Anaerobic Digestion of Broiler Litter from Different Commercial Farm Flocks
Abstract
1. Introduction
2. Materials and Methods
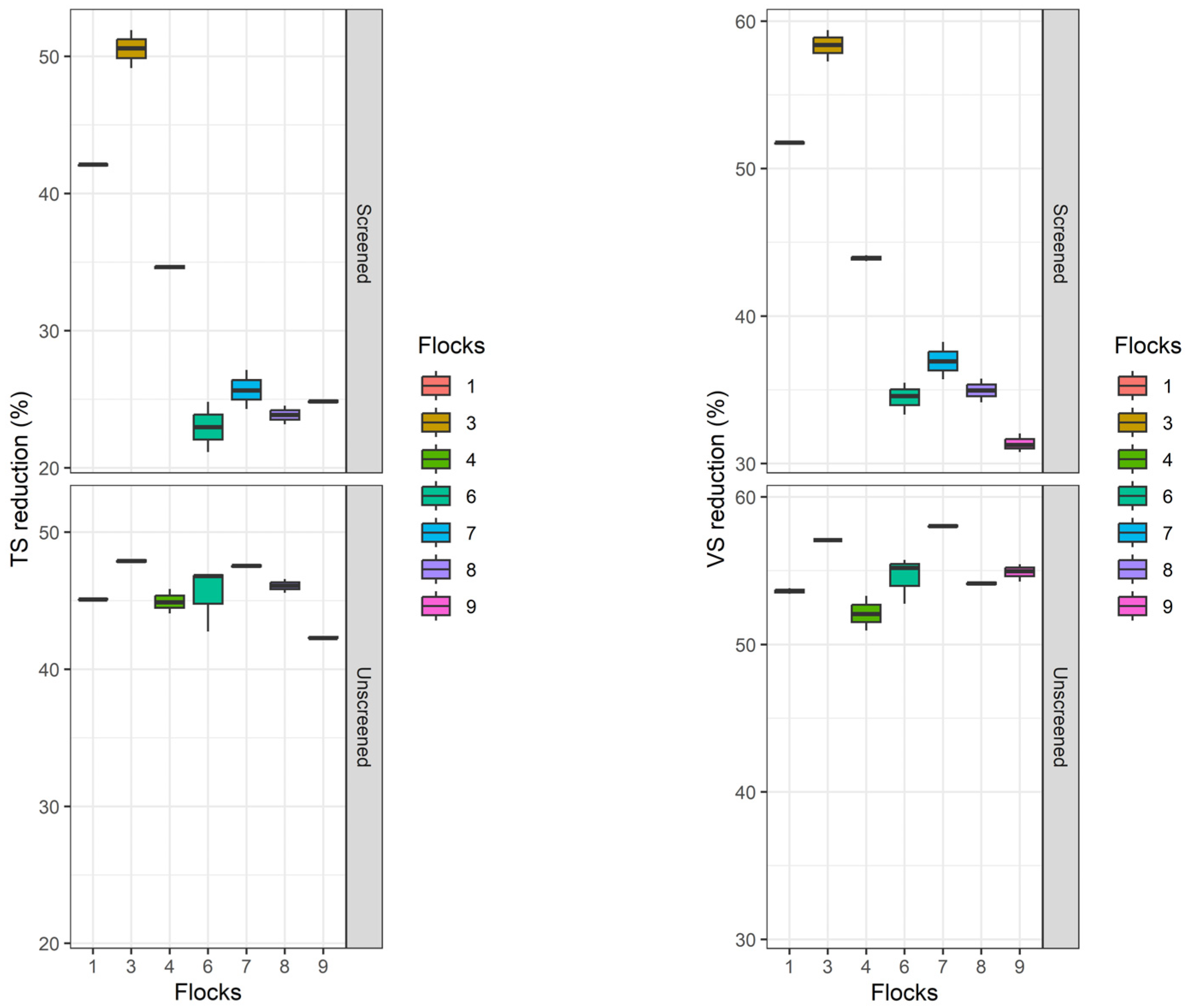
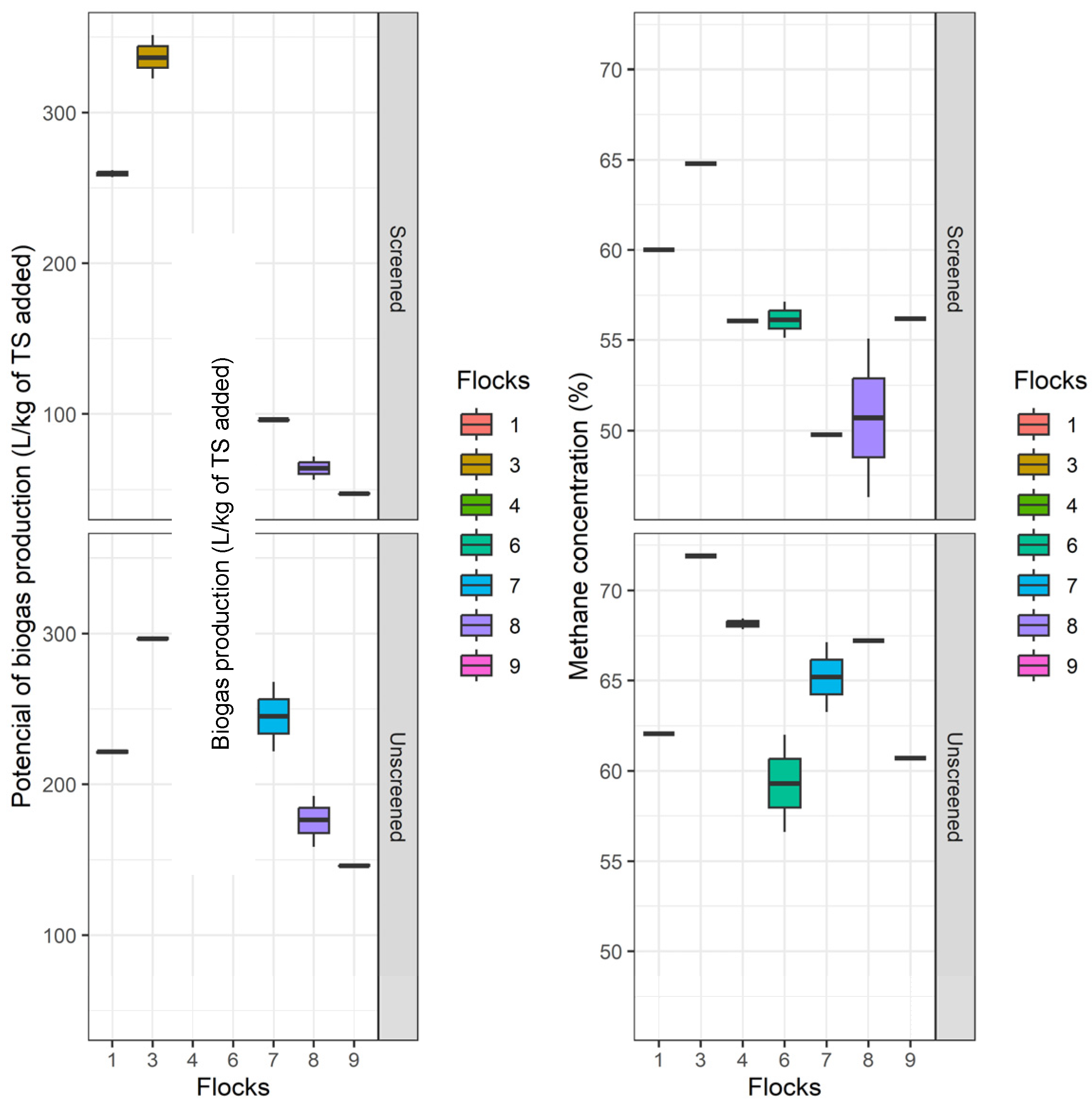
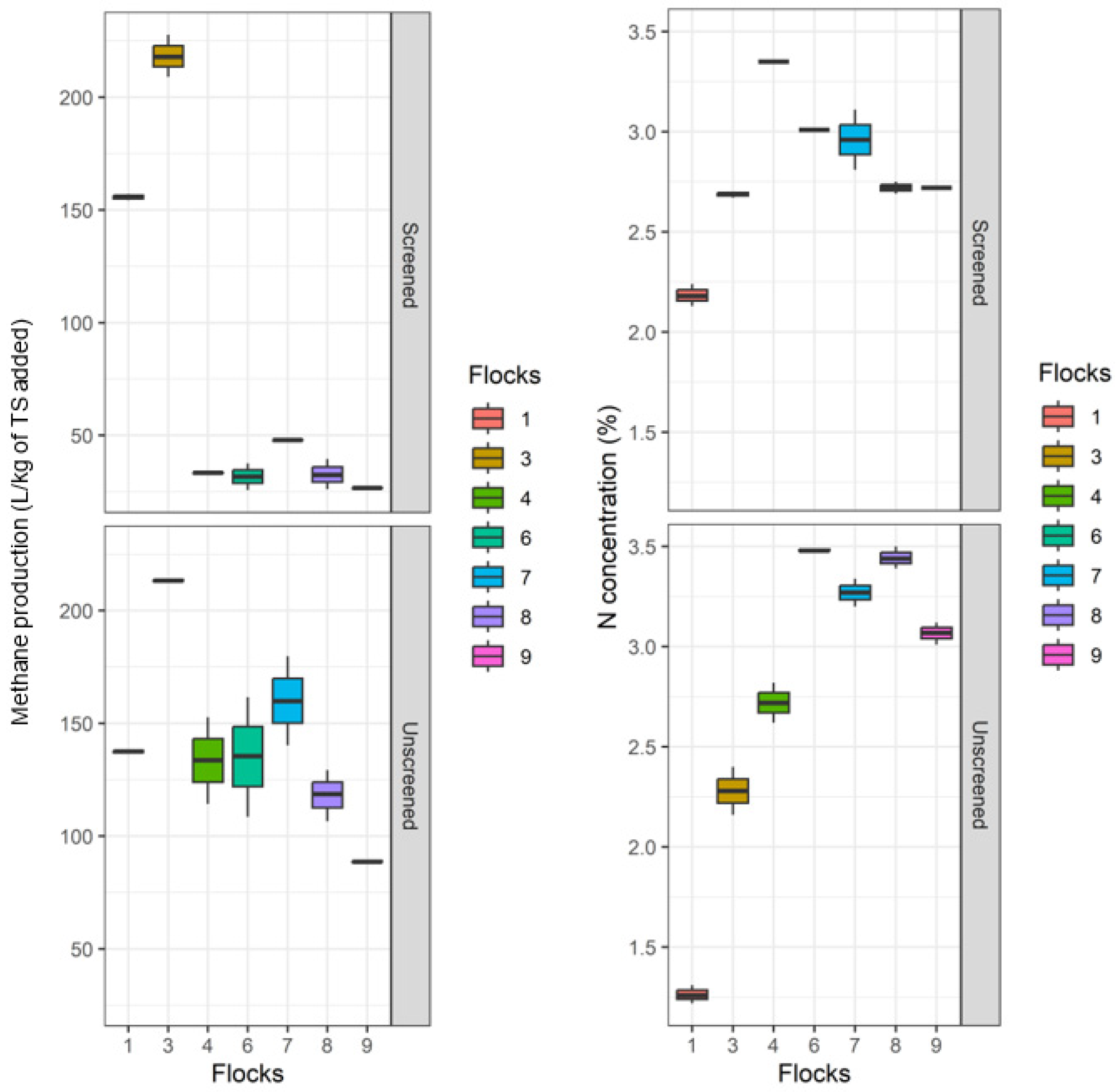
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, C.E.; Thomas, R.; Williams, M.; Zalasiewicz, J.; Edgeworth, M.; Miller, H.; Coles, B.; Foster, A.; Burton, E.J.; Marume, U. The Broiler Chicken as a Signal of a Human Reconfigured Biosphere. R. Soc. Open Sci. 2018, 5, 180325. [Google Scholar] [CrossRef] [PubMed]
- ABPA. Associação Brasileira de Proteína Animal—Relatório Anual; Relatório Anual-ABPA. 2024. Available online: https://abpa-br.org/abpa-relatorio-anual/ (accessed on 1 March 2025).
- Orrico, A.C.A.; Schwingel, A.W.; de M. Costa, M.S.S.; Orrico Junior, M.A.P.; Borquis, R.R.A.; Alves, G.P.; de Oliveira, J.D.; Leite, B.K.V.; Garcia, R.G.; Vilela, R.N.S. Characterization and Valuing of Hatchery Waste from the Broiler Chicken Productive Chain. Waste Manag. 2020, 105, 520–530. [Google Scholar] [CrossRef]
- Jurgutis, L.; Slepetiene, A.; Volungevicius, J.; Amaleviciute-Volunge, K. Biogas Production from Chicken Manure at Different Organic Loading Rates in a Mesophilic Full Scale Anaerobic Digestion Plant. Biomass Bioenergy 2020, 141, 105693. [Google Scholar] [CrossRef]
