Do Laying Hens Form Stable Social Networks?—A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Set-Up
2.3. Behaviour Scoring and Data Analysis
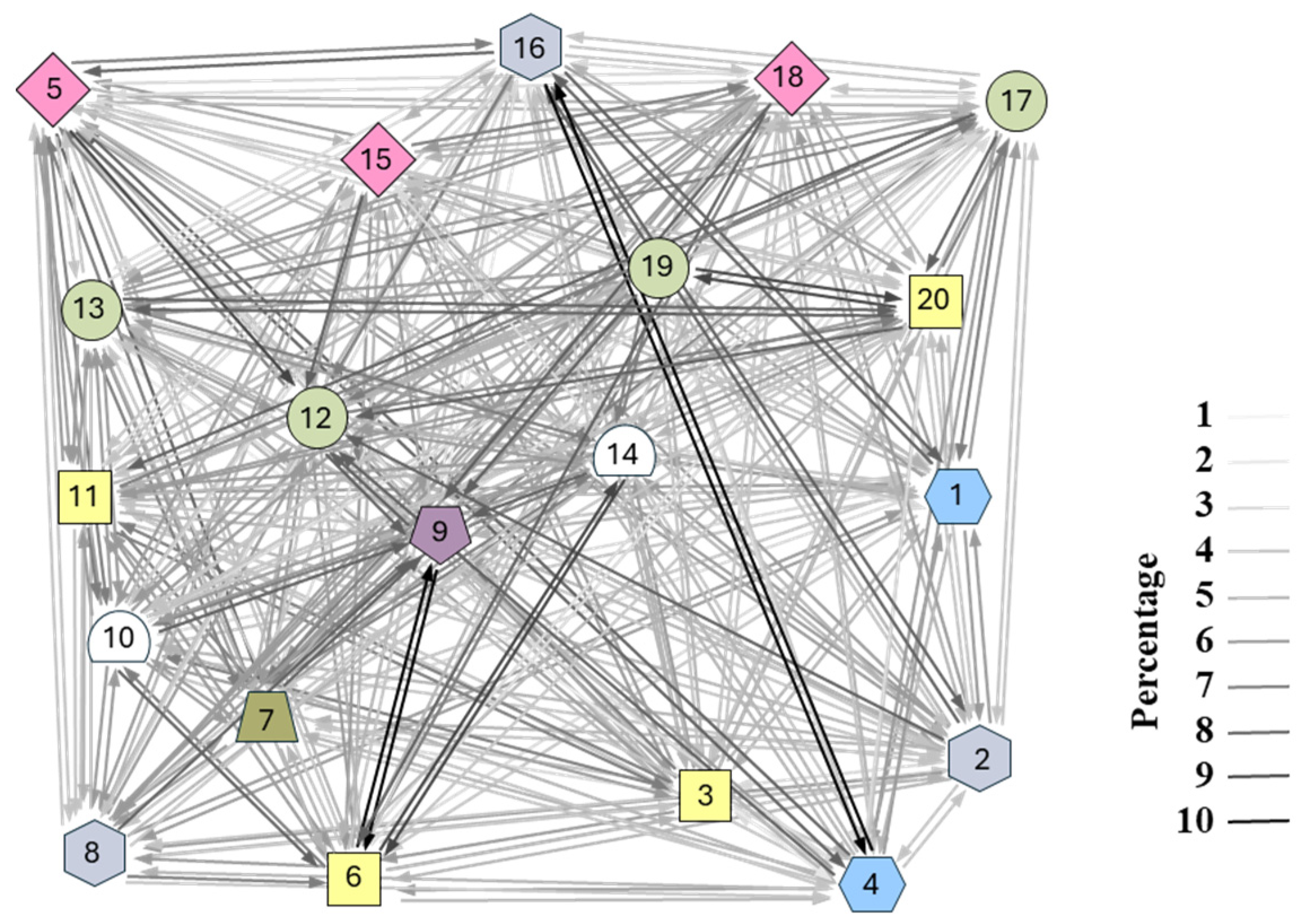
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krause, J.; Lusseau, D.; James, R. Animal social networks: An introduction. Behav. Ecol. Sociobiol. 2009, 63, 967–973. [Google Scholar] [CrossRef]
- Massen, J.; Sterck, E.; de Vos, H. Close social associations in animals and humans: Functions and mechanisms of friendship. Behaviour 2010, 147, 1379–1412. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Kaiser, S.; Sachser, N. Social buffering of the stress response: Diversity, mechanisms, and functions. Front. Neuroendocrinol. 2009, 30, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.P.; Badyaev, A.V. Structure of social networks in a passerine bird: Consequences for sexual selection and the evolution of mating strategies. Am. Nat. 2010, 176, E80–E89. [Google Scholar] [CrossRef]
- Cardoso, S.D.; Faustino, A.I.; Costa, S.S.; Valério, F.; Gonçalves, D.; Oliveira, R.F. Social network predicts loss of fertilizations in nesting males of a fish with alternative reproductive tactics. Acta. Ethol. 2017, 20, 59–68. [Google Scholar] [CrossRef]
- Wacker, S.; Ness, M.H.; Östlund-Nilsson, S.; Amundsen, T. Social structure affects mating competition in a damselfish. Coral. Reefs. 2017, 36, 1279–1289. [Google Scholar] [CrossRef]
- McDonald, G.C.; Spurgin, L.G.; Fairfield, E.A.; Richardson, D.S.; Pizzari, T. Differential female sociality is linked with the fine-scale structure of sexual interactions in replicate groups of red junglefowl. Gallus gallus. Proc. R. Soc. B 2019, 286, 20191734. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Keeling, L.J. Social discrimination and aggression by laying hens in large groups: From peck orders to social tolerance. Appl. Anim. Behav. Sci. 2003, 84, 197–212. [Google Scholar] [CrossRef]
