Buriti Oil (Mauritia flexuosa L.) as Functional Feed for Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment I
Determining the Nutritional Composition and Metabolizable Energy of BO
2.2. Experiment II
2.2.1. Birds and Experimental Design
2.2.2. Diet
2.2.3. Performance and Carcass Yield
2.2.4. Nutrient Digestibility
2.2.5. Meat’s Physical Analyses
2.2.6. Blood Parameters
2.2.7. Intestinal Morphometry
2.2.8. Collagen Concentrate
2.2.9. Histological Analyses
2.2.10. Economic Analysis
2.2.11. Statistical Analyses
3. Results
3.1. Nutritional Composition and Metabolizable Energy of BO
3.2. Performance
3.3. Nutrient Digestibility
3.4. Carcass and Meat Cuts Yield
3.5. Meat’s Physical Analyses
3.6. Blood Parameters
3.7. Intestinal Morphometry
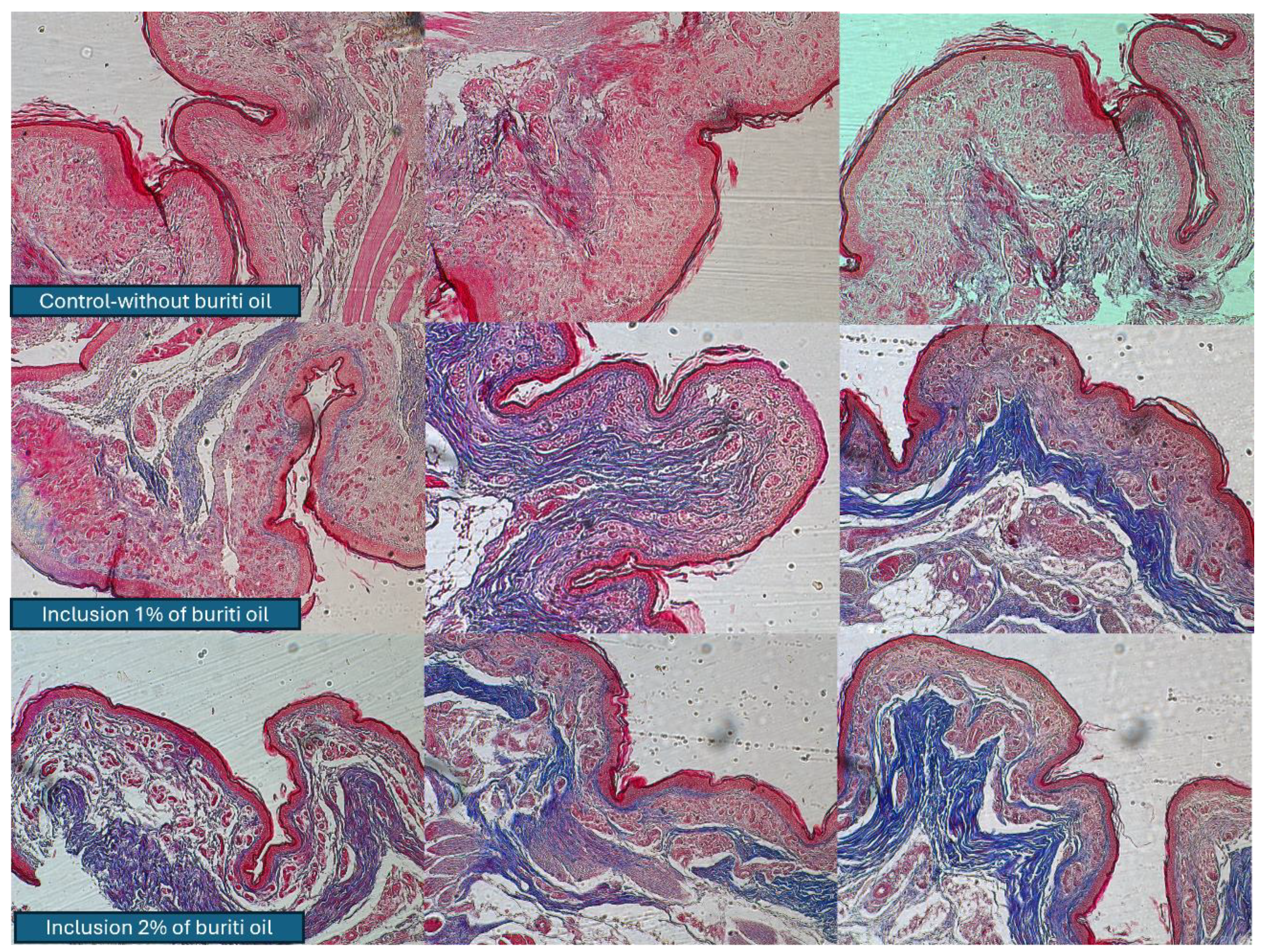
3.8. Collagen Concentration and Histological Analyses
3.9. Economic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wickramasuriya, S.S.; Ault, J.; Ritchie, S.; Gay, C.G.; Lillehoj, H.S. Alternatives to Antibiotic Growth Promoters for Poultry: A Bibliometric Analysis of the Research Journals. Poult. Sci. 2024, 103, 103987. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Heal. Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Lee, Y.; Lu, M.; Lillehoj, H.S. Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines 2022, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Bess, F.; Favero, A.; Vieira, S.L.; Torrent, J. The Effects of Functional Oils on Broiler Diets of Varying Energy Levels. J. Appl. Poult. Res. 2012, 21, 567–578. [Google Scholar] [CrossRef]
- Su, G.; Wang, L.; Zhou, X.; Wu, X.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Mao, X.; Zheng, P.; et al. Effects of Essential Oil on Growth Performance, Digestibility, Immunity, and Intestinal Health in Broilers. Poult. Sci. 2021, 100, 101242. [Google Scholar] [CrossRef]
- Barbalho, R.L.C.; Castaneda, C.; Araújo, L.F.; Kiess, A.S.; Carvalho, R.S.B.; Barbalho, C.B.; Borges, L.L.; Bonato, M.A. Β—Glucans and MOS, Essential Oil, and Probiotics in Diets of Broilers Challenged with Eimeria spp. and Clostridium perfringens. Poult. Sci. 2023, 102, 102541. [Google Scholar] [CrossRef]
- Choi, J.; Singh, A.K.; Chen, X.; Lv, J.; Kim, W.K. Application of Organic Acids and Essential Oils as Alternatives to Antibiotic. Growth Promoters in Broiler Chickens. Animals 2022, 12, 2178. [Google Scholar] [CrossRef]
- Corduk, M.; Sarica, S.; Yarim, G.F. Effects of Oregano or Red Pepper Essential Oil Supplementation to Diets for Broiler Chicks with Delayed Feeding after Hatching. 1. Performance and Microbial Population. J. Appl. Poult. Res. 2013, 22, 738–749. [Google Scholar] [CrossRef]