- Do, A.D.T.; Lozano, A.; Van Laar, T.A.; Mero, R.; Lopez, C.; Hisasaga, C.; Lopez, R.; Franco, M.; Celeste, R.; Tarrant, K.J. Evaluating Microbiome Patterns, Microbial Species, and Leg Health Associated with Reused Litter in a Commercial Broiler Barn. J. Appl. Poult. Res. 2024, 33, 100490. [Google Scholar] [CrossRef]
- Coufal, C.D.; Chavez, C.; Niemeyer, P.R.; Carey, J.B. Measurement of Broiler Litter Production Rates and Nutrient Content Using Recycled Litter. Poult. Sci. 2006, 85, 398–403. [Google Scholar] [CrossRef]
- Alves, A.S.; Schultz, N.; Conforto, B.A.A.F.; Zonta, E.; de Carvalho, D.F. Soil, Water and Nutrient Loss under Simulated Rainfall Patterns in an Area Fertilised with Chicken Litter. J. Hydrol. 2023, 620, 129543. [Google Scholar] [CrossRef]
- Kamp, J.N.; Feilberg, A. Covering Reduces Emissions of Ammonia, Methane, and Nitrous Oxide from Stockpiled Broiler Litter. Biosyst. Eng. 2024, 248, 73–81. [Google Scholar] [CrossRef]
- Collins, B.A.; Birzer, C.H.; Kidd, S.P.; Hall, T.; Medwell, P.R. The Influence of Biochar Position in a Leach Bed System Anaerobically Digesting Chicken Litter. J. Environ. Manag. 2023, 344, 118404. [Google Scholar] [CrossRef]