- Collias, N.E.; Collias, E.C. Social organization of a red junglefowl, Gallus gallus, population related to evolution theory. Anim. Behav. 1996, 51, 1337–1354. [Google Scholar] [CrossRef]
- Javed, S.; Rahmani, A.R. Flocking and habitat use pattern of the Red Junglefowl Gallus gallus in Dudwa National Park, India. Trop. Ecol. 2000, 41, 11–16. [Google Scholar]
- Zakaria, M.; Arshad, M.I.; Sajap, A.S. Population size of red junglefowl (Gallus gallus Spadiceus) in agriculture areas. Pakistan. J. Sci. Ind. R. 2003, 46, 52–57. [Google Scholar]
- Sullivan, M.S. Flock structure in red junglefowl. Appl. Anim. Behav. Sci. 1991, 30, 381–386. [Google Scholar] [CrossRef]
- Lannhard Öberg, Å. Marknadsrapport animalieprodukter—Utvecklingen i Sverige till och med 2023. Swed. Board Agric. 2019. Available online: https://jordbruksverket.se/download/18.2a5bd7eb188fa97ada65a69f/1727779283303/Marknadsrapport-animalieprodukter-utvecklingen-tga.pdf (accessed on 1 December 2024).
- Svenska Ägg. Nulägesanalys av svensk äggproduktion, Bilaga 1. Sven. Ägg. 2015. Available online: https://www.svenskaagg.se/wp-content/uploads/2024/10/Nulagesanalys-2015.pdf (accessed on 1 December 2024).
- Tillstånd enligt 9 kap. miljöbalken för befintlig och utökad produktion av ägg och unghöns. Länsstyrelsen I Kalmar Län 2018. Available online: https://jordbruksverket.se/download/18.2c58b19f1807734f8f8bc11e/1651500109244/M%C3%B6nster%C3%A5s_AB%20CA%20Cedergren_Fj%C3%A4derf%C3%A4_Tillst%C3%A5nd_2018-05-30-tgau.pdf (accessed on 1 December 2024).
- Statens jordbruksverks föreskrifter och allmänna råd om fjäderfähållning inom lantbruket m.m., saknr L 111. Swed. Board Agric. 2019, 19. Available online: https://jvdoc.sharepoint.com/:b:/s/sjvfs/EY546msTN69JnQQhUP6e_u8BuLDlke8zVP0nZKnip4446A?download=1 (accessed on 1 December 2024).
