Abstract
Changes in tongue and hand strength measurements of men with Parkinson’s Disease and agedmatched controls across multiple days were examined. The Iowa Oral Performance Instrument measured tongue and hand strength during four consecutive days and at day 11. Peak tongue strength measurements occurred on day 3 with a small decrease on day 4, which was maintained at day 11, indicating a significant increase in tongue strength measurements with task repetition in multiple days. No change in hand strength measurements was noted over days. Significant differences in mean tongue and hand strength measurements between the PD and age-matched control group were found. Tongue and hand strength measurements were lower for the PD group compared with the control group on average across days.
INTRODUCTION
The various functions of the tongue include oral intake, sensory processing of taste and touch, motor skills in the mastication and deglutition of food, and articulation during speech. The tongue musculature appears to work in a gradient fashion with several muscles executing the bulk of the movement while other muscles mediate movement, hold nearby structures in place, or oppose the main movement. Dworkin (1980), evaluating tongue force using a straingauge transducer, found that measurements were greater in males than females among normal individuals. Similarly, Crow and Ship (1996) found greater tongue strength measurements in normal males than normal females using the Iowa Oral Performance Instrument (IOPI). Tongue strength decreases with age in normals (Crow & Ship, 1996). Tongue function in swallowing and articulation appears to remain relatively stable during aging in the absence of neurological pathology or structural changes (Robbins, Hamilton, Lof, & Kempster, 1992; Sonies, Stone, & Shawker, 1984; Tracy et al., 1989).
Reduced tongue strength has been reported for persons with a variety of neurological disorders that result in disordered speech such as amyotrophic lateral sclerosis (ALS: DePaul & Brooks, 1993; Langmore & Lehman, 1994), stroke (Thompson, Murdoch, & Stokes, 1995), and traumatic brain injury (TBI; Stierwalt, Robin,
Solomon, Weiss, & Max, 1995). Studies that attempt to correlate tongue strength with speech disorders have shown conflicting results. Previous research has shown reduced tongue strength and hand strength for persons with Parkinson’s Disease (PD) (Solomon, Lorell,
Robin, Rodnitzky, & Luschei, 1995; Solomon,
Robin, & Luschei, 2000).
Reduced tongue function in those who have PD is manifested as dysarthric speech or oral stage disorders during deglutition. Attempts to correlate speech deficits related to PD with reduced tongue strength measurements using air pressure measurement instruments, such as the IOPI have revealed mixed results (Solomon et al., 1995; Solomon et al., 2000). Studies using the IOPI have shown that tongue force control (Gentil, Perrin, Tournier, & Pollack, 1999) and tongue strength measurements were lower in individuals with PD than age-matched controls (Solomon, Robin, Lorell, Rodnitzky, & Luschei,
1994; Solomon et al., 1995; Solomon et al., 2000). Solomon et al. (1995) found a positive correlation between tongue strength measurements and disease severity. Other researchers have suggested that speech dysfunction is one of the few symptoms of PD that does not correspond to the disease progression (Metter & Hanson, 1986; Morrison,
Rigrodsky, & Mysak, 1970).
In addition to measuring tongue strength, hand strength has been measured in controls and individuals with PD. The peak hand strength measurement for men occurs between 35 and 39 years of age (Butler, 1997). In the normal adult population, research has been designed to determine if differences exist between agematched controls across age and gender, and if a relationship exists between tongue and hand strength measurements. Using a Jamar dynamometer, hand strength measurements have been found to progressively decline with age (Desrosiers, Bravo, Hebert, & Dutil, 1995;
Fiebert, Roach, Armstrong, Mandel, & Donohue,
1995; Horowitz, Tollin, & Cassidy, 1997; Shiffman, 1992). However, a more recent study found only a weak negative correlation between age and hand strength measurements in 20 to 64 year old individuals (Hanten et al., 1999).
A significant relationship has been shown between hand width, hand length and finger length with hand strength (Everett & Sills, 1952). Hand circumference has been shown to be a predictor of hand strength (Desrosiers et al., 1995). Other factors correlated with hand strength measurement are height, weight, hand dominance, and overall physical fitness (Crosby,
Wehbe, & Mawr, 1994; Kellor, Frost, Silverberg,
Iversen, & Cummings, 1971; Schmidt & Toews, 1970).
Solomon et al. (1995) reported on tongue and hand strength measurements using the IOPI, showed that individuals with mild to moderate PD had lower hand strength measurements than age-matched controls. In a subsequent study, Solomon et al. (2000) found no significant differences in hand strength measurements between a group of individuals with mild and moderate PD and a control group. When Solomon et al. (2000) combined their data with retrospective data; results showed tongue strength of the PD group was significantly lower than the control group.