- Nacimento, R.A.; Rojas Moreno, D.A.; Luiz, V.T.; Avelar de Almeida, T.F.; Rezende, V.T.; Bazerla Andreta, J.M.; Ifuki Mendes, C.M.; Giannetti, B.F.; Gameiro, A.H. Sustainability Assessment of Commercial Brazilian Organic and Conventional Broiler Production Systems under an Emergy Analysis Perspective. J. Clean. Prod. 2022, 359, 132050. [Google Scholar] [CrossRef]
- Li, Z.; You, Z.; Zhang, L.; Chen, H. Effect of Total Solids Content on Anaerobic Digestion of Waste Activated Sludge Enhanced by High-Temperature Thermal Hydrolysis. J. Environ. Manag. 2024, 359, 120980. [Google Scholar] [CrossRef]
- Gyadi, T.; Bharti, A.; Basack, S.; Kumar, P.; Lucchi, E. Influential Factors in Anaerobic Digestion of Rice-Derived Food Waste and Animal Manure: A Comprehensive Review. Bioresour. Technol. 2024, 413, 131398. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. A Review on Enhanced Biogas Production from Anaerobic Digestion of Lignocellulosic Biomass by Different Enhancement Techniques. Process Biochem. 2019, 84, 81–90. [Google Scholar] [CrossRef]