- Silva, S.M.; Sampaio, K.A.; Taham, T.; Rocco, S.A.; Ceriani, R.; Meirelles, A.J.A. Characterization of Oil Extracted from Buriti Fruit (Mauritia flexuosa) Grown in the Brazilian Amazon Region. J. Am. Oil Chem. Soc. 2009, 86, 611–616. [Google Scholar] [CrossRef]
- Souza Aquino, J.; Pontes Pessoa, D.C.N.; Lourdes, G.V.; Araújo, K.; Epaminondas, P.S.; Schuler, A.R.P.; Souza, A.G.; Stamfordb, T.L.M. Refining of Buriti Oil (Mauritia flexuosa) Originated from the Brazilian Cerrado: Physicochemical, Thermal-Oxidative and Nutritional Implications. J. Braz. Chem. Soc. 2012, 23, 212–219. [Google Scholar] [CrossRef]
- Klemm, R.D.W.; Labrique, A.B.; Christian, P.; Rashid, M.; Shamim, A.A.; Katz, J.; Sommer, A.; West, K.P. Newborn Vitamin a Supplementation Reduced Infant Mortality in Rural Bangladesh. Pediatrics 2008, 122, e242–e250. [Google Scholar] [CrossRef] [PubMed]
- Mariath, J.G.R.; Lima, M.C.C.; Santos, L.M.P. Vitamin A Activity of Buriti (Mauritia vinifera Mart) and Its Effectiveness in the Treatment and Prevention of Xerophthalmia. Am. J. Clin. Nutr. 1989, 49, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.L.S.; Guedes, I.; Alcantara, P.; Moreira, S.G.C.; Barbosa Neto, N.M.; Correa, D.S.; Zilio, S.C. Characterization of Buriti (Mauritia flexuosa L.) Oil by Absorption and Emission Spectroscopies. J. Braz. Chem. Soc. 2005, 16, 1113–1117. [Google Scholar] [CrossRef]
- Ferreira, M.O.G.; Lima, I.S.; Ribeiro, A.B.; Lobo, A.O.; Rizzo, M.S.; Osajima, J.A.; Estevinho, L.M.; Silva-Filho, E.C. Biocompatible Gels of Chitosan-Buriti Oil for Potential Wound Healing Applications. Materials 2020, 13, 1977. [Google Scholar] [CrossRef]
- Novak, A.F.; Clark, G.C.; Dupuy, H.P. Antimicrobial Activity of Some Ricinoleic Acid Oleic Acid Derivatives. J. Am. Oil Chem. Soc. 1961, 38, 321–324. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Darnet, S.; Silva, L.H.M. Fatty Acid Profiles and Tocopherol Contents of Buriti (Mauritia flexuosa), Patawa (Oenocarpus bataua), Tucuma (Astrocaryum vulgare), Mari (Poraqueiba paraensis) and Inaja (Maximiliana maripa) Fruits. J. Braz. Chem. Soc. 2010, 21, 2000–2004. [Google Scholar] [CrossRef]
- Cruz, M.B.; Oliveira, W.S.; Araújo, R.L.; Honório França, A.C.; Pertuzatti, P.B. Buriti (Mauritia flexuosa L.) Pulp Oil as an Immunomodulator against Enteropathogenic Escherichia coli. Ind. Crops Prod. 2020, 149, 112330. [Google Scholar] [CrossRef]
- Pighinelli, L. Application of Chitosan and Buriti Oil (Mauritia flexuosa L.) in Skin Wound Healing. J. Appl. Biotechnol. Bioeng. 2017, 3, 272–279. [Google Scholar] [CrossRef]
- Carvalho, F.L.A.; Lopes, P.M.; Moura, F.A.S.; Dourado, L.B.R.; Souza, R.G.; Feitoza, A.C.; Oliveira, A.N.; Biagiotti, D. Buriti Oil as an Alternative to the Use of Antimicrobials in Broiler Diets. An. Acad. Bras. Cienc. 2024, 96, e20230577. [Google Scholar] [CrossRef]