Most studies measuring tongue and hand strength have been based on multiple measurements during a single day of testing. Recent studies of tongue and hand strength measurement in children and cycling strength in adults have investigated strength measurement learning effects using repeated trials over several days. A study of tongue strength measurement in children reported a learning curve across three consecutive days with repeated measures of tongue strength measurement across four consecutive days of testing. No significant difference in strength measurement was found between the third and fourth day of testing (Weathers, 2000). Research on cycling strength showed a learning effect over the first two consecutive days of testing (Capriotti, Sherman, & Lamb, 1999; Martin, Diedrich, & Coyle, 2000). Martin et al. (2000) found that the increase in maximal cycling strength was maintained up to six days later.
A review of the literature reveals little normative data about adult tongue strength measurement related to the normal population and even less related to individuals with neurological disorders. There are few research reports on tongue strength measurement across days in the adult population (Robin, Somodi, & Luschei, 1991)). Speech-language pathologists (SLP’s) would benefit from a greater knowledge of the nature of tongue strength measurement in normal adults and disordered populations. SLP’s often use subjective observations to recommend oral motor exercises for individuals with PD who experience dysarthria. Typically, rehabilitation programs for individuals with dysarthria characterized by force “dyscontrol” of the tongue involve force physiology training. While systematic training is often recommended for dysarthria, the training regimen may vary in intensity and length based on the SLP’s clinical impressions (Tonkovich, Latham, & Rambow, 1982). Factors such as age, gender, general medical status, and motivation affect the prognosis for improvement, treatment planning, and treatment outcomes. Treatment outcomes vary greatly. The same treatment program often results in different levels of improvement from patient to patient even when controlled for age and medical status (Dworkin, 1991).
Establishment of expected procedural effects would allow better determination of treatment efficacy.
Previous studies of tongue and hand strength measurement have determined first day pressure data (Robin, Goel, Somodi, & Luschei,1992; Solomon et al., 1995; Solomon et al., 2000). In light of these studies, the intent of the present project was to compare maximum hand and tongue strength measurements of normal males and male individuals with PD over five days of testing. The performance of the control participants was compared to the performance of participants with PD.
The research questions explored a change in tongue or hand strength measurement over testing days of control and PD participants. The results were examined for a difference in the amount of change over days in tongue or hand strength measurement between the control and PD groups. Tongue strength measurement could be a possible diagnostic indicator for differentiating individuals with PD from agematched, healthy peers. Low tongue strength measurement might be an indication of pathology that may require further diagnostic testing. Hand strength measurement by disease and control group was examined for a correlation between hand size measurements.
METHOD
Participants
Twenty native English speakers were recruited from the community for this project. The PD group consisted of ten male adults diagnosed with PD ranging in age from 52-79 years. The control group consisted of ten participants who were age-matched to the PD participants within nine years.
Inclusion criteria for participants in the control groups were performance within normal limits on the Folstein Mini-Mental Status Exam (MMSE: Folstein, Folstein, & McHugh, 1975), Oral Speech Mechanism Screening Examination-Revised (OSMSE-R: St. Louis & Ruscello, 1987), and The Goldman Fristoe Test of Articulation 2 (GFTA-2: Goldman & Fristoe, 2000). One control participant did not perform within normal limits on the OSMSE-R and was excluded from the study. Exclusionary criteria for control participants included neurogenic illnesses as determined by participant interview. Inclusionary criterion for participants in the PD group was a medical diagnosis of PD by each participant's physician. All participants with PD were screened for dementia using the MMSE. If the score fell below normal limits, the legal guardian signed the consent form. Only one participant with PD scored below the normal or borderline range on the MMSE and required consent from a legal guardian. Exclusionary criteria for PD participants included neurogenic illnesses other than PD.
IOPI Apparatus
The Iowa Oral Performance Instrument (IOPI), model 1.5, was developed to measure tongue and hand strength and endurance. The IOPI is a portable, battery-powered device that provides a digital display of pressure in kilopascals (kPa). An arrow symbol appears in the digital display when the voltage drops to 7.75. When the arrow appears, the pressure reading may be impacted by approximately one percent of error; therefore, the battery level was maintained above this level to reduce measurement of error.