- Alonso, L.L.; Podio, N.S.; Marino, D.J.G.; Almada, N.S.; Gange, J.M.; Bernigaud, I.; Mórtola, N.; Wunderlin, D.A. Evaluating Antibiotic Occurrence, Degradation, and Environmental Risks in Poultry Litter within Argentina’s Agricultural Hub. Sci. Total Environ. 2024, 920, 170993. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Ran, H.; Peng, P.; Wang, Y.; Peng, G.; Wu, Y.; Wen, X. Residual Ciprofloxacin in Chicken Manure Inhibits Methane Production in an Anaerobic Digestion System. Poult. Sci. 2025, 104, 104539. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, A.; Caselli, E.; Lucchi, A.; Sala, C.; Parisi, A.; Manfreda, G.; Mazzacane, S. Impact of a Probiotic-Based Cleaning Product on the Microbiological Profile of Broiler Litters and Chicken Caeca Microbiota. Poult. Sci. 2019, 98, 3602–3610. [Google Scholar] [CrossRef]
- de Oliveira, J.D.; Orrico, A.C.A.; Leite, B.K.V.; Schwingel, A.W.; Orrico Junior, M.A.P.; de Avila, M.R.; Machado, J.F.; Dias, A.M.D.F.; Macena, I.A.; dos Santos, W. Anaerobic Co-Digestion of Swine Manure and Forage at Two Harvesting Ages. Ciênc. Rural 2022, 52, e20200760. [Google Scholar] [CrossRef]
- VDI-4630; Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Verlag des Vereins Deutscher Ingenieure: Düsseldorf, Germany, 2006.