- McBride, G.; Foenander, F. Territorial behaviour in flocks of domestic fowls. Nature 1962, 194, 102. [Google Scholar] [CrossRef]
- Odén, K.; Vestergaard, K.; Algers, B. Space use and agonistic behaviour in relation to sex composition in large flocks of laying hens. Appl. Anim. Behav. Sci. 2000, 67, 307–320. [Google Scholar] [CrossRef]
- Campbell, D.L.; Horton, B.J.; Hinch, G.N. Using radio-frequency identification technology to measure synchronised ranging of free-range laying hens. Animals 2018, 8, 210. [Google Scholar] [CrossRef]
- Gómez, Y.; Berezowski, J.; Jorge, Y.A.; Gebhardt-Henrich, S.G.; Vögeli, S.; Stratmann, A.; Toscano, M.J.; Voelkl, B. Similarity in temporal movement patterns in laying hens increases with time and social association. Animals 2022, 12, 555. [Google Scholar] [CrossRef]
- Väisänen, J.; Håkansson, J.; Jensen, P. Social interactions in Red Junglefowl (Gallus gallus) and White Leghorn layers in stable groups and after re-grouping. Br. Poult. Sci. 2005, 46, 156–168. [Google Scholar] [CrossRef]
- Nicol, C.; Gregory, N.; Knowles, T.; Parkman, I.; Wilkins, L. Differential effects of increased stocking density, mediated by increased flock size, on feather pecking and aggression in laying hens. Appl. Anim. Behav. Sci. 1999, 65, 137–152. [Google Scholar] [CrossRef]
- Hughes, B.; Carmichael, N.; Walker, A.; Grigor, P. Low incidence of aggression in large flocks of laying hens. Appl. Anim. Behav. Sci. 1997, 54, 215–234. [Google Scholar] [CrossRef]
- Estevez, I.; Newberry, R.C.; De Reyna, L.A. Broiler chickens: A tolerant social system. Etologia 1997, 5, 19–29. [Google Scholar]
- Dawkins, M.S. How do hens view other hens? The use of lateral and binocular visual fields in social recognition. Behaviour 1995, 132, 591–606. [Google Scholar] [CrossRef]
- Bradshaw, R. Discrimination of group members by laying hens Gallus domesticus. Behav. Process 1991, 24, 143–151. [Google Scholar] [CrossRef]
- Hughes, B. Selection of group size by individual laying hens. Br. Poult. Sci. 1977, 18, 9–18. [Google Scholar] [CrossRef]
- Abeyesinghe, S.M.; Drewe, J.A.; Asher, L.; Wathes, C.M.; Collins, L.M. Do hens have friends? Appl. Anim. Behav. Sci. 2013, 143, 61–66. [Google Scholar] [CrossRef]
- Koene, P.; Ipema, B. Social networks and welfare in future animal management. Animals 2014, 4, 93–118. [Google Scholar] [CrossRef]
- Veissier, I.; Le Neindre, P. Weaning in calves: Its effects on social organization. Appl. Anim. Behav. Sci. 1989, 24, 43–54. [Google Scholar] [CrossRef]
- van Dierendonck, M.C. The Importance of Social Relationships in Horses; Utrecht University: Utrecht, The Netherlands, 2006. [Google Scholar]
- Petersen, H.; Vestergaard, K.; Jensen, P. Integration of piglets into social groups of free-ranging domestic pigs. Appl. Anim. Behav. Sci. 1989, 23, 223–236. [Google Scholar] [CrossRef]
- Whitehead, H.; Dufault, S. Techniques for analyzing vertebrate social structure using identified individuals: Review and recommendations. Adv. Stud. Behav. 1999, 28, 3374. [Google Scholar]
- Silk, M.J. Conceptual representations of animal social networks: An overview. Anim. Behav. 2023, 201, 157–166. [Google Scholar] [CrossRef]
- Murniyati; Mutiara, A.B.; Wirawan, S.; Yusnitasari, T.; Anggraini, D. Expanding Louvain Algorithm for Clustering Relationship Formation. IJACSA 2023, 14, 701–708. [Google Scholar]
- Johnsson, M.; Henriksen, R.; Fogelholm, J.; Höglund, A.; Jensen, P.; Wright, D. Genetics and Genomics of Social Behavior in a Chicken Model. Genetics 2018, 209, 209–221. [Google Scholar] [CrossRef]




| Week 2 | Week 3 | Week 4 | ||
| Week 1 | Pearson’s r | 0.55 | 0.74 | 0.72 |
| df | 18 | 18 | 18 | |
| p-value | 0.011 | <0.001 | <0.001 | |
| Week 2 | Pearson’s r | 0.49 | 0.71 | |
| df | 18 | 18 | ||
| p-value | 0.027 | <0.001 | ||
| Week 3 | Pearson’s r | 0.47 | ||
| df | 18 | |||
| p-value | 0.033 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedlund, L.; Wright, D.; Jensen, P. Do Laying Hens Form Stable Social Networks?—A Case Study. Poultry 2025, 4, 7. https://doi.org/10.3390/poultry4010007
Hedlund L, Wright D, Jensen P. Do Laying Hens Form Stable Social Networks?—A Case Study. Poultry. 2025; 4(1):7. https://doi.org/10.3390/poultry4010007
Chicago/Turabian StyleHedlund, Louise, Dominic Wright, and Per Jensen. 2025. "Do Laying Hens Form Stable Social Networks?—A Case Study" Poultry 4, no. 1: 7. https://doi.org/10.3390/poultry4010007
APA StyleHedlund, L., Wright, D., & Jensen, P. (2025). Do Laying Hens Form Stable Social Networks?—A Case Study. Poultry, 4(1), 7. https://doi.org/10.3390/poultry4010007