Procedure
Participants signed an informed consent and completed a short interview regarding age, gender, presence of neurological deficits, medical history related to PD, and hand dominance. The procedures for the PD and control participants included hand size measurements, dementia screening, oral motor examination, articulation testing, initial hand and tongue strength measurements and repeated hand and tongue strength measurements.
All participants were evaluated in a quiet environment. Controlling for time of day for evaluating control participants was not a factor in similar studies and was not controlled in this study. The time of day for evaluating participants with PD was determined via phone conversation prior to the first testing day. All participants with PD were evaluated at the time of day when they self-reported achieving maximum benefits from their Parkinson-related medications. Three participants with PD reported the absence of peak benefits from their medications. The interview, screening, and testing were audio and videotaped using a Sony TRV67 Hi-8 video camcorder with a clip-on microphone.
Hand size of all participants was measured using a flexible tape measure. Three hand measurements were taken. Hand length was measured from the proximal end of the scaphoid bone, which connects to the radius, to the tip of the middle phalange. Finger length was measured from the joint at the knuckle of the middle phalange to the tip of the middle phalange. Hand circumference was a radius measurement at the level of the head of the first four metacarpals (Marieb, 1992).
The PD participants were shown GFTA-2 Sounds-in-Words pictures and asked to name or describe what they saw (Goldman & Fristoe, 2000). A narrative speech sample was elicited from the participants with PD using the cookie theft picture (BDAE: Goodglass & Kaplan, 1972). The responses to the GFTA-2 and the cookie theft picture were reviewed for the presence of misarticulations and dysarthria. No misarticulations were noted. Participants with PD were judged to have minimal dysarthria characteristics consistent with PD. No formal evaluation for dysarthria was performed.
The IOPI was used to obtain all tongue and hand strength measurements (Crow & Ship, 1996; Robin et al., 1991; Robin et al., 1992). The IOPI was calibrated prior to each testing situation using the built-in calibration feature of the IOPI. Prior to positioning the IOPI tongue pressure sensor bulb, one control participant removed a partial dental prosthesis for two molars. The IOPI tongue pressure sensor bulb was positioned medially on the anterior portion of the tongue. The tongue pressure sensor bulb was positioned behind the central incisors. Participants were instructed to allow the central incisors to rest on the clear plastic collar without applying pressure. The clear plastic collar connects the tongue pressure sensor bulb to the connecting tube. Consistent with other similar research, a bite block was not employed (Crow & Ship, 1996; Robin et al., 1992; Solomon et al., 2000; Solomon & Munson, 2004). Correct positioning of the tongue pressure sensor bulb was verified upon visual inspection.
The IOPI displayed the tongue strength measurement when the participant pushed the tongue pressure sensor bulb against the anterior portion of the hard palate using the anterior portion of the tongue dorsum (Crow & Ship, 1996; Robin et al., 1992; Solomon et al., 2000). Participants were instructed to use the anterior portion of the tongue, but not the tongue tip (Robin et al., 1992). Maximum tongue and hand strength measurements were determined based on a previously published paradigm (Robbins et al., 1995; Robin et al., 1991, 1992). Each participant was instructed to press against the tongue pressure bulb as hard as possible for one second and then relax. A one-minute break was provided between each of three measurements (Innes, 1999).
Hand strength measurements were measured using the IOPI with the 10 ml rubber syringe bulb. Participants were asked to remove any rings before positioning the hand pressure sensor bulb in the hand. Based on recommendations by Mathiowetz, Weber, Volland, and Kashman (1984), which were established by the American Society of Hand Therapists, the participants were asked to sit in a chair with a 90-degree angle at the pelvis, knees, and elbow. The shoulder was in a neutral position and the arm was unsupported with the hand held forward. The IOPI hand pressure sensor bulb was positioned in the palm of the dominant hand. In accordance with the IOPI instructional manual, each participant was instructed to fold the fingers over the hand pressure sensor bulb, make a fist with the thumb outside of the fingers, and squeeze. Each participant was told not to press with his fingertips. The participants were instructed to squeeze the hand pressure sensor bulb as hard as possible for one second and then relax (Hamilton, Balnave, & Adams, 1994). Hand strength testing instructions were based on a review of the literature, which suggested providing instructions using the same tone and volume each time during each trial, and providing a 60 second rest period between trials (Innes, 1999). Each subject performed a submaximal grip to become familiar with the device without using significant strength as suggested by Innes (1999). The maximum strength was determined to be the greatest pressure generated during one of the three trials each day (Clark, Henson, Barber, Stierwalt, & Sherrill, 2003; Solomon, Drager, & Luschei, 2000; Solomon, Robin & Luschei, 2000). The time of the day for testing hand strength was not a consideration since testing time was not found to have a significant impact on hand strength measurement based on previous studies (Innes, 1999).