- Santis, R.; Albuquerque, F.C.F.; Silva, R.L.S.; Mesquita, L.R.; Ferreira, I.D.; Dourado, L.R.B.; Ferreira, G.J.B.C. Intestina l Morphometry and Performance of Broiler Chickens Subjected to Diets with Buriti Oil. Arq. Bras. Med. Veterinária e Zootec. 2022, 74, 359–366. [Google Scholar] [CrossRef]
- Medeiros, R.M.; Cavalcanti, E.P.; Duarte, J.F.M. Classificação Climática De Köppen Para O Estado Do Piauí—Brasil. Rev. Equador 2020, 9, 82–99. [Google Scholar] [CrossRef]
- Rostagno, H.S.; Albino, L.F.T.; Donzele, J.L.; Gomes, P.C.; Oliveira, R.D.; Lopes, D.C.; Euclides, R.F. Tabelas Brasileiras Para Aves e Suínos: Composição de Alimentos e Exigências Nutricionais; 4a.; Federal University of Viçosa: Viçosa, Brazil, 2017. [Google Scholar]
- Sakomura, N.K.; Rostagno, H.S. Métodos de Pesquisa Em Nutrição de Monogástricos; 2a.; Foundation to Support Research, Teaching and Extension: Jaboticabal, Brazil, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Matterson, L.D.; Potter, L.M.; Stutz, M.W. Metabolizable Energy and Digestibility Coefficients of Barley for Chicks as Influenced by Water Treatment or by Presence of Fungal Enzyme. Poult. Sci. 1965, 44, 565–573. [Google Scholar] [CrossRef]
- Thrall, M.A.; Baker, D.C.; Campbell, T.W.; Denicola, D.; Fettman, M.J.; Lasse, E.D.; Rebar, A.; Weiser, G. Hematologia e Bioquímica Clínica Veterinária; rded: Roca, Brazil, 2015. [Google Scholar]
- Sousa, S.C.; Silva, G.G.; Sousa Moura, F.A.; Pereira, D.R.; Machado, L.P.; Santos Silva, L.; Silva Delgado, F.; Bezerra, R.M.; Dourado, L.R.B. Synbiotic Supplements as Antibiotic Alternatives in Broiler Diets. Semin. Agrar. 2023, 44, 1859–1877. [Google Scholar] [CrossRef]
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory Methods in Histotechnology (Armed Forces Institute of Phatology); American Registry of Pathology: Washington, DC, USA, 1992. [Google Scholar]
- Paiva, J.G.A.; Fank Carvalho, S.M.; Magalhães, M.P.; Graciano-Ribeiro, D. Verniz Vitral Incolor 500®: Uma Alternativa de Meio de Montagem Economicamente Viável. Acta Bot. Brasilica 2006, 20, 257–264. [Google Scholar] [CrossRef]
- Della Torre, J.C.; Lichtig, J.; Beraquet, N.J. Validação Do Método Espectrofotométrico Para Quantificação Do Aminoácido Hidroxiprolina Em Conservas de Carne. Rev. Inst. Adolfo Lutz 2004, 63, 35–42. [Google Scholar] [CrossRef]
- Macovescu, G.; Chelaru, C.; Albu Kaya, M.G.; Albu, L. Medical Bioproducts Collagen Quantification by Hydroxyproline Determination. In Proceedings of the ICAMS 2016—6th International Conference on Advanced Materials and Systems, Bucharest, Romania, 20–22 October 2016; pp. 105–110. [Google Scholar] [CrossRef]