- Kunz, A.; Steinmetz, L.R.R.; do Amaral, A.C. Sociedade Brasileira Dos Especialistas Em Resíduos Das Produções Agropecuária e Agroindustrial-Sbera. In Fundamentos da Digestão Anaeróbia, Purificação do Biogás, Uso e Tratamento do Digestato; Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Detmann, E.; Souza, M.A.; Valadares Filho, S.C.; Queiroz, A.C.; Berchielli, T.T.; Saliba, E.O.S.; Cabral, L.S.; Pina, D.S.; Ladeira, M.M.; Azevedo, J.A.G. Métodos para Análise de Alimentos; Suprema: Visconde do Rio Branco, Brazil, 2012. [Google Scholar]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Kirby, M.E.; Mirza, M.W.; Leigh, T.; Oldershaw, L.; Reilly, M.; Jeffery, S. Destruction of Staphylococcus Aureus and the Impact of Chlortetracycline on Biomethane Production during Anaerobic Digestion of Chicken Manure. Heliyon 2019, 5, e02749. [Google Scholar] [CrossRef]
- Leite, B.K.V.; Orrico, A.C.A.; Orrico Junior, M.A.P.; Aspilcueta Borquis, R.R.; da Costa, É.C.P.; da, S. Menezes, I.; de Oliveira, J.D.; Macena, I.A. Effect of Monensin Supplementation in the Bovine Diet on the Composition and Anaerobic Digestion of Manure with and without Screening. Fermentation 2024, 10, 474. [Google Scholar] [CrossRef]
- Marti, E.; Gros, M.; Boy-Roura, M.; Ovejero, J.; Busquets, A.M.; Colón, J.; Petrovic, M.; Ponsá, S. Pharmaceuticals Removal in an On-Farm Pig Slurry Treatment Plant Based on Solid-Liquid Separation and Nitrification-Denitrification Systems. Waste Manag. 2020, 102, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, K.C.; Mascarenhas, N.M.H.; dos Santos da Rocha, P.A.; Ton, A.P.S.; do Amaral, A.G.; Schneider, R.M.; dos Santos Lima de Brito, A.N.; Furtado, D.A.; do Nascimento, J.W.B. Chicken Bed Reuse. Environ. Sci. Pollut. Res. 2023, 30, 39537–39545. [Google Scholar]
- Aires, A.M. Biodigestão Anaeróbia da Cama de Frangos de Corte Com Ou Sem Separação das Frações Sólida e Líquida. 134 f. Dissertação (Mestrado em Zootecnia)–Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias. 2009. Available online: http://repositorio.unesp.br/handle/11449/96593 (accessed on 1 March 2025).
- Oliveira, M.C.; Ferreira, H.A.; Cancherini, L.C. Efeito de Condicionadores Químicos Sobre a Qualidade Da Cama de Frango [Effect of Chemical Conditioners on Poultry Litter Quality]. Arq. Bras. Med. Vet. Zootec. 2009, 56, 536–541. [Google Scholar]
- Dornelas, K.C.; Schneider, R.M.; do Amaral, A.G. Biogas from Poultry Waste—Production and Energy Potential. Environ. Monit. Assess. 2017, 189, 407. [Google Scholar] [CrossRef]
- Orrico Júnior, M.A.P.; Orrico, A.C.A.; de Lucas Júnior, J. Biodigestão Anaeróbia Dos Resíduos Da Produção Avícola: Cama de Frangos e Carcaças. Eng. Agríc. Jaboticabal 2010, 30, 546–554. [Google Scholar] [CrossRef]
- Adnane, I.; Taoumi, H.; Elouahabi, K.; Lahrech, K.; Oulmekki, A. Valorization of Crop Residues and Animal Wastes: Anaerobic Co-Digestion Technology. Heliyon 2024, 10, e26440. [Google Scholar]
- Orrico Júnior, M.A.P.; Orrico, A.C.A.; de Lucas Júnior, J. Compostagem Dos Resíduos Da Produção Avícola: Cama de Frangos e Carcaças de Aves. Eng. Agríc. Jaboticabal 2010, 30, 538–545. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic Co-Digestion of Animal Manures and Lignocellulosic Residues as a Potent Approach for Sustainable Biogas Production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar]
- Caetano, B.C.; Santos, N.D.S.A.; Hanriot, V.M.; Sandoval, O.R.; Huebner, R. Energy Conversion of Biogas from Livestock Manure to Electricity Energy Using a Stirling Engine. Energy Convers. Manag. X 2022, 15, 100224. [Google Scholar] [CrossRef]
- Vicente Júnior, D.J. Biodigestão e Co-Digestão Anaeróbias de Cama de Frangos Com Água Residuária de Suinocultura. 68 f. Dissertação (Mestrado em Engenharia Agrícola)-Universidade Estadual do Oeste do Paraná. 2012. Available online: https://tede.unioeste.br/handle/tede/2876 (accessed on 1 March 2025).