Tongue and hand strength measurements were obtained on four consecutive days and (after a seven-day interim on day 11) on an additional day. The highest pressure generated of three measurements each day was determined to reflect the potential maximum strength measurement. The strength measurements were not disclosed to the participants until after all data were collected on the last day of testing.
RESULTS
The independent variables were group (one control group and one PD group) and day (5 testing days). The dependent variable was the IOPI tongue or hand strength measurements reported in kPa.
Tongue Strength Measurement
Means of groups by day are presented in Table 1. An analysis of variance (ANOVA) examining groups and days with repeated measures on days was performed. Statistically significant results were found for groups, F (1,18) = 5.64, p = .0288 and days, F (4, 72) = 4.09, p = .0048 (Table 2). No statistically significant interaction was found for group by days (Figure 1). Eta Squared analysis determined effect sizes. The maximum tongue strength measurement of the control group (M = 58.7) was significantly higher than of the PD group (M = 44.26). A Least Squares Means comparison analysis was performed on the group means (Figure 1). The control group had significantly greater maximum tongue strength compared to the PD group for days one through four.
Because the repeated days were not independent, a Helmert analysis was used to probe the maximum tongue strength means for each day. The Helmert does not analyze all pairwise comparisons, but shows the point at which responses cease to change (Table 1). Therefore, the Helmert compared the mean of each day to the means of all subsequent days. The first day mean tongue strength measurement was significantly lower than every other day of testing regardless of group, F (1, 18) = 6.03, p = .0244. The mean tongue strength measurement on the third day was significantly higher (M = 47.1) than the means of day four (M = 45) and day 11 (M = 45.2) regardless of group, F (1,18) = 4.81, p = .0417. No significant difference was found between mean tongue strength measurements of days four and eleven.
Hand Strength Measurement
Means of groups by day are presented in Table 1. An ANOVA (groups, days) with repeated measures on days was performed. Statistically significant results were found for groups, F (1,18) = 5.15, p = .0358 (Table 2). The mean hand strength measurement of the control group maximum hand strength measurement mean (M = 136.34) was significantly higher than the PD group (M = 108.68). Statistical significance was not found for group maximum hand strength measurement mean over five days of testing regardless of group. No statistically significant interaction was found for maximum hand strength of group by day.
Correlation Analyses of Hand Size and Hand Strength Measurement
A Pearson’s correlation was performed to examine potential relationships between hand measurements and maximum hand strength measurements. No significant correlations were found between hand length, finger length, or palm circumference with the hand strength measurements for the control or PD group.
DISCUSSION
The ANOVA with repeated measures and the multiple comparisons show that the control group had a significantly higher maximum tongue strength compared to the PD group. The fact that the control group differed significantly from the PD group suggested that tongue strength measurement would be a good diagnostic indicator for differentiating individuals with PD from age-matched, healthy peers. Accordingly, an adult with a low tongue strength measurement may signal the need for additional diagnostic testing for possible neuromotor pathology.
In 1995, Solomon et al. reported that individuals with mild to moderate PD had lower tongue and hand strength measurements than age-matched controls. Subsequently, Solomon et al. (2000) found no significant differences in tongue and hand strength measurements between a group of individuals with mild and moderate PD and a control group. However, when they combined the data from the 2000 study with data from their 1995 study, their results demonstrated that tongue strength of the PD group was found to be significantly lower than for the control group (Solomon et al., 2000).
Significant increases from day one to day two and from day two to day three were found in the mean tongue strength measurement across days for the PD group. This was in agreement with findings reported by Weathers (2000), who analyzed tongue strength measurement in children across four days of testing. She suggested that children demonstrated a learning effect across four days when performing a novel task such as using the IOPI. Therefore, both adults and children exhibit an increased tongue strength measurement across testing days without therapeutic intervention.
For this project, the smallest mean tongue strength measurement was found on the first day of testing for the PD group. Similar to Weathers (2000), the greatest mean tongue strength measurement was found on the third day of testing. A statistically significant decrease was found from the third to the fourth day of testing in this project; however, Weathers found no significant difference between day three and day four of testing children. In studies of other groups of skeletal muscles, Capriotti et al. (1999) and Martin et al. (2000) suggested that a learning effect existed over the first two consecutive days of testing cycling strength. Their results showed that the highest cycling strength scores were found on the third day of testing followed by a plateau in performance. This project supported these studies, which suggested a learning effect to the equipment and the testing procedure.