- Pasquali, G.A.M.; Oliveira, R.F.; Aiello, P.A.B.; Polycarpo, G.V.; Crivellari, R.; Cruz-Polycarpo, V.C. Performance and Economic Viability of Broiler Chicken Fed Diets with Multienzyme Complexes. Acta Sci. Anim. Sci. 2017, 39, 91. [Google Scholar] [CrossRef]
- Nascif, C.C.C.; Gomes, P.C.; Albino, L.F.T.; Rostagno, H.S. Determinação Dos Valores Energéticos de Alguns Óleos e Gorduras Para Pintos de Corte Machos e Fêmeas Aos 21 Dias de Idade. Rev. Bras. Zootec. 2004, 33, 375–385. [Google Scholar] [CrossRef]
- Almeida, A.P.S.; Pinto, M.F.; Poloni, L.B.; Ponsano, E.H.G.; Garcia Neto, M. Efeito Do Consumo de Óleo de Linhaça e de Vitamina E No Desempenho e Nas Características de Carcaças de Frangos de Corte. Arq. Bras. Med. Veterinária e Zootec. 2009, 61, 698–705. [Google Scholar] [CrossRef]
- Murakami, K.T.T.; Pinto, M.F.; Ponsano, E.H.G.; Garcia Neto, M. Desempenho Produtivo e Qualidade Da Carne de Frangos Alimentados Com Ração Contendo Óleo de Linhaça. Pesqui. Agropecuária Bras. 2010, 45, 401–407. [Google Scholar] [CrossRef]
- Lara, L.J.C.; Baião, N.C.; Aguilar, C.A.L.; Cançado, S.V.; Fiuza, M.A.; Ribeiro, B.R.C. Rendimento, Composição e Teor de Ácidos Graxos Da Carcaça de Frangos de Corte Alimentados Com Diferentes Fontes Lipídicas. Arq. Bras. Med. Veterinária e Zootec. 2006, 58, 108–115. [Google Scholar] [CrossRef]
- Perini, J.Â.L.; Stevanato, F.B.; Sargi, S.C.; Visentainer, J.V.J.E.L.; Dalalio, M.M.d.O.; Matshushita, M.; Souza, N.E.; Visentainer, J.V.J.E.L.; Souza, N.E.; Visentainer, J.V.J.E.L. Ácidos Graxos Poli-Insaturados n-3 e n-6: Metabolismo Em Mamíferos e Resposta Imune. Rev. Nutr. 2010, 23, 1075–1086. [Google Scholar] [CrossRef]
- Manhezi, A.C.; Bachion, M.M.; Pereira, Â.L. Utilização de Ácidos Graxos Essenciais No Tratamento de Feridas. Rev. Bras. Enferm. 2008, 61, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Joseph, M.; Alfaro-Wisaquillo, M.C.; Quintana-Ospina, G.A.; Patiño, D.; Vu, T.; Dean, L.L.; Fallen, B.; Mian, R.; Taliercio, E.; et al. Effects of High Oleic Full-Fat Soybean Meal on Broiler Live Performance, Carcass and Parts Yield, and Fatty Acid Composition of Breast Fillets. Poult. Sci. 2024, 103, 103399. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.S. Óleo de Buriti Em Dietas Para Frangos de Corte: Desempenho e Características Da Carne. Ph.D. Thesis, Universidade Federal do Piauí, Bom Jesus, Brazil, 2017. [Google Scholar]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, Color, and Texture of Thai Indigenous and Broiler Chicken Muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- Schneider, J.P. Carne Análoga Ao DFD Em Frangos (OU) Carne DFD Em Frangos. Ph.D. Thesis, Faculdade de Ciências Farmacêuticas, São Paulo, Brazil, 2004. [Google Scholar]