- Rani, J.; Pandey, K.P.; Kushwaha, J.; Priyadarsini, M.; Dhoble, A.S. Antibiotics in Anaerobic Digestion: Investigative Studies on Digester Performance and Microbial Diversity. Bioresour. Technol. 2022, 361, 127662. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Osman, A.I.; Ai, P.; Zhou, Z.; Meng, F.; Rooney, D.W. Bioenergy Production from Chicken Manure: A Review. Environ. Chem. Lett. 2023, 21, 2707–2727. [Google Scholar]
- Bi, S.; Westerholm, M.; Hu, W.; Mahdy, A.; Dong, T.; Sun, Y.; Qiao, W.; Dong, R. The Metabolic Performance and Microbial Communities of Anaerobic Digestion of Chicken Manure under Stressed Ammonia Condition: A Case Study of a 10-Year Successful Biogas Plant. Renew. Energy 2021, 167, 644–651. [Google Scholar] [CrossRef]
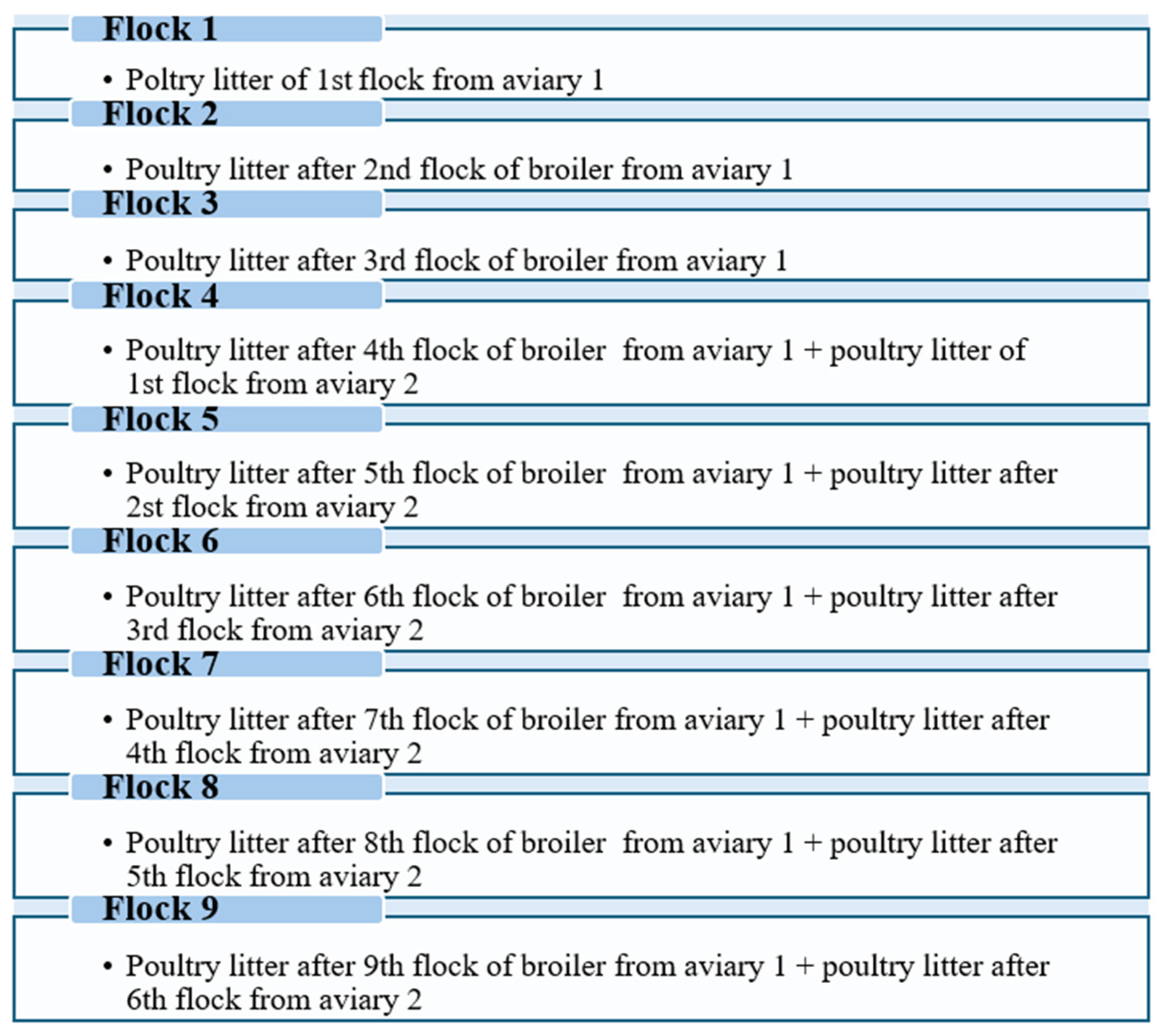
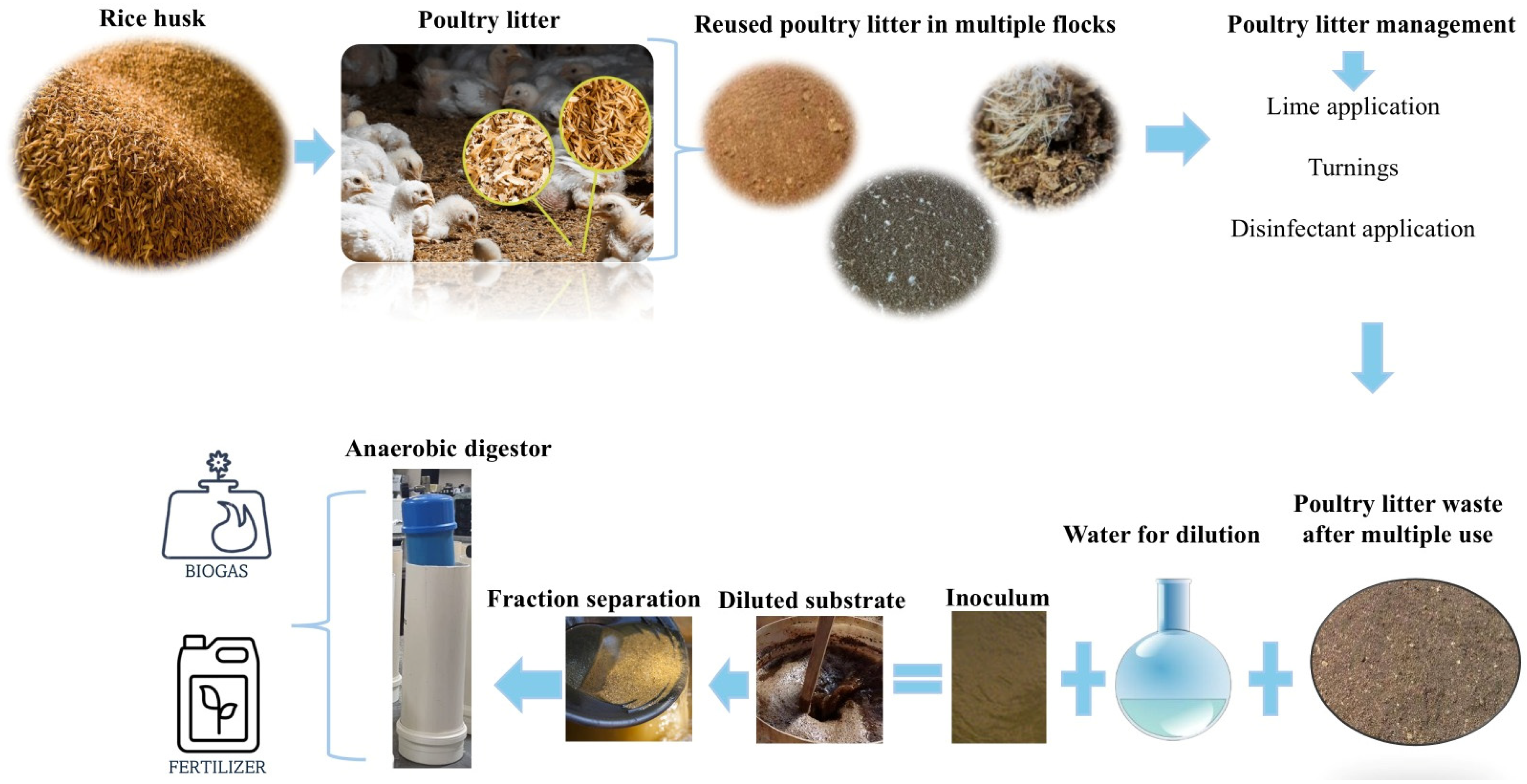
| Fresh Poultry Litter | pH | TS (%) | VS (%) * | C (%) * | N (%) * | C/N Ratio | NDF (%) * | ADF (%) * |
|---|---|---|---|---|---|---|---|---|
| Flock 1 | 9.70 | 80.23 | 78.32 | 19.12 | 2.01 | 9.51 | 58.48 | 39.15 |
| Flock 2 | 9.55 | 77.88 | 74.74 | 17.71 | 2.11 | 8.39 | 52.76 | 31.69 |
| Flock 3 | 8.89 | 86.38 | 83.45 | 17.48 | 2.27 | 7.70 | 41.76 | 20.53 |
| Flock 4 | 8.35 | 85.13 | 87.31 | 20.17 | 2.82 | 7.15 | 38.60 | 15.89 |
| Flock 5 | 8.93 | 78.25 | 78.44 | 22.65 | 2.61 | 8.68 | 48.77 | 22.12 |
| Flock 6 | 8.80 | 77.44 | 80.72 | 22.30 | 2.70 | 8.26 | 41.20 | 15.22 |
| Flock 7 | 8.86 | 77.74 | 80.53 | 19.20 | 2.70 | 7.11 | 44.54 | 15.68 |