Efficacy of therapy depends on attributing increases in tongue strength measurement to treatment and not to a learning effect to the strength measuring equipment alone. If tongue strength were measured as part of a strengthening training program, these results suggest that improvement in tongue strength measurement may not be attributed to therapy alone. In the first three days of treatment, a learning effect for the equipment or testing procedures may account for part or all of the increase in tongue strength measurement.
In contrast to a significant increase in tongue strength measurement across days, no significant change in hand strength measurement across days was found. The tongue pressure task may be more unique compared to the hand pressure task. Positioning a plastic bulb on the tongue and manipulating it is an unusual experience. Grasping and gripping a variety of items is a typical daily occurrence and may be less likely to involve a learning effect to a hand pressure sensor bulb. The task of measuring tongue strength takes only a few minutes. A physiological change, even when testing occurred over several days, probably could not be attributed to improved muscle strength from daily participation. The greatest increase in tongue strength measurement was seen on the second day of testing. This suggests that if significant changes were due to therapy, they would be present after only three trials on the second day of therapy. No significant change was seen after the fourth day of testing. According to these results, only improvement in tongue strength measurement obtained after the fourth day of therapeutic intervention and compared to the fourth day baseline may be due to the intervention. Martin et al. (2000) found that the increase in maximal cycling strength was maintained up to six days. This is consistent with the findings in this project, which show no significant difference in tongue strength measurement means between the fourth day of testing and testing after a one week interim.
The repeated measures ANOVA and multiple comparison analysis revealed that the control group had a significantly higher hand strength measurement compared to the PD group. The significant difference between the maximum hand strength measurement of the control group and the PD group suggested that maximum hand strength measurement would be a good diagnostic indicator for differentiating individuals with PD from their peers. Previous research using larger groups of participants have shown reduced tongue strength and hand strength for persons with PD (Solomon et al., 1995).
No significant correlation was found between hand size and hand strength measurement for control groups and PD group. This is in contrast to previous research that reports a significant relationship between hand width, hand length, and finger length with hand strength (Everett & Sills, 1952). Hand circumference has been shown to be a predictor of hand strength (Desrosiers et al., 1995).

Table 1.
Maximum Tongue and Hand Strength Measurement Means by Day and Group (in kPa).
Table 1.
Maximum Tongue and Hand Strength Measurement Means by Day and Group (in kPa).
| Tongue | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | M | SD |
| PD Group | |||||||
| M | 38.7 | 45.3 | 47.1 | 45 | 45.2 | 44.26 | 13.27 |
| SD | 13.15 | 13.55 | 11.42 | 12.89 | 16.12 | ||
| Control Group | |||||||
| M | 55.1 | 58.5 | 62.1 | 58.8 | 59 | 58.7 | 15.26 |
| SD | 18.86 | 14.65 | 13.62 | 15.59 | 15.57 | ||
| Hand | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | M | SD |
| PD Group | |||||||
| M | 105.9 | 106.1 | 110.5 | 109.2 | 111.7 | 108.68 | 33.01 |
| SD | 32.93 | 28.93 | 38.55 | 31.62 | 38.67 | ||
| Control | |||||||
| Group | |||||||
| M | 133.2 | 139.3 | 136.9 | 134.2 | 137.5 | 136.34 | 22.65 |
| SD | 25.62 | 25.27 | 24.03 | 23.71 | 18.09 |

Table 2.
Repeated Measures Analysis of Variance of Maximum Tongue and Hand Strength Measurement.
Table 2.
Repeated Measures Analysis of Variance of Maximum Tongue and Hand Strength Measurement.
| Variable Tongue | F | df | p | ES |
|---|---|---|---|---|
| Group by Day | .21 | 4, 72 | .9312 | .1476 |
| Group | 5.64 | 1, 18 | .0288 | .2225 |
| Day | 4.09 | 4, 72 | .0048 | .1825 |
| Hand | ||||
| Group by Day | .4 | 4, 72 | .8053 | .0095 |
| Group | 5.15 | 1, 18 | .0358 | .2387 |
| Day | .5 | 4, 72 | .7363 | .1833 |
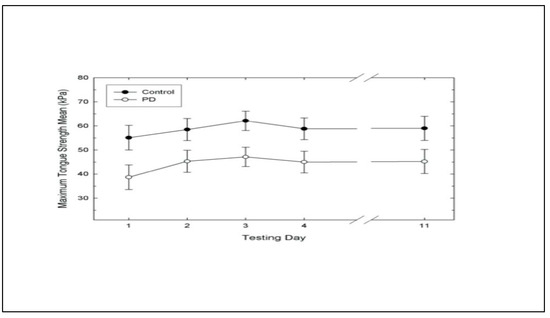
Figure 1.