- Pérez-Vendrell, A.M.; Hernández, J.M.; Llauradó, L.; Schierle, J.; Brufau, J. Influence of Source and Ratio of Xanthophyll Pigments on Broiler Chicken Pigmentation and Performance. Poult. Sci. 2001, 80, 320–326. [Google Scholar] [CrossRef]
- Costa, R.G.; Santos, N.M.; Sousa, W.H.; Queiroga, R.d.C.R.E.; Azevedo, P.S.; Cartaxo, F.Q. Qualidade Física e Sensorial Da Carne de Cordeiros de Três Genótipos Alimentados Com Rações Formuladas Com Duas Relações Volumoso: Concentrado. Rev. Bras. Zootec. 2011, 40, 1781–1787. [Google Scholar] [CrossRef]
- Carvalho, F.L.A. Óleo De Buriti Como Alternativa Aos Antibióticos Melhoradores De Desempenho Em Dietas Para Frangos De Corte. Ph.D. Thesis, Universidade Federal do Piauí, Bom Jesus, Brazil, 2019. [Google Scholar]
- Cardoso, A.L.S.P.; Tessari, E.N.C. Interação Entre Imunidade e Nutrição Das Aves: Revisão de Literatura. Rev. Científica Med. Veterinária 2015, 24, 20. [Google Scholar]
- Batista, S.; Olinda, R.G.; Medeiros, V.B.; Rodrigues, C.M.F.; Oliveira, A.F.; Paiva, E.S.; Freitas, C.I.A.; da Cunha Medeiros, A. Atividade Antibacteriana e Cicatrizante Do Óleo de Buriti Mauritia flexuosa L. Cienc. Rural 2012, 42, 136–141. [Google Scholar] [CrossRef]
- Delbem, N.L.C. Desempenho, Rendimento de Carcaça e Qualidade Da Carne de Frangos Suplementados Com Óleo de Soja e Antioxidantes. Ph.D. Thesis, Universidade Estadual Paulista, Botucatu, Brazil, 2014. [Google Scholar]
- Falcão, A.O.; Speranza, P.; Ueta, T.; Martins, I.M.; Macedo, G.A.; Macedo, J.A. Antioxidant Potential and Modulatory Effects of Restructured Lipids from the Amazonian Palms on Liver Cells. Food Technol. Biotechnol. 2017, 55, 553–561. [Google Scholar] [CrossRef]
- Kuzmuk, K.N.; Swanson, K.S.; Tappenden, K.A.; Schook, L.B.; Fahey, G.C. Diet and Age Affect Intestinal Morphology and Large Bowel Fermentative End-Product Concentrations in Senior and Young Adult Dogs. J. Nutr. 2005, 135, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Vieira, A.B.D.; Kamada, I. Treatment of Open Wounds Using Mosqueta Rose: A Review. Rev. Bras. Enferm. 2009, 62, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.V.; Diogenes, L.V.; Oliveira, R.L.; Souza, M.N.S.; Mazza, P.H.S.; Silva Júnior, J.M.; Pereira, E.S.; Parente, M.O.M.; Araújo, M.J.; Oliveira, J.P.F.; et al. Effect of Dietary Buriti Oil on the Quality, Fatty Acid Profile and Sensorial Attribut es of Lamb Meat. Meat Sci. 2022, 186, 108734. [Google Scholar] [CrossRef] [PubMed]
- Parente, M.O.M.; Rocha, K.S.; Bessa, R.J.B.; Parente, H.N.; Zanine, A.M.; Machado, N.A.F.; Lourenço Júnior, J.B.; Bezerra, L.R.; Landim, A.V.; Alves, S.P. Effects of the Dietary Inclusion of Babassu Oil or Buriti Oil on Lamb Performance, Meat Quality and Fatty Acid Composition. Meat Sci. 2020, 160, 107971. [Google Scholar] [CrossRef]