| Flock 8 | 8.74 | 78.27 | 82.12 | 21.28 | 2.70 | 7.88 | 43.11 | 16.06 |
| Flock 9 | 8.87 | 80.65 | 83.25 | 18.71 | 2.57 | 7.28 | 39.62 | 16.80 |
| Substrates | pH | TS (%) | VS (%) * | N (%) * | COD (mg/O2/L) | BOD (mg/O2/L) | HRT (Days) |
|---|---|---|---|---|---|---|---|
| Flock 1 SC | 9.45 | 2.55 | 69.61 | 2.77 | 17,106.67 | 7700.00 | 98 |
| Flock 2 SC | 9.37 | 2.52 | 67.49 | 2.29 | 15,313.33 | 8940.00 | 167 |
| Flock 3 SC | 8.91 | 2.17 | 73.61 | 2.97 | 18,886.67 | 8560.00 | 133 |
| Flock 4 SC | 8.46 | 2.49 | 82.77 | 4.21 | 20,160.00 | 8377.00 | 167 |
| Flock 5 SC | 8.97 | 2.89 | 72.77 | 2.76 | 12,000.00 | 7240.00 | 167 |
| Flock 6 SC | 8.78 | 2.82 | 71.82 | 2.77 | 12,626.67 | 8480.00 | 121 |
| Flock 7 SC | 8.78 | 2.97 | 75.44 | 2.54 | 19,480.00 | 8200.00 | 121 |
| Flock 8 SC | 8.83 | 2.78 | 78.17 | 2.66 | 17,540.00 | 8680.00 | 98 |
| Flock 9 SC | 8.82 | 2.36 | 70.08 | 3.68 | 16,533.33 | 8560.00 | 161 |
| Flock 1 US | 9.43 | 4.53 | 79.26 | 1.96 | 20,250.00 | 15,550.00 | 70 |
| Flock 2 US | 9.28 | 4.78 | 77.28 | 1.48 | 25,133.33 | 21,850.00 | 121 |
| Flock 3 US | 8.98 | 4.12 | 79.94 | 2.17 | 28,433.33 | 18,750.00 | 133 |
| Flock 4 US | 8.53 | 4.36 | 83.27 | 3.02 | 29,833.33 | 20,400.00 | 98 |
| Flock 5 US | 9.01 | 4.32 | 78.19 | 2.42 | 22,333.33 | 16,800.00 | 121 |
| Flock 6 US | 8.86 | 4.09 | 81.87 | 2.42 | 26,900.00 | 22,250.00 | 121 |
| Flock 7 US | 8.85 | 4.72 | 82.64 | 2.44 | 28,700.00 | 18,100.00 | 167 |