Maximum tongue strength with +/− one standard error measurement across days for control and Parkinson’s Disease Groups.
The results of this project and other studies (Baum & Bodner, 1983; Crow & Ship, 1996) suggest that decreasing tongue strength is a function of aging. Despite this, decreasing tongue strength does not appear to adversely affect speech or swallowing in a healthy older population. However, when aging and pathology factors occur together in the older population, dysphagia and difficulty forming and positioning a bolus are common (Robbins et al., 1992; Sonies et al., 1984; Tracy et al., 1989). This is evidenced by lingual hesitancy (Calne, Shaw, Spiers, & Stern, 1970) and piecemeal deglutition (Calne et al., 1970; Logemann, 1998), which are commonly associated with PD.
CONCLUSIONS
The control and PD participants demonstrated a significant change in tongue strength over days. Statistical significance was found for a difference in mean tongue strength maximum measurements over days. The mean tongue strength measurement on the first day was significantly lower than on subsequent days.
Mean tongue strength measurement was significantly higher on the third day compared to the fourth day. Performance level was maintained on the fourth day and after a one-week interim on day 11.
This study shows that the best performance for tongue strength is not found during the first day of testing. Tongue strength measurements during the first day may be confounded by unfamiliarity with the task, novelty of the task, or a lack of confidence. Therefore, testing tongue strength is subject to changes across time. Multiple days of strength testing indicate an individual’s capability.
Statistical significance was found for a difference in mean tongue strength measurement between groups. A statistically significant difference was found in mean tongue strength measurement between the control group and the PD group across the first four days of testing. The mean tongue strength measurement of the control participants was significantly greater than the PD group across the first four days of testing.
Hand strength measurements were significantly different between the control and PD participants. The mean hand strength measurement of the control participants was significantly higher than mean hand strength measurement of the PD participants. However, no difference was found in maximum hand strength measurement over days for the control and PD groups. Therefore, testing hand strength does not appear to be subject to changes across time.
Future Research
Future research projects may focus on comparing the tongue strength measurement of groups of individuals with PD of various ages.
Of particular interest is whether younger individuals with PD respond similarly to peers in a control group. Also of interest would be a comparison of performance measurements of younger and older individuals with PD. PD severity staging would provide information about tongue strength measurement expected as PD progresses, which would assist clinicians in determining realistic goal levels for approximation during treatment. To reliably report improvement in tongue strength due to treatment, patterns of tongue strength measurement across days are needed. Further testing is needed to show if therapeutic intervention has an effect on tongue strength. A comparative analysis would show the differences between the two patterns of improvement due to a learning effect of the equipment and improvement due to therapeutic intervention. A variety of therapy regimens could be analyzed to determine the effectiveness of each in attempting to increase tongue strength measurement in individuals with PD to approximate normal levels.
References
- Baum, B. J., and L. Bodner. 1983. Aging and oral motor function; evidence for altered performance among older persons. Journal of Dental Restoration 62: 2–6. [Google Scholar] [CrossRef] [PubMed]
- Butler, M. 1997. Hand strength: A comparative study. New Zealand Journal of Occupational Therapy 48, 1: 5–12. [Google Scholar]
- Calne, D. B., D. G. Shaw, A. S. D. Spiers, and G. M. Stern. 1970. Swallowing in Parkinsonism. British Journal of Radiology 43: 456–457. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, V. P., W. M. Sherman, and D. R. Lamb. 1999. Reliability of power output during intermittent high-intensity cycling. Medical Science of Sports Exercise 31, 6: 913–915. [Google Scholar] [CrossRef]