- Darnet, S.H.; Silva, L.H.M.; Rodrigues, A.M.C.; Lins, R.T. Composição Centesimal, Em Ácidos Graxos e Tocoferóis Das Polpas Amazônicas de Buriti (Mauritia flexuosa) e de Patauá (Oenocarpus bataua). Cienc. e Tecnol. Aliment. 2011, 31, 488–491. [Google Scholar] [CrossRef]
- Silva, N.R.R.N.; Cavalcante, R.B.M.; Silva, F.A. Nutritional Properties of Buriti (Mauritia flexuosa) and Health Benefits. J. Food Compos. Anal. 2023, 117, 105092. [Google Scholar] [CrossRef]
- Araújo, I.M.; de Alencar Silva, A.; Pereira-de-Morais, L.; de Menezes Dantas, D.; de Oliveira Barbosa, M.; Leite GM, L.; Nonato, C.d.F.A.; da Costa, J.G.M.; Pereira, R.L.S.; Mendonça, M.R.K.; et al. Phytochemical characterization, toxicity and pharmacological profile of the central effects of the Fixed Fruit Pulp Oil of Mauritia flexuosa L.F. (Buriti). Fitoterapia 2025, 180, 106303. [Google Scholar] [CrossRef]
- Ferreira, B.S.; Almeida, C.G.; Faza, L.P.; Almeida, A.; Diniz, C.G.; Silva, V.L.; Grazul, R.M.; Le Hyaric, M. Comparative Properties of Amazonian Oils Obtained by Different Extraction Methods. Molecules 2011, 16, 5875–5885. [Google Scholar] [CrossRef]
| Reference Diet * | Pre-Starter | Starter | |
|---|---|---|---|
| Corn (7.8% CP) | 65.434 | 52.491 | 56.321 |
| Soybean meal (48% CP) | 30.023 | 38.232 | 35.511 |
| Soybean oil | 0.659 | 3.429 | 3.528 |
| Dicalcium phosphate | 1.474 | 1.857 | 1.641 |
| Limestone | 0.994 | 0.986 | 0.903 |
| Salt | 0.481 | 0.534 | 0.517 |
| Vitamin and mineral premix 1 | 0.400 | 0.500 | 0.400 |
| L-Lysine HCL | 0.263 | 0.229 | 0.248 |
| DL-Methionine | 0.272 | 0.356 | 0.3396 |
| L-Threonine | - | 0.086 | 0.085 |
| L-Arginine | - | - | 0.007 |
| Buriti oil | - | 0.000 | 0.000 |
| Inert | - | 1.300 | 0.500 |
| Metabolizable Energy (Mcal/kg) | 3002 | 2975 | 3050 |
| Linoleic acid | 1.835 | 3.101 | 3.205 |
| Ca | 0.860 | 0.971 | 0.878 |
| Available P | 0.384 | 0.463 | 0.419 |
| K | 0.823 | 0.975 | 0.929 |
| Cl | 0.341 | 0.385 | 0.377 |
| Na | 0.210 | 0.225 | 0.225 |
| Crude Protein (%) | 20.00 | 23.00 | 22.00 |
| Digestible Lysine | 1.141 | 1.307 | 1.256 |
| Digestible Methionine + Cysteine | 0.822 | 0.967 | 0.929 |
| Digestible Methionine | 0.539 | 0.657 | 0.630 |
| Digestible Arginine | 1.117 | 1.413 | 1.344 |
| Gross energy (kcal/kg of NM) | 8375 |
| Metabolizable energy (kcal/kg of NM) | 6854 ± 521 |
| Apparent metabolizable energy (kcal/kg DM) | 6924 ± 516 |
| Dry matter | 99.88 |
| Crude protein | 0.75 |
| Ether extract | 99.3 |
| Fatty acids (%) | |
| C12:0 | - |
| C14:0 | 1.14 |
| C15:0 | - |
| C16:0 | 18.57 |
| C16:1 cis9 | 0.54 |
| C17:0 | - |
| C18:0 | 2.23 |
| C18:1 cis9 | 76.2 |
| C18:1 cis11–13 | 1.53 |
| C18:2 n–6 | 1.88 |
| C18:3 n–3 | 1.01 |
| C20:0 | 0.80 |
