynamics of interlip coupling in speakers with a repaired unilateral cleft-lip history. Journal of Speech Language And Hearing Research 45: 5–19. [Google Scholar]
- Van Lieshout, P., P. J. Alfonso, W. Hulstijn, and H. F. Peters. 1994. Edited by F.J. Maarse, A. E. Akkerman, A. N. Brand, L. J. M. Mulder and M. J. Van der Stelt. Electromagnetic Midsagittal Articulography (EMMA). In Computers in psychology: Applications, methods, and instrumentation. Swets & Zeitlinger: Lisse: pp. 62–76. [Google Scholar]
- Wohlert, A. B. 1996. Perioral muscle activity in young and older adults during speech and nonspeech tasks. Journal of Speech and Hearing Research 39: 761–770. [Google Scholar] [PubMed]
- Yamaguchi, A., M. Nasu, Y. Esaki, H. Shimada, and S. Yoshiki. 1982. Amyloid Deposits in the Aged Tongue-A Postmortem Study of 107 Individuals Over 60 Years of Age. Journal of Oral Pathology & Medicine 11: 237–244. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2008 by the authors. 2008 Janice W. Bennett, Pascal H.H.M. van Lieshout, Catriona M. Steele
Share and Cite
Bennett, J.W.; van Lieshout, P.H.H.M.; Steele, C.M. Tongue Control for Speech and Swallowing in Healthy Younger and Older Subjects. Int. J. Orofac. Myol. Myofunct. Ther. 2007, 33, 5-18. https://doi.org/10.52010/ijom.2007.33.1.1
Bennett JW, van Lieshout PHHM, Steele CM. Tongue Control for Speech and Swallowing in Healthy Younger and Older Subjects. International Journal of Orofacial Myology and Myofunctional Therapy. 2007; 33(1):5-18. https://doi.org/10.52010/ijom.2007.33.1.1
Chicago/Turabian StyleBennett, Janice W., Pascal H. H. M. van Lieshout, and Catriona M. Steele. 2007. "Tongue Control for Speech and Swallowing in Healthy Younger and Older Subjects" International Journal of Orofacial Myology and Myofunctional Therapy 33, no. 1: 5-18. https://doi.org/10.52010/ijom.2007.33.1.1
APA StyleBennett, J. W., van Lieshout, P. H. H. M., & Steele, C. M. (2007). Tongue Control for Speech and Swallowing in Healthy Younger and Older Subjects. International Journal of Orofacial Myology and Myofunctional Therapy, 33(1), 5-18. https://doi.org/10.52010/ijom.2007.33.1.1