- Clark, H. M., P.A. Henson, W. D. Barber, J. A. G. Stierwalt, and M. Sherrill. 2003. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairment. American Journal of Speech-Language Pathology 12: 40–50. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C. A., M. A. Wehbe, and B. Mawr. 1994. Hand strength: Normative values. Journal of Hand Surgery 19A: 665–670. [Google Scholar] [CrossRef]
- Crow, H. C., and J. A. Ship. 1996. Tongue strength and endurance in different aged individuals. Journal of Gerontology: Medical Sciences 51, 5: M247–M250. [Google Scholar] [CrossRef]
- DePaul, R., and B. R. Brooks. 1993. Multiple orofacial indices in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research 36: 1158–1167. [Google Scholar] [CrossRef]
- Desrosiers, J., G. Bravo, R. Hebert, and E. Dutil. 1995. Normative data for hand strength of elderly men and women. The American Journal of Occupational Therapy 49, 7: 637–644. [Google Scholar] [CrossRef]
- Dworkin, J. P. 1980. Tongue force in normals and in dysarthric patients with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research 23: 828–837. [Google Scholar] [CrossRef]
- Dworkin, J. P. 1991. Edited by D. K. Marshall. Motor Speech Disorders: A treatment guide. St. Louis: Mosby-Year Book, Inc.: 220. [Google Scholar]
- Everett, P. W., and F. D. Sills. 1952. Relationship of grip strength to stature, somato-type components and anthropometric measurements of the hand. Research Quarterly 23: 1610166. [Google Scholar]
- Fiebert, I. M., K. E. Roach, T. Armstrong, D. W. Mandel, and M. Donohue. 1995. Dynamometric hand strength assessment of subjects sixty years and older. Physical & Occupational Therapy in Geriatrics 13, 4: 27–40. [Google Scholar]
- Folstein, M., S. Folstein, and P. McHugh. 1975. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12: 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gentil, M., S. Perrin, C. Tournier, and P. Pollak. 1999. Lip, tongue, and forefinger force control in Parkinson's disease. Clinical Linguistics and Phonetics 13, 1: 45–54. [Google Scholar]
- Goldman, R., and M. Fristoe. 2000. Goldman Fristoe-2 Test of Articulation. In Circle pines. Minnesota: American Guidance Service, Inc. [Google Scholar]
- Goodglass, H., and E. Kaplan. 1972. The assessment of aphasia and related disorders. Philadelphia: Lea & Febriger. [Google Scholar]
- Hamilton, A., R. Balnave, and R. Adams. 1994. Grip strength testing reliability. Journal of Hand Therapy 7: 163–170. [Google Scholar] [CrossRef]
- Hanten, W. P., W. Chen, A. A. Austin, R. E. Brooks, H. Carter, C. A. Law, M. K. Morgan, D. J. Sanders, C. A. Swan, and A. L. Vanderslice. 1999. Maximum hand strength in normal subjects from 20 to 64 years of age. Journal of the American Society of Hand Therapists 12, 3: 193–200. [Google Scholar] [CrossRef]
- Horowitz, B., R. Tollin, and G. Cassidy. 1997. Hand strength: Collection of Normative data with community dwelling elders. Physical and Occupational Therapy in Geriatrics 15, 1: 53–64. [Google Scholar]
- Innes, E. 1999. Hand literature. Australian Occupational Therapy Journal 46: 120–140. [Google Scholar] [CrossRef]
- Kellor, M., J. Frost, N. Silverberg, I. Iversen, and R. Cummings. 1971. Hand strength and dexterity: Norms for clinical use. American Journal of Occupational Therapy 25: 77–83. [Google Scholar]
- Langmore, S. E., and M. E. Lehman. 1994. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research 37: 28–37. [Google Scholar] [CrossRef]
- Logemann, J. A. 1998. Evaluation and treatment of swallowing disorders, 2nd Ed. ed. Austin, Texas: Pro-Ed, Inc., pp. 334–336. [Google Scholar]
- Martin, J. C., D. Diedrich, and E. F. Coyle. 2000. Time course of learning to produce maximum cycling power. International Journal of Sports Medicine 21, 7: 485–487. [Google Scholar] [CrossRef]
- Mathiowetz, V., K. Weber, G. Volland, and N. Kashman. 1984. Reliability and validity of hand strength and pinch strength evaluations. The Journal of Hand Surgery 9A: 222–226. [Google Scholar] [CrossRef]
- Marieb, E. N. 1992. The appendicular skeleton. In Human anatomy laboratory manual. Redwood City, California: The Benjamin/Cummings Publishing Company, Inc. [Google Scholar]