| C22:0 | - |
| C24:0 | - |
| Others | 2.48 |
| BO% | Variables | |||
|---|---|---|---|---|
| BW, g/bird | BWG, g/bird | FI, g/bird | FC | |
| 0 | 1070.0 a | 1025.3 a | 1362.8 | 1.32 a |
| 1 | 991.6 b | 947.3 b | 1352.6 | 1.43 b |
| 2 | 1021.6 b | 977.6 b | 1364.5 | 1.39 b |
| Probability | 0.0223 | 0.0217 | 0.5895 | 0.0483 |
| Regression | ns | ns | ns | ns |
| SEM | 12.4 | 12.31 | 4.86 | 0.02 |
| CV % | 4.23 | 4.38 | 1.55 | 4.76 |
| BO% | Variables | |||||
|---|---|---|---|---|---|---|
| AMEn | GEDC | DMDC | CPDC | EEDC | MMR | |
| (Kcal kg−1) | (%) | |||||
| 0 | 3043 | 84.48 | 83.15 | 83.21 | 89.0 | 66.4 |
| 1 | 3216 | 85.54 | 85.54 | 83.42 | 88.7 | 66.3 |
| 2 | 3227 | 85.73 | 84.03 | 84.20 | 90.7 | 67.8 |
| Probability | <0.0001 | 0.1190 | 0.3062 | 0.3958 | 0.6625 | 0.2979 |
| Regression | Q | L | ns | ns | ns | ns |
| SEM | 22.7 | 0. 26 | 15.1 | 0.30 | 0.90 | 0.43 |
| CV (%) | 1.40 | 1.14 | 1.14 | 1.53 | 4.07 | 2.44 |
| BO (%) | Variables (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CY | BY | TY | DY | WY | RH | RL | RAF | RG | |
| 0 | 73.9 | 33.7 | 13.5 | 14.8 | 11.9 | 0.82 | 2.98 | 1.32 | 3.47 |
| 1 | 73.2 | 33.9 | 13.6 | 14.9 | 11.8 | 0.76 | 3.20 | 1.34 | 3.68 |
| 2 | 74.8 | 33.9 | 13.4 | 14.9 | 11.8 | 0.79 | 3.00 | 1.39 | 3.46 |
| Probability | 0.2307 | 0.8806 | 0.7107 | 0.8845 | 0.8284 | 0.5193 | 0.2716 | 0.7264 | 0.2380 |
| Regression | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| SEM | 0.37 | 0.28 | 0.08 | 0.11 | 0.08 | 0.02 | 0.06 | 0.04 | 0.06 |
| CV % | 2.08 | 3.69 | 2.73 | 3.34 | 3.06 | 9.95 | 8.09 | 12.31 | 6.74 |
| BO% | SF | CL | Breast Filet Color | |||||
|---|---|---|---|---|---|---|---|---|
| Kgf/cm | % | Raw | Cooked | |||||
| L* | a* | b* | L* | a* | b* | |||
| 0 | 2.271 | 27.88 | 55.0 | 1.23 a | 12.75 a | 56.44 | 2.15 b | 13.50 |
| 1 | 2.399 | 29.46 | 55.2 | 1.58 a | 14.88 b | 56.01 | 2.05 b | 14.66 |
| 2 | 2.523 | 28.17 | 53.6 | 2.42 b | 14.26 b | 55.03 | 3.17 a | 15.11 |
| Probability | 0.7581 | 0.8015 | 0.4337 | <0.0001 | 0.0202 | 0.5844 | 0.0065 | 0.0672 |
| Regression | ns | ns | ns | L | Q | ns | L | L |
| SEM | 129.5 | 0.98 | 0.57 | 0.13 | 0.31 | 0.55 | 0.17 | 0.29 |
| CV (%) | 34.37 | 16.10 | 5.65 | 23.73 | 9.57 | 5.52 | 30.63 | 10.34 |
| BO% | Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PCV | TPP | RBC | LEUC | HET | LYMPH | EOS | MON | HL | |
| (%) | (g/dL) | (×106/µL) | (n/µL) | ||||||
| 0 | 28.00 | 3.36 | 1.48 | 28,333 | 13,825 | 12,295 | 740 | 1916 | 1.17 |
| 1 | 27.83 | 3.23 | 1.51 | 23,500 | 13,243 | 8796 | 797 | 928 | 1.59 |
| 2 | 28.83 | 3.33 | 1.55 | 21,333 | 11,313 | 7566 | 850 | 1745 | 1.59 |
| Probability | 0.6374 | 0.7503 | 0.9236 | 0.3260 | 0.6297 | 0.1098 | 0.9800 | 0.2887 | 0.3370 |
| Regression | ns | ns | ns | ns | ns | L | ns | ns | ns |