| Flock 8 US | 8.73 | 4.60 | 81.96 | 3.24 | 24,383.33 | 17,010.00 | 121 |
| Flock 9 US | 8.80 | 4.46 | 81.41 | 2.77 | 27,116.66 | 13,370.00 | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orrico, A.C.A.; Leite, B.K.V.; Oliveira, J.D.d.; Blans, K.F.; Menezes, I.d.S.; Souza, V.; Gimenes, R.M.T.; Aspilcueta Borquis, R.R.; Orrico Junior, M.A.P. Anaerobic Digestion of Broiler Litter from Different Commercial Farm Flocks. Poultry 2025, 4, 19. https://doi.org/10.3390/poultry4020019
Orrico ACA, Leite BKV, Oliveira JDd, Blans KF, Menezes IdS, Souza V, Gimenes RMT, Aspilcueta Borquis RR, Orrico Junior MAP. Anaerobic Digestion of Broiler Litter from Different Commercial Farm Flocks. Poultry. 2025; 4(2):19. https://doi.org/10.3390/poultry4020019
Chicago/Turabian StyleOrrico, Ana Carolina Amorim, Brenda Kelly Viana Leite, Juliana Dias de Oliveira, Karina Fidelis Blans, Isabella da Silva Menezes, Vanessa Souza, Régio Marcio Toesca Gimenes, Rusbel Raul Aspilcueta Borquis, and Marco Antônio Previdelli Orrico Junior. 2025. "Anaerobic Digestion of Broiler Litter from Different Commercial Farm Flocks" Poultry 4, no. 2: 19. https://doi.org/10.3390/poultry4020019
APA StyleOrrico, A. C. A., Leite, B. K. V., Oliveira, J. D. d., Blans, K. F., Menezes, I. d. S., Souza, V., Gimenes, R. M. T., Aspilcueta Borquis, R. R., & Orrico Junior, M. A. P. (2025). Anaerobic Digestion of Broiler Litter from Different Commercial Farm Flocks. Poultry, 4(2), 19. https://doi.org/10.3390/poultry4020019