- Metter, E. J., and W. R. Hanson. 1986. Clinical and acoustic variability in hypokinetic dysarthria. Journal of Communication Disorders 19: 347–366. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E. B., S. Rigrodsky, and E. D. Mysak. 1970. Parkinson’s disease: Speech disorder and released infantile oroneuromotor activity. Journal of Speech and Hearing Research 13: 655–666. [Google Scholar] [CrossRef]
- Robin, D. A., A. Goel, L. B. Somodi, and E. S. Luschei. 1992. Tongue strength and endurance: Relation to highly skilled movements. Journal of Speech and Hearing Research 35: 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Robin, D. A., L. B. Somodi, and E. S. Luschei. 1991. Edited by C. A. Moore, K. M. Yorkston and D. R. Beukelman. Measurement of strength and endurance in normal and articulation disordered subjects. In Dysarthria and apraxia of speech: Perspectives on management. Baltimore: Paul H. Brookes, pp. 173–184. [Google Scholar]
- Robbins, J., J. W. Hamilton, G. L. Lof, and G. B. Kempster. 1992. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 103: 823–829. [Google Scholar] [CrossRef]
- Robbins, J., R. Levine, J. Wood, E. B. Roecker, and E. Luschei. 1995. Age effects on lingual pressure generation as a risk factor for dysphagia. Journal of Gerontology: Medical Sciences 50A: M257–M262. [Google Scholar] [CrossRef]
- Schmidt, R., and J. Toews. 1970. Grip strength as measured by the Jamar Dynamometer. Archives of Physical Medicine and Rehabilitation 51: 321–327. [Google Scholar]
- Shiffman, L. M. 1992. Effects of aging on adult hand function. The American Journal of Occupational Therapy 46, 9: 785–792. [Google Scholar] [CrossRef]
- St. Louis, K. O, and D. M. Ruscello. 1987. Oral speech mechanism screening examination-revised. Austin, Texas: Pro-Ed. [Google Scholar]
- Solomon, N. P., D. A. Robin, D. M. Lorell, R. L. Rodnitzky, and E. S. Luschei. 1994. Edited by J. A. Till, K. M. Yorkston and D. R. Beukelman. Tongue function testing in Parkinson's Disease. In Motor speech disorders. Baltimore: Paul H. Brookes Publishing Co, pp. 147–160. [Google Scholar]
- Solomon, N. P., D. M. Lorell, D. A. Robin, R. L. Rodnitzky, and E. S. Luschei. 1995. Tongue strength and endurance in mild to moderate Parkinson's disease. Journal of Medical Speech-Language Pathology 3: 15–26. [Google Scholar]
- Solomon, N. P., D. A. Robin, and E. S. Luschei. 2000. Strength, endurance, and stability of the tongue and hand in Parkinson's Disease. Journal of Speech, Language, and Hearing Research 43, 1: 256–267. [Google Scholar] [CrossRef]
- Solomon, N. P., K. D. R. Drager, and E. S. Luschei. 2000. Sustaining a Constant Effort by the Tongue and Hand: Effects of Acute Fatigue. Journal of Speech, Language and Hearing Research 45: 613–624. [Google Scholar] [CrossRef] [PubMed]
- Solomon, N. P., and B. Munson. 2004. The effect of jaw position on measures of tongue strength and endurance. Journal of Speech, Language, and Hearing Research 47: 584–594. [Google Scholar] [CrossRef] [PubMed]
- Sonies, B. C., M. Stone, and T. Shawker. 1984. Speech and swallowing in the elderly. Gerontology 3: 115–123. [Google Scholar] [CrossRef] [PubMed]
- Stierwalt, J. A. G., D. A. Robin, N. P. Solomon, A. L. Weiss, and J. E. Max. 1995. Edited by D. A. Robin, K. M. Yorkston and D. R. Beukelman. Tongue strength and endurance: Relation to the speaking ability of children and adolescents following traumatic grain injury. In Disorders of motor speech: Recent advances in assessment, treatment, and clinical characterization. Baltimore: Paul H. Brookes, pp. 243–258. [Google Scholar]
- Thompson, E. C., B. E. Murdoch, and P. D. Stokes. 1995. Tongue function in subjects with upper motor neuron type dysarthria following cerebrovascular accident. Journal of Medical Speech-Language Pathology 3: 27–40. [Google Scholar]
- Tonkovich, J. D., T. J. Latham, and M. W. Rambow. 1982. Dysarthria rehabilitation. Austin, Texas: Pro Ed. [Google Scholar]
- Tracy, J. F., J. A. Logemann, P. J. Kahrilas, P. Jacob, M. Kobara, and C. Krugler. 1989. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia 4: 90–94. [Google Scholar] [CrossRef]
- Weathers, M. 2000. Oral motor abilities in children performing a novel task. Unpublished doctoral dissertation, University of South Carolina, Columbia. [Google Scholar]
© 2008 by the authors. 2008 Carol O’Day, Elaine Frank, Allen Montgomery, Michele Nichols, Hiram McDade