| SEM | 0.43 | 0.07 | 0.07 | 1905 | 1064 | 961 | 213 | 268 | 0.13 |
| CV (%) | 6.82 | 9.48 | 20.86 | 32.73 | 36.44 | 39.24 | 108.7 | 71.82 | 37.28 |
| BO% | VH | VW | CD | CW | VCR | MW |
|---|---|---|---|---|---|---|
| Duodenum (μm) | ||||||
| 0 | 1688 | 193 | 167 | 58 | 10.0 | 267 |
| 1 | 1722 | 167 | 184 | 55 | 9.4 | 265 |
| 2 | 1653 | 189 | 190 | 60 | 8.8 | 267 |
| Probability | 0.8678 | 0.0533 | 0.0632 | 0.1005 | 0.1717 | 0.9737 |
| Regression | ns | Q | L | ns | L | ns |
| SEM | 47.03 | 4.65 | 4.26 | 1.13 | 0.26 | 4.20 |
| CV (%) | 11.51 | 7.98 | 8.24 | 7.01 | 9.88 | 7.12 |
| Jejunum (μm) | ||||||
| 0 | 1817 | 177 | 188 | 58 | 9.72 | 272 |
| 1 | 1815 | 172 | 180 | 52 | 10.1 | 283 |
| 2 | 1796 | 183 | 176 | 56 | 10.4 | 279 |
| Probability | 0.9676 | 0.3797 | 0.1232 | 0.3903 | 0.5867 | 0.8684 |
| Regression | ns | ns | L | ns | ns | ns |
| SEM | 32.30 | 3.32 | 2.44 | 1.93 | 0.23 | 7.41 |
| CV (%) | 7.24 | 7.23 | 4.93 | 14.39 | 8.95 | 11.32 |
| BO% | Variables | |||
|---|---|---|---|---|
| Cost (BRL/bird) | Gross Revenue (BRL/bird) | Profit (BRL/bird) | Profit Index (%) | |
| 0 | 4.43 c | 5.99 a | 1.56 a | 26.11 a |
| 1 | 4.91 b | 5.55 b | 0.63 b | 11.18 b |
| 2 | 5.56 a | 5.79 ab | 0.32 c | 5.53 c |
| Probability | <0.0001 | 0.0181 | <0.0001 | <0.0001 |
| SEM | 0.104 | 0.069 | 0.143 | 2.33 |
| CV (%) | 0.70 | 3.99 | 27.43 | 25.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourado, L.R.B.; Brauna, A.S.A.; Bezerra, R.M.; Sousa, I.S.; Carvalho, F.L.A.; Silva, G.G.; Moura, F.A.S.; Sousa, S.C.; Santos, R.A.; Silva, S.C.; et al. Buriti Oil (Mauritia flexuosa L.) as Functional Feed for Broiler Chickens. Poultry 2025, 4, 6. https://doi.org/10.3390/poultry4010006
Dourado LRB, Brauna ASA, Bezerra RM, Sousa IS, Carvalho FLA, Silva GG, Moura FAS, Sousa SC, Santos RA, Silva SC, et al. Buriti Oil (Mauritia flexuosa L.) as Functional Feed for Broiler Chickens. Poultry. 2025; 4(1):6. https://doi.org/10.3390/poultry4010006
Chicago/Turabian StyleDourado, Leilane R. B., Adriana S. A. Brauna, Roseane M. Bezerra, Iara S. Sousa, Franscica Luana A. Carvalho, Gabriela G. Silva, Francinete A. S. Moura, Samara C. Sousa, Renato A. Santos, Silvokleio C. Silva, and et al. 2025. "Buriti Oil (Mauritia flexuosa L.) as Functional Feed for Broiler Chickens" Poultry 4, no. 1: 6. https://doi.org/10.3390/poultry4010006
APA StyleDourado, L. R. B., Brauna, A. S. A., Bezerra, R. M., Sousa, I. S., Carvalho, F. L. A., Silva, G. G., Moura, F. A. S., Sousa, S. C., Santos, R. A., Silva, S. C., Silva, L. S., Gonçalves, L. M. F., & Miranda, R. S. (2025). Buriti Oil (Mauritia flexuosa L.) as Functional Feed for Broiler Chickens. Poultry, 4(1), 6. https://doi.org/10.3390/poultry4010006